Intrauterine correction of open forms of spina bifida: comparative analysis of the results of V.I. Kulakov NMRC for OG&P of Minzdrav of Russia and the MOMS multicenter randomized trial
Petrova U.L., Shmakov R.G., Zinenko D.Yu., Gladkova K.A., Kostyukov K.V., Nikolaeva A.V., Sakalo V.A., Ostrik K.A., Chugunova L.A., Shramko A.V., Tarasenko Yu.I., Berdichevskaya E.M., Balashova E.N., Ionov O.V.
Objective: This study aimed to evaluate the results of intrauterine correction of open forms of spina bifida performed at V.I. Kulakov NMRC for OG&P of Minzdrav of Russia and compare them with the results of the MOMS trial.
Materials and methods: This study included 30 patients with a confirmed diagnosis of meningomyelocele or fetal rachischisis who underwent intrauterine treatment at the center. The inclusion criteria were completion of a perinatal consultation at the center, maternal age over 18 years, singleton pregnancy, normal fetal karyotype, gestational age between 19 and 25+6 weeks, presence of Arnold–Chiari malformation type II, cervical length >20 mm, absence of fetal kyphosis >30°, and signed informed consent for surgical intervention. Comparisons were made with 78 women from the MOMS trial whose fetuses underwent intrauterine meningomyelocele repair. Statistical analysis was performed using the StatTech v 3.1.10 (Stattech LLC, Russia) and Prism v. software. 8.0.1 (GraphPad Software, USA).
Results: The most common complication of intrauterine correction for open forms of spina bifida was preterm birth. The mean gestational age at delivery was 33.3 weeks. Premature placental abruption and premature rupture of membranes were observed in 13.3% and 30% of cases, respectively. Delivery was performed via cesarean section in all cases. Correction of Arnold–Chiari II malformation was observed in 100% of the cases, and CSF shunt surgery was performed in one case for intraventricular hemorrhage. Obstetric and neurological outcomes were comparable to those of the MOMS randomized multicenter trial.
Conclusion: Timely diagnosis and careful selection of fetuses for intrauterine correction of open forms of spina bifida lead to regression of Arnold–Chiari II syndrome with subsequent improvements in neurological outcomes in children managed with an integrated multidisciplinary approach.
Authors' contributions: Petrova U.L., Shmakov R.G. – conception and design of the study; Tarasenko Yu.I., Ostrik K.A., Shramko A.V., Balashova E.N., Nikolaeva A.V. – material collection and processing; Petrova U.L., Sakalo V.A. – statistical analysis; Petrova U.L., Chugunova L.A., Berdichevskaya E.M. – drafting of the manuscript; Shmakov R.G., Zinenko D.Yu., Gladkova K.A., Kostyukov K.V., Ionov O.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Petrova U.L., Shmakov R.G., Zinenko D.Yu., Gladkova K.A., Kostyukov K.V., Nikolaeva A.V., Sakalo V.A., Ostrik K.A., Chugunova L.A., Shramko A.V., Tarasenko Yu.I., Berdichevskaya E.M.,
Balashova E.N., Ionov O.V. Intrauterine correction of open forms of spina bifida: comparative analysis of the results of V.I. Kulakov NMRC for OG&P of Minzdrav of Russia and the MOMS multicenter randomized trial.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 27-36 (in Russian)
https://dx.doi.org/10.18565/aig.2024.114
Keywords
The 21st century is known for its effective, safe, evidence-based, and multidisciplinary approach for disease treatment. Currently, the etiology, pathogenesis, and treatment of diseases are considered at the intersection of various scientific disciplines.
Spina bifida is the most common central nervous system developmental anomaly, affecting 1 in 1000 fetuses at 12 weeks of pregnancy [1]. This condition leads to lifelong disability and occurs due to impaired neurulation in the 3rd–4th week of embryogenesis.
Although the exact causes of spina bifida are not fully understood, many risk factors have been identified, including genetic and epigenetic factors [2]. Chromosomal abnormalities such as trisomy 13 and 18 and triploidy contribute to less than 10% of all cases of neural tube defects (NTDs) [3]. Most cases are non-syndromic and occur sporadically [4]. Women who have had a previous pregnancy affected by spina bifida have a 3% empirical risk of recurrence in the next pregnancy, which increases to 10% if a second embryo with NTD is conceived [4].
Certain types of NTDs show a gender bias. For example, spina bifida occurs more frequently in female fetuses, possibly because of sex-related genetic or epigenetic effects. One factor alone is not sufficient to disrupt normal neurulation, but a combination of factors has a synergistic effect, leading to abnormal embryonic development [5]. In addition to genetic factors, environmental factors also play a role in NTDs. Modifiable risk factors include folic acid and vitamin deficiencies (such as vitamin B12 and vitamin C), excessive caffeine consumption, alcohol and nicotine intake, valproic acid use, hyperthermia (from saunas, hot water, or fever), obesity, and hyperglycemia. Despite programs promoting folic acid intake before conception and in the first trimester of pregnancy, some forms of spina bifida do not respond to folate treatment.
Spina bifida can be classified as occulta (closed) or aperta (open). Open spina bifida can take different forms, including hernial protrusions through defects in the spine and surrounding tissues (meningocele, which contains fluid, and meningomyelocele, which includes the spinal cord tissue). There is also a form without herniation called rachischisis, which is the most severe form in which the spinal cord remains open in the spinal canal. Open forms of spina bifida have a highly variable prognosis, depending on the level of damage. Clinical manifestations include neurological, motor, gastrointestinal, urological, skin, and orthopedic disorders [6]. Over 75% of children with meningomyelocele and rachischisis also have Arnold–Chiari malformation type II (CM-II). CM-II is characterized by herniation of the cerebellum and brainstem into the foramen magnum (FM). This condition often leads to hydrocephalus, which requires postnatal ventriculoperitoneal shunting. Unfortunately, this procedure is associated with a high risk of infection, and significantly worsens the underlying disease.
In the 1950s, the mortality rate of children with meningomyelocele was approximately 90%. However, owing to advancements in medicine and surgical interventions, survival rates have greatly improved. By the early 2000s, mortality rates had dropped to 24–51% [7–9]. Recent reports from 2011 and 2019 indicate that the mortality rate varies depending on the country's socio-economic status, ranging from 2.15% to 37% [10, 11]. The main causes of death included infectious complications, hydrocephalus, and complications associated with CM-II. Among patients diagnosed with hydrocephalus, the overall survival rate is 56%, whereas in those without hydrocephalus, it is noticeably higher at 86.7% [12]. In cases of symptomatic CM-II, the mortality rate increases to 25%, with 30% of deaths occurring within the first three months of life. The causes of death include infection of the central nervous system, cardiorespiratory disorders, renal failure, and unknown etiologies in over 30% of cases [13–15].
Spina bifida can often be diagnosed during fetal development through ultrasound examination, typically in the second trimester of pregnancy. However, some cases may be detected as early as the first trimester of pregnancy. Despite advances in the early diagnosis of chromosomal abnormalities and malformations, there is still a need to improve the prenatal diagnosis of neural tube defects [16–18]. With recent progress in fetal medicine and the possibility of intrauterine correction of open forms of spina bifida, the focus of diagnosis has shifted to the intracranial criteria. Classic ultrasound signs of CM-II include a "lemon" shaped skull, "banana" deformations of the cerebellum from its herniation, ventriculomegaly, and open spina bifida (meningomyelocele or rachischisis). Recent studies have also found an association between spinal dysraphism and various structural abnormalities of the central nervous system, such as impaired neuronal migration, cerebellar dysplasia, and corpus callosum abnormalities [19–27].
The standard treatment for meningomyelocele involves surgical closure of the defect after birth in the first 48 h of life [28]. Fetal surgery has become a pinnacle of medical development for the treatment of open forms of spina bifida. Evidence that amniotic fluid has long-term toxicity and progressive destruction of exposed neural tissue has led to an ethically and surgically challenging, cutting-edge 21st-century study. The advantages and disadvantages of intrauterine correction became known in 2011 when the results of a multicenter prospective randomized study (MOMS trial) were first published. In this study, the outcomes were compared between intrauterine meningomyelocele closure and postpartum closure. The study was terminated prematurely because of the apparent benefits of prenatal correction. Intrauterine treatment led to a reduction in the need for ventriculoperitoneal shunting, regression of the hindbrain herniation, improvement in mental development and motor functions, and increased the likelihood of the child walking independently [29]. The success of the MOMS study was a landmark achievement in the field of fetal medicine, and it set a new standard in the international field of maternal-fetal surgery. In 2021, the MOMS 2 study was published, which compared children from the MOMS trial aged 5–10 years. As a result of the study, encouraging data were obtained: children who underwent meningomyelocele surgery in utero more often walked without orthopedic or assistive devices (29% vs. 11%; p=0.06), had higher average functional rehabilitation scores when assessing sensory-neurological indicators (92±9 vs. 85±18; p<0.001), hindbrain herniation occurred less frequently (60% vs. 87%; p<0.001), ventriculoperitoneal shunting due to hydrocephalus was required less often (49% vs. 85%, p<0.001), and patients with installed shunts underwent revision less often (47% vs. 70%; p=0.02) than those in the postnatal group. Parents of children with intrauterine correction have reported a higher average quality of life [30]. The results obtained prove the effectiveness and importance of timely intrauterine treatment of meningomyeloceles.
First intrauterine correction of meningomyelocele at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, was performed in February 2019, together with neurosurgeons from the Veltischev Research and Clinical Institute for Pediatrics and Pediatric Surgery under the guidance of an experienced surgeon specializing in these types of operations, Professor Sergio Cavalheiro from the University of São Paulo (Brazil).
Materials and methods
Between February 2019 and September 2023, 30 intrauterine corrections of the open forms of spina bifida were performed at the Center. Gestational age was determined according to crown rump length during ultrasound screening in the first trimester of pregnancy. All fetuses underwent expert examination of anatomy, extended two-dimensional echocardiography, morphometric assessment of brain structures, and Doppler measurements. Ultrasound examination was performed using a 3.5 MHz 2D convex probe and a 5–9 MHz 3D convex probe on Voluson E8 and Samsung Medison WS80.
All patients underwent a perinatal consultation at the Center, during which the benefits, risks, and possible complications of in utero treatment were explained, followed by obtaining informed consent for surgical treatment. At the preoperative preparation stage, a comprehensive clinical, laboratory, and instrumental examination was carried out in a hospital setting, including magnetic resonance imaging (MRI) of the fetus and amniocentesis for chromosomal microarray analysis.
Clinical data on the condition of the children were obtained during the examination of newborns in inpatient treatment in the NICU[1] and the DPNPI[2] of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, as well as medical records and/or telephone interviews with parents and/or doctors at the place of residence. The psychomotor development of children (cognitive function, speech, and motor skills) was assessed using the CAT/CLAMS scale [21].
Statistical analysis
Accumulation, adjustment, and systematization of source information were performed using Microsoft Office Excel spreadsheets. Statistical analysis was performed using StatTech v. 2.6.5 (Stattech LLC, Russia). The critical level of significance of the null hypothesis was taken to be p˂0.05 (95% confidence level). Data distributions were tested for normality using the Shapiro–Wilk test. Continuous variables showing a normal distribution were expressed as mean (M), standard deviation (SD), and 95% confidence interval (95% CI). Categorical variables are described as frequencies and percentages. Normally distributed continuous variables were compared between the two groups using Student’s t-test, provided equal variance. The obtained values of Student's t-test were assessed by comparison with the critical values. Differences between the groups were considered statistically significant at p<0.05.
Course of the operation
The surgery was performed by a multidisciplinary team including obstetrician-gynecologists, neurosurgeons from the Veltischev Research and Clinical Institute of Pediatrics and Pediatric Surgery, an ultrasound diagnostic specialist, an anesthesiologist-intensivist, and doctors from the neonatal intensive care unit.
The operation can be roughly divided into three stages: the first and third stages are obstetrical and the second is neurosurgical. The first stage involves opening the anterior abdominal wall layer-by-layer and exteriorizing the uterus. Ultrasound navigation determines the location of placental tissue and fetal heart rate. When necessary, the fetus was rotated. After selecting the optimal site for the incision, two separate ligatures were placed on the uterus, between which an incision was made with a monopolar coagulator, then 4 Alice clamps were then placed, and the amniotic membranes were sutured with separate Vicryl sutures. The uterine wall between the sutures was cut with scissors to expose the fetus. The fetus was anesthetized by intramuscular injection of a fentanyl solution, followed by assessment of the fetal heart rate by ultrasound and periodically throughout the surgical procedure. The second stage was performed by the neurosurgeons. The soldered section of the spinal cord was separated from the hernial sac, the spinal cord was immersed in the spinal canal, plastic surgery was performed with local tissues, and the skin was restored using separate interrupted sutures (Fig. 1). Throughout the procedure, the leaking amniotic fluid was replaced with warm sterile saline solution. An antibacterial drug is administered intra-amniotically to reduce the risk of septic complications. In the third stage, the integrity of the uterus and anterior abdominal wall was restored layer-by-layer. At the end of the surgical procedure, fetal heart rate and amniotic fluid volume were monitored.
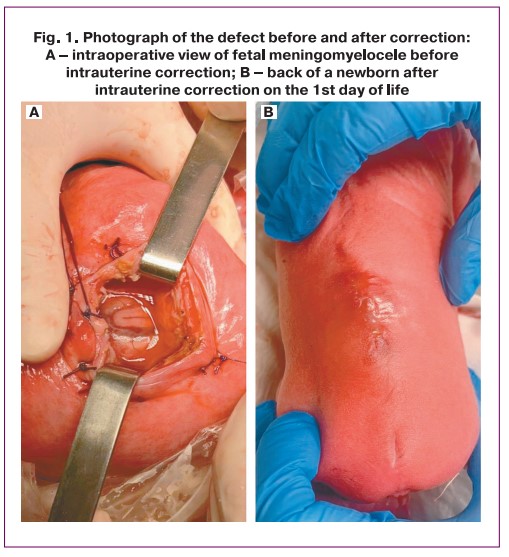
Results
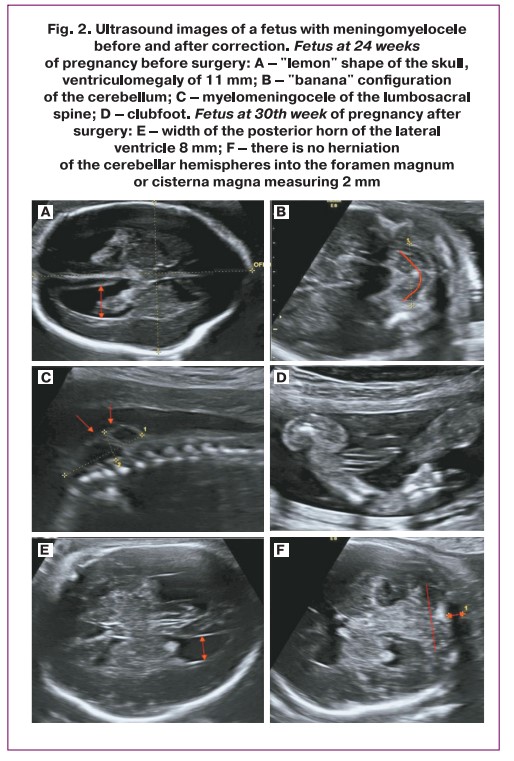
CM-II was diagnosed in all fetuses at 19–21 weeks of gestation. In all cases, the generally accepted echographic signs of CM-II were identified, including cerebellar herniation into the foramen magnum with a sign of deformation (“banana” shape), changes in the configuration of the skull bones (“lemon” shape), ventriculomegaly, and the presence of spina bifida (Fig. 2). In most fetuses, the initial level of spina bifida (myelomeningocele/rachischisis) was located at the L2–L3 vertebra.
All 30 fetuses had the following abnormalities: ventriculomegaly (10–17 mm regardless of the level of spinal cord damage), "beak-shaped" tectum (roof of the brain), "pointed" pointed to occipital horns of the lateral ventricles, delayed formation of sulci. Slight shortening of the length of the corpus callosum was observed in all fetuses, of which 3/30 (10%) were diagnosed with dysgenesis and 1/30 (3.3%) with partial agenesis. Dorsal cyst-like enlargement of the third ventricle was observed in half of the fetuses, confirming the hypothesis of weakening of the caudal part of the corpus callosum in this defect. Agenesis of the septum pellucidum was observed in 6/30 (20%) fetuses.
Echographic evaluation of brain structures after intrauterine correction revealed several characteristic features. In the majority of fetuses, there was a 2–3 mm increase in the size of the lateral ventricles at 6 weeks postoperatively, with no increase up to the time of delivery. One fetus with severe holoprosencephaly developed hydrocephalus. Caudal displacement of the cerebellar tonsils without herniation into the foramen magnum was noted in all the cases. A large cistern (dimensions 2–3 mm) was visualized in half of the subjects.
Abnormal opercularization of the Sylvian fissure was observed in 80% of cases (24 patients).
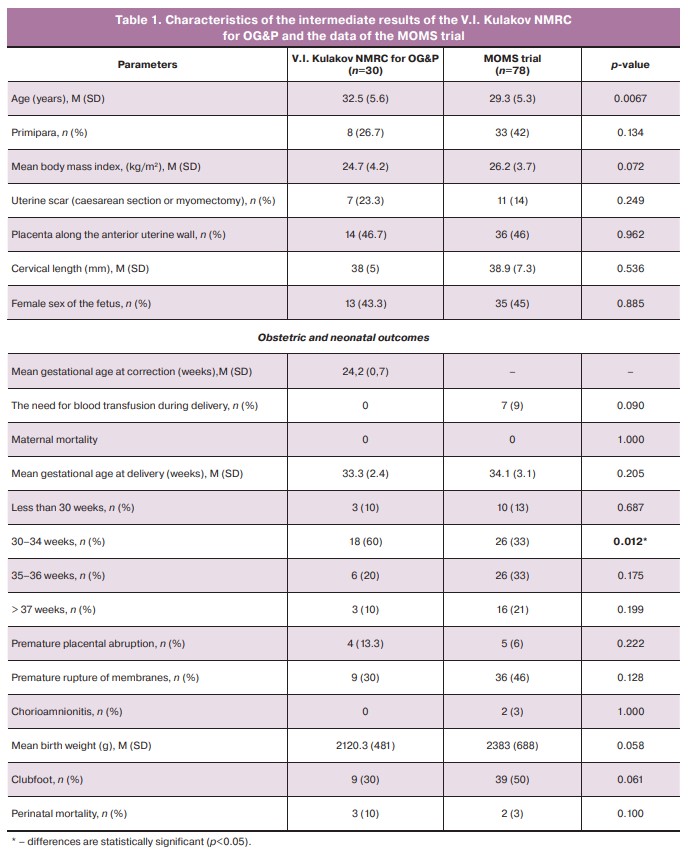
In 100% of the cases (30 patients), pregnant women underwent cesarean section, of which 28/30 (93.3%) underwent emergency cesarean deliveries. The indications for delivery were the onset of regular labor in 11/30 (36.7%) cases, placental abruption in 4/30 (13.3%) cases, suspected uterine scarring after correction in 3/30 (10%) cases, and premature rupture of membranes in 9/30 (30%) cases. Of these, 4/9 (44.4%) pregnancies were prolonged in the pathology department of pregnancy under the supervision of clinical and laboratory examinations and functional diagnostic tests before regular labor. The mean age of the pregnant women was 32.5 (5.6) years, and 8/30 (26.7%) women were primigravida. The mean gestational age during intrauterine treatment was 24.2 (0.7) weeks and 33.3 (2.4) weeks at delivery (Table 1).
Twenty-seven children are under observation, of whom 3/30 (10%) died in the early neonatal period due to prematurity. The oldest child is 4.5 years old. After birth, 28/30 newborns (2 patients gave birth at their place of residence) were treated in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, where they underwent ultrasound examination of the central nervous system, kidneys, and bladder, and consultations with a neurosurgeon, neurologist, orthopedist, and MRI for diagnostic purposes. Follow-up MRI showed, that CM-II was corrected in 100% of the cases. Upon discharge, each patient received a patient referral form with recommendations.
Further observation of patients was carried out full-time or in correspondence format due to the remoteness of the regions. Neuropsychological and motor development was assessed using the international scale "The Cognitive Adaptive Test/Clinical Linguistic and Auditory Milestone Scale" (CAT/CLAMS), adapted in Russia, unlike from Bayley III Scale, which is used in all international studies, but at the time of writing this article has not been adapted and validated in the Russian Federation. These scales correlate well with one another and are highly sensitive and specific. Observations by a neurosurgeon were also carried out in a part-time format: patients provided neurosonography and MRI data within the prescribed time frame.
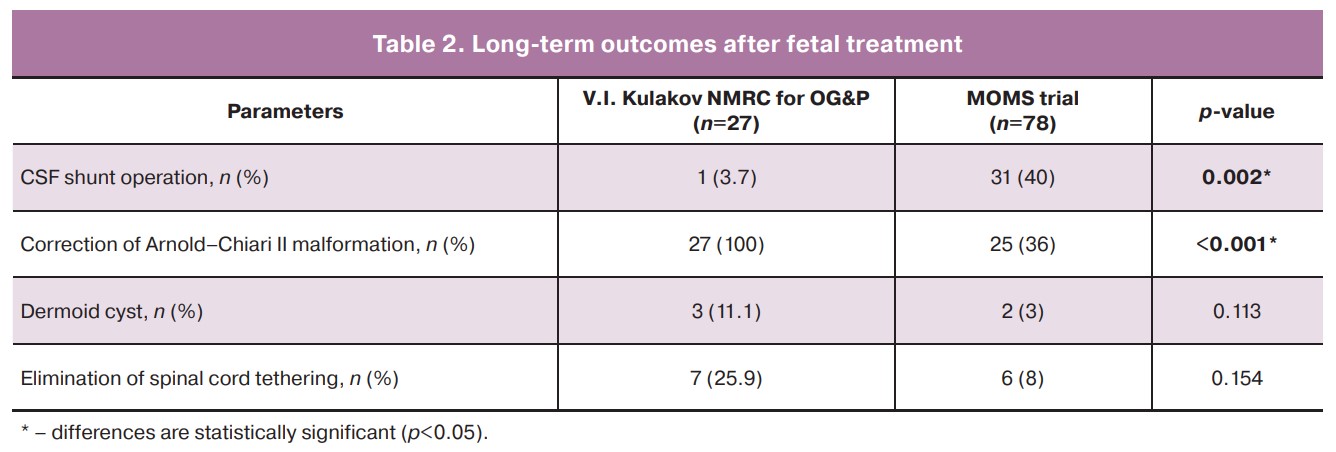
Only one child required CSF shunt surgery due to intraventricular hemorrhage (3.7%), and CM-II was corrected in 100% of cases after birth (Table 2). Removal of the tethered spinal cord was performed in 7/27 (25.9%) patients (Table 2). Assessment of the developmental characteristics of the patients showed a significant effect compared to the traditional approach. Of the 27 children, 24 reached the age of 12 months or more. The main criteria for the success of fetal operations were verticalization, independent walking, and walking with support, which were observed in 12/24 (50%), 5/24 (20.8%), 5/24 (20.8%) children, respectively. It is important to note that 10/30 patients (33.3%) had rachischisis, which is the most severe form of spina bifida. Repeated suturing of the suture defect was required in 1/27 (3.7%) patient.
Assessment of cognitive functions using the CAT/CLAMS scale showed that in 95% of our patients the scores ranged from 85 to 100%, which is normal. This trend continues as children grow older.
Discussion
This is the first study to evaluate the outcomes of intrauterine correction of open forms of spina bifida. The study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, in comparison with the MOMS trial. Our study showed that obstetric and neonatal outcomes are comparable to those of the MOMS trial and are even superior in terms of neurological outcomes.
Most of the assessed parameters did not differ significantly between the groups. However, some parameters exhibited significant differences. Although complications such as premature rupture of membranes (30% vs. 46%, p=0.105) and premature abruption of a normally located placenta (13.3% vs. 6%, p=0.240) were more common in our patient cohort, these differences were not statistically significant.
Preterm birth is the most common complication of intrauterine correction of open forms of spina bifida. In our study, late preterm birth (34+0–36+6 weeks) occurred in 40% of the cases, which resulted in the most favorable neonatal outcomes. The mean gestational age at the time of delivery was not significantly different (p=0.205) and was 33.3 and 34.1 weeks, respectively. When assessing the timing of delivery, we found that the rate of delivery at less than 30 weeks of gestation in our group of patients and the MOMS group was comparable (10% and 13%, respectively, p=1,000). At 30–34 weeks, operative delivery occurred in 60% of our group compared with 33% of the MOMS trial group; this difference was statistically significant (p=0.011). At 35–36 weeks, 20% of the pregnant women in our group gave birth compared to 33% in the MOMS group (p=0.123). At full term, operative delivery occurred in 10% of the cases in our group compared to 21% in the MOMS group (p=0.246). The mean body weight of the newborns in our group was 2120.3 g, which did not differ significantly from that of the MOMS group (2383 g, p=0.058).
In our group, obstetric complications, such as chorioamnionitis, endometritis, sepsis, uterine rupture, and maternal mortality, were not diagnosed.
In addition to the well-known echographic features, we identified structural changes in the brain, including abnormalities in the corpus callosum, configuration of the tectum, formation of sulci, interhemispheric holoprosencephaly, and dorsal expansion of the third ventricle, in all fetuses with CM-II. These findings are consistent with those of previous studies [5–12]. The change in the topography of the structures of the posterior cranial fossa after intrauterine correction of spina bifida revealed during a dynamic ultrasound examination, namely, the absence of herniation of the cerebellum into the foramen magnum, the formation of the cistern magna, and the stable dimensions of the lateral ventricles, indicating the restoration of liquor exchange in this category of patients.
CM-II correction in 100% of cases by the time of birth is of great importance as it eliminates the need for another surgical intervention, decompression of the posterior cranial fossa, in contrast to patients operated on after birth (approximately 40%). In addition, a number of publications have been devoted to decreased learning ability in patients with CM-II. Correction of spinal cord tethering was performed in 25.9% of the patients and did not differ significantly from the MOMS trial group (p=0.154).
In our study, intrauterine surgical treatment was performed in 33.3% of the fetuses with rachischisis. Children who are not operated on prenatally are born with complete paralysis of the lower extremities and gross dysfunction of the pelvic organs. Unfortunately, subsequent surgical interventions do not lead to improvement of function but are only aimed at restoring the anatomical integrity of the system. Our patients became vertical after fetal correction at the age of ≥ 1 year.
Conclusion
Open forms of spina bifida are extremely complex defects associated with multiple pathologies, including those of the central nervous system and other organs and systems. A comprehensive interdisciplinary approach to the intrauterine treatment of meningomyelocele and spina bifida, as well as a proven algorithm for subsequent rehabilitation, provides the most favorable outcomes for children with open spina bifida. Our results confirm the need to continue the development of fetal surgery in the Russian Federation. The medical community must change its attitude toward this cohort of patients as syndromic and unpromising, which leads to worsening disability and decreased quality of life in children with spina bifida and their families.
[1] Neonatal intensive care unit.
[2] Department of pathology of newborns and preterm infants.
References
- https://www.fetalmedicine.org/education/fetal-abnormalities/spine/open-spina-bifida
- Avagliano L., Massa V., George T.M., Qureshy S., Bulfamante G.P., Finnell R.H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019; 111(19): 1455-67. https://dx.doi.org/10.1002/bdr2.1380.
- Kennedy D., Chitayat D., Winsor E.J., Silver M., Toi A. Prenatally diagnosed neural tube defects: ultrasound, chromosome, and autopsy or postnatal findings in 212 cases. Am. J. Med. Genet. 1998; 77(4): 317-21. https://dx.doi.org/10.1002/(sici)1096-8628(19980526)77:4<317::aid-ajmg13>3.0.co;2-l.
- Copp A.J., Adzick N.S., Chitty L.S., Fletcher J.M., Holmbeck G.N., Shaw G.M. Spina bifida. Nat. Rev. Dis. Primers. 2015; 1: 15007. https://dx.doi.org/10.1038/nrdp.2015.7.
- Harris M.J., Juriloff D.M. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2007; 79(3): 187-210. https://dx.doi.org/10.1002/bdra.20333.
- Alabi N.B., Thibadeau J., Wiener J.S., Conklin M.J., Dias M.S., Sawin K.J. et al. Surgeries and health outcomes among patients with spina bifida. Pediatrics. 2018; 142(3): e20173730. https://dx.doi.org/10.1542/peds.2017-3730.
- McLone D.G. Treatment of myelomeningocele: arguments against selection. Clin. Neurosurg. 1986; 33: 359-70.
- Bowman R.M., McLone D.G., Grant J.A., Tomita T., Ito J.A. Spina bifida outcome: a 25-year prospective. Pediatr. Neurosurg. 2001; 34(3): 114-20. https://dx.doi.org/10.1159/000056005.
- Oakeshott P., Hunt G.M. Long-term outcome in open spina bifida. Br. J. Gen. Pract. 2003; 53(493): 632-6.
- Spoor J.K.H., Gadjradj P.S., Eggink A.J., DeKoninck P.L.J., Lutters B., Scheepe J.R. et al. Contemporary management and outcome of myelomeningocele: the Rotterdam experience. Neurosurg. Focus. 2019; 47(4): E3. https://dx.doi.org/10.3171/2019.7.FOCUS19447.
- Warf B.C., Wright E.J., Kulkarni A.V. Factors affecting survival of infants with myelomeningocele in southeastern Uganda. J. Neurosurg. Pediatr. 2011; 7(2): 127-33. https://dx.doi.org/10.3171/2010.11.PEDS10428.
- Tennant P.W., Pearce M.S., Bythell M., Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010; 375(9715): 649-56. https://dx.doi.org/10.1016/S0140-6736(09)61922-X.
- Borgstedt-Bakke J.H., Fenger-Grøn M., Rasmussen M.M. Correlation of mortality with lesion level in patients with myelomeningocele: a population-based study. J. Neurosurg. Pediatr. 2017; 19(2): 227-31. https://dx.doi.org/10.3171/2016.8.PEDS1654.
- McDowell M.M., Blatt J.E., Deibert C.P., Zwagerman N.T., Tempel Z.J., Greene S. Predictors of mortality in children with myelomeningocele and symptomatic Chiari type II malformation. J. Neurosurg. Pediatr. 2018; 21(6): 587-96. https://dx.doi.org/10.3171/2018.1.PEDS17496.
- Bowman R.M., Lee J.Y., Yang J., Kim K.H., Wang K.C. Myelomeningocele: the evolution of care over the last 50 years. Childs Nerv. Syst. 2023; 39(10): 2829-45. https://dx.doi.org/10.1007/s00381-023-06057-1.
- Paladini D., Malinger G., Birnbaum R., Monteagudo A., Pilu G., Salomon L.J. et al. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet. Gynecol. 2021; 57(4): 661-71. https://dx.doi.org/10.1002/uog.23616.
- Volpe N., Dall'Asta A., Di Pasquo E., Frusca T., Ghi T. First-trimester fetal neurosonography: technique and diagnostic potential. Ultrasound Obstet. Gynecol. 2021; 57(2): 204-14. https://dx.doi.org/10.1002/uog.23149.
- Kozlowski P., Burkhardt T., Gembruch U., Gonser M., Kähler C., Kagan K.O. et al. DEGUM, ÖGUM, SGUM and FMF Germany Recommendations for the implementation of first-trimester screening, detailed ultrasound, cell-free DNA screening and diagnostic procedures. Ultraschall. Med. 2019; 40(2): 176-93. https://dx.doi.org/10.1055/a-0631-8898.
- Memet Özek M., Cinalli G., Maixner W.J., eds. Spina bifida: management and outcome. Springer Science & Business Media; 2008.
- Callen A.L., Filly R.A. Supratentorial abnormalities in the Chiari II malformation, I: the ventricular "point". J. Ultrasound Med. 2008; 27(1): 33-8. https://dx.doi.org/10.7863/jum.2008.27.1.33.
- Callen A.L., Stengel J.W., Filly R.A. Supratentorial abnormalities in the Chiari II malformation, II: tectal morphologic changes. J. Ultrasound Med. 2009; 28(1): 29-35. https://dx.doi.org/10.7863/jum.2009.28.1.29.
- Wong S.K., Barkovich A.J., Callen A.L., Filly R.A. Supratentorial abnormalities in the Chiari II malformation, III: The interhemispheric cyst. J. Ultrasound Med. 2009; 28(8): 999-1006. https://dx.doi.org/10.7863/jum.2009.28.8.999.
- Filly M.R., Filly R.A., Barkovich A.J., Goldstein R.B. Supratentorial abnormalities in the Chiari II malformation, IV: the too-far-back ventricle. J. Ultrasound Med. 2010; 29(2): 243-8. https://dx.doi.org/10.7863/jum.2010.29.2.243.
- Finn M., Sutton D., Atkinson S., Ransome K., Sujenthiran P., Ditcham V. et al. The aqueduct of Sylvius: a sonographic landmark for neural tube defects in the first trimester. Ultrasound Obstet. Gynecol. 2011; 38(6): 640-5. https://dx.doi.org/10.1002/uog.10088.
- Leibovitz Z., Shkolnik C., Haratz K.K., Malinger G., Shapiro I., Lerman-Sagie T. Assessment of fetal midbrain and hindbrain in mid-sagittal cranial plane by three-dimensional multiplanar sonography. Part 2: application of nomograms to fetuses with posterior fossa malformations. Ultrasound Obstet. Gynecol. 2014; 44(5): 581-7. https://dx.doi.org/10.1002/uog.13312.
- Pugash D., Hendson G., Dunham C.P., Dewar K., Money D.M., Prayer D. Sonographic assessment of normal and abnormal patterns of fetal cerebral lamination. Ultrasound Obstet. Gynecol. 2012; 40(6): 642-51. https://dx.doi.org/10.1002/uog.11164.
- Pooh R.K., Machida M., Nakamura T., Uenishi K., Chiyo H., Itoh K. et al. Increased Sylvian fissure angle as early sonographic sign of malformation of cortical development. Ultrasound Obstet. Gynecol. 2019; 54(2): 199-206. https://dx.doi.org/10.1002/uog.20171.
- Морозов С.Л., Полякова О.В., Яновская Н.В., Зверева А.В., Длин В.В. Spina Bifida. Современные подходы и возможности к диагностике, лечению и реабилитации. Практическая медицина. 2020; 18(3): 32-7. [Morozov S.L., Polyakova O.V., Yanovskaya N.V., Zvereva A.V., Dlin V.V. Spina Bifida. Modern approaches and opportunities for diagnosis, treatment and rehabilitation. Practical Medicine. 2020; 18(3): 32-7. (in Russian)]. https://dx.doi.org/10.32000/2072-1757-2020-3-32-37.
- Adzick N.S., Thom E.A., Spong C.Y., Brock J.W. 3rd, Burrows P.K., Johnson M.P. et al.; MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011; 364(11): 993-1004. https://dx.doi.org/10.1056/NEJMoa1014379.
- Houtrow A.J., MacPherson C., Jackson-Coty J., Rivera M., Flynn L., Burrows P.K. et al. Prenatal repair and physical functioning among children with myelomeningocele: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2021; 175(4): e205674. https://dx.doi.org/10.1001/jamapediatrics.2020.5674.
Received 13.05.2024
Accepted 04.06.2024
About the Authors
Uliana L. Petrova, PhD, Junior Researcher at the Innovation Development Department of the Department of Regional Cooperation and Integration, obstetrician-gynecologist at the 2nd Physiological Obstetric Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(991)118-24-31, u_petrova@oparina4.ru, https://orcid.org/0000-0003-0388-3104Roman G. Shmakov, Dr. Med. Sci., Professor of the Russian Academy of Sciences, Director of the V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, Ministry of Health of Russia, 101000, Russia, Moscow, Pokrovka str., 22A; Professor at the Academician G.M. Savelyeva Department of Obstetrics and Gynecology of Pediatric Faculty, N.I. Pirogov Russian National Research University, Ministry of Health of Russia; Chief Non-staff Specialist in Obstetrics at Ministry of Health of Russia, mdshmakov@mail.ru, https://orcid.org/0000-0002-2206-1002
Dmitriy Yu. Zinenko, Dr. Med. Sci., Professor, Head of the Department of Neurosurgery, Veltischev Research and Clinical Institute for Pediatrics and Pediatric Surgery of Pirogov Russian National Research Medical University, 125412, Russia, Moscow, Taldomskaya str., 2, +7(985)769-22-88, zinenko1959@mail.ru,
https://orcid.org/0000-0003-0477-1016
Kristina A. Gladkova, PhD, Senior Researcher at the Department of Fetal Medicine of the Institute of Obstetrics, Head of the 1st Obstetric Department of Pregnancy Pathology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495) 438-07-88, k_gladkova@oparina4.ru,
https://orcid.org/0000-0001-8131-4682
Kirill V. Kostyukov, Dr. Med. Sci., Head of the Department of the Ultrasound and Functional Diagnosis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparina str., 4, k_kostyukov@oparina4.ru
Anastasia V. Nikolaeva, PhD, Chief Physician, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(916)653-43-09, a_nikolaeva@oparina4.ru
Victoria A. Sakalo, PhD, Junior Researcher at the Department of Obstetric and Extragenital Pathology, obstetrician-gynecologist at the 1st Obstetric Department of Pregnancy Pathology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-07-88, v_sakalo@oparina4.ru,
https://orcid.org/0000-0002-5870-4655
Kirill A. Ostrik, anesthesiologist and intensive care physician at the Department of Anesthesiology and Intensive Care, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, k_ostrik@oparina4.ru, https://orcid.org/0009-0005-6064-665С
Liliyana A. Chugunova, PhD, Senior Researcher at the Department of Ultrasound and Functional Diagnosis, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str.4, Liliyana79@mail.ru
Artem V. Shramko, neurosurgeon at the Neurosurgery Department, Veltischev Research and Clinical Institute for Pediatrics and Pediatric Surgery of Pirogov Russian National Research Medical University, 125412, Russia, Moscow, Taldomskaya str., 2, +7(905)508-49-85, Shramko@pedklin.ru
Yuliya I. Tarasenko, Junior Researcher at the Innovation Development Department of the Department of Regional Cooperation and Integration, obstetrician-gynecologist at the Obstetric Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(968)864-67-82,
yu_tarasenko@oparina4.ru, https://orcid.org/0009-0005-1945-2108
Evgenia M. Berdichevskaya, neurologist, neurophysiologist at the Neurosurgery Department, Veltischev Research and Clinical Institute for Pediatrics and Pediatric Surgery of Pirogov Russian National Research Medical University, 125412, Russia, Moscow, Taldomskaya str., 2, +7(916)666-21-45, dr.maratovna@gmail.com,
https://orcid.org/0000-0001-6285-1968
Ekaterina N. Balashova, PhD, Leading Researcher at the Prof. A.G. Antonov Department of Anesthesiology and Intensive Care, Associate Professor at the Department of Neonatology of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-22-77, e_balashova@oparina4.ru, https://orcid.org/0000-0002-3741-0770
Oleg V. Ionov, Dr. Med. Sci., Head of the Prof. A.G. Antonov Department of Anesthesiology and Intensive Care, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor at the Department of Neonatology, Faculty of Pediatrics, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119435 Russia, Moscow, Bolshaya Pirogovskay str., 2-4, +7(495)438-22-77, o_ionov@oparina4.ru,
https://orcid.org/0000-0002-4153-133X



