Intrauterine use of granulocyte colony-stimulating factor in patients with thin endometrium in frozen embryo transfer programs
Objective. To determine the efficacy of intrauterine use of granulocyte colony-stimulating factor (G-CSF) for the increase of endometrial thickness and pregnancy rates in patients with thin endometrium in frozen embryo transfer programs.Dzhincharadze L.G., Abubakirov A.N., Mishieva N.G.
Materials and methods. The main group included 15 patients with thin endometrium, which was refractory to standard methods of treatment; embryo transfer cycles were cancelled several times due to insufficient endometrial thickness in these patients. In addition to hormone replacement therapy (HRT), the patients were given an intrauterine injection of G-CSF on the 5–6th and 12–13th days of the menstrual cycle. The control group included 17 patients with thin endometrium, they received only HRT. The endometrial thickness was measured by ultrasound on the 14–15th and 20–21st (the day of embryo transfer) days of the menstrual cycle. The primary outcome was an increase in endometrial thickness greater than 7 mm on the day of embryo transfer, the secondary outcome was pregnancy rate.
Results. Endometrial thickness greater than 7 mm was observed in 7 patients (46.67%) in G-CSF group, and in 8 (47.06%) patients in the control group; the difference was not statistically significant (p=0.983). The average increase in endometrial thickness compared to the previous cycle was 0.6 mm. The average thickness of the endometrium was 7.32 mm in the main group. Embryo transfer was performed in 8 (53.33%) patients in G-CSF group, pregnancy occurred in 4 (50%) patients, clinical pregnancy in 3 (37.5%) patients, one patient had a biochemical pregnancy. At the time of the article’s publication there were two (25%) livebirths and one ongoing pregnancy. In the control group, the average thickness of the endometrium was 6.9 mm. Embryo transfer was performed in 4 patients (23.53%). But no pregnancy was achieved in any of the patients, the difference was not statistically significant (p=0.84).
Conclusion. Despite the results that were not statistically significant, patients in G-CSF group showed a tendency for the increase in endometrial thickness and pregnancy rates, as well as the decrease in embryo transfer cycle cancellations. It is worth conducting studies with a larger sample of patients to obtain more reliable results.
Keywords
In clinical practice, the minimum thickness of the endometrium associated with higher pregnancy rates, is considered to be 7 mm at the end of the follicular phase of the menstrual cycle [1]. Endometrial thickness greater than this threshold level is associated with high pregnancy rates in in vitro fertilization (IVF) and frozen embryo transfer programs [2]. The study [3] showed that thin endometrium plays a negative role in ART programs, reducing the pregnancy rates by 9.1 times.
Despite the fact that the incidence of thin endometrium is not high and is equal to 2.4%, according to Kasius et al. [4], currently this problem remains unsolved and such patients have a high frequency of canceling embryo transfer (ET) cycles. But nowadays, there is no consensus on what endometrial thickness is sufficient for successful implantation. Moreover, it is likely that this thickness can be different for cycles with ovarian stimulation and for cycles with endometrial preparation in oocyte donation programs, surrogacy and frozen embryo transfer programs [5]. In the stimulated cycles of standard IVF programs involving the use of freshly obtained patient’s own oocytes, the average endometrial thickness is 8–12 mm on the day of ovulation trigger if there is at least one normally developing follicle in the ovaries, according to the ultrasound assessment [6].
A lot of treatment protocols have been proposed to increase the endometrial thickness, including the use of low-dose aspirin, vasodilators, intravaginal administration of vitamin E, L-arginine and sildenafil citrate [7], intrauterine administration of stem and progenitor cells. But, despite the large number of prescribed medications, there is no reliably effective method for treating thin endometrium.
In 2011, Gleicher et al. were the first to suggest using granulocyte colony-stimulating factor for the treatment of thin endometrium in patients in IVF programs [8].
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein that belongs to the group of colony-stimulating factors and stimulates the development of a colony of granulocytes. G-CSF is an amino acid polypeptide which is produced by many cells, including endothelial monocytes and endometrial cells [9]. G-CSF is supposed to play an important role in endometrial decidualization, trophoblast development and placental metabolism. G-CSF promotes the mobilization, migration and differentiation of stem cells. It may also contribute to the regeneration of the endometrium by stimulating angiogenesis and reducing the apoptotic activity of endometrial cells. It is suggested that G-CSF influences the embryo implantation and the course of pregnancy through the temporary suppression of the immune response since G-CSF affects lymphocytes, macrophages and T-helpers 2 [10]. G-CSF has been successfully used for more than 20 years to treat neutropenia in patients with cancer after chemotherapy by intravenous and subcutaneous administration [11, 12].
After the publication of results of the studies carried out by foreign researchers on the successful use of G-CSF for treating patients with thin endometrium in a frozen embryo transfer programs, as well as the absence of a negative effect on the chromosome set of the embryo [8, 13–20], we decided to conduct our own study to reveal the efficacy of this treatment method.
The objective of the study was to determine the efficacy of intrauterine use of G-CSF in order to increase endometrial thickness and pregnancy rates in patients with thin endometrium in frozen embryo transfer programs.
Materials and Methods
It was a non-randomized clinical trial. It was approved by the Local Ethics Committee of the V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Moscow, Russia. It should be noted that G-CSF is an off lable medication and according to the instructions, it is not intended for intrauterine administration and treatment of thin endometrium.
The main group included 15 patients with thin endometrium which was refractory to standard methods of treatment in frozen embryo transfer programs. For these patients, the embryo transfer cycle was repeatedly canceled due to insufficient endometrium thickness. The control group included 17 patients with thin endometrium, they received only hormone replacement therapy.
The participants of the study met the following inclusion criteria:
- age 20–42 years;
- regular ovulatory menstrual cycle (25–34 days);
- body mass index: 18–30 kg/m2
- infertility caused by tubal and/or male factor and/or external genital endometriosis;
- idiopathic infertility;
- a history of canceling embryo transfer due to the thin endometrium;
- absence of intrauterine pathology based on hysteroscopy;
- presence of at least three vitrified blastocyst classes: 1) excellent (≥3AA); 2) good (3, 4, 5, 6, AB and BA) and/or average (3, 4, 5, 6 BB, AC and CA) (according to blastocyst scoring system of Gardner and Schoolcraft (1999));
- absence of individual intolerance to G-CSF and contraindications according to the instructions to this medical preparation;
- informed consent to participate in the study.
Patients were excluded from the study if they had a history of cancer, severe external genital endometriosis or adenomyosis, intrauterine synechia, polyps, submucous uterine fibroids, and congenital malformations of the uterus before surgical treatment.
All patients in both groups received hormone replacement therapy (HRT): estradiol valerate (Bayer Schering Pharma, France) from 6 mg per day with a maximum dose of up to 12 mg per day, and a micronized progesterone (CYNDEA PHARMA, S.L., Spain) at a dose of 400 mg per day and dydrogesterone (Solvay Pharmaceuticals, Netherlands) at a dose of 40 mg per day in the luteal phase of the menstrual cycle. In addition to HRT, the patients of the main group were given an intrauterine injection of G-CSF (Filgrastim) at a dose of 300 mcg (Leukostim, Biocad (Russia)) using an insemination catheter (Smiths Medical International Ltd.) on days 5–6 and 12–13 of the menstrual cycle. Evaluation of the endometrial thickness was performed using ultrasonography (GE Voluson E8) on the 14–15th day of the menstrual cycle and on the day of embryo transfer, that is on the 20–21st day of the menstrual cycle. Embryo transfer was carried out when endometrial thickness was more than 7 mm. Blood tests for the beta subunit of human chorionic gonadotropin was performed 14 days after embryo transfer, pelvic ultrasound assessment was performed 28 days after embryo transfer to determine clinical pregnancy. The primary outcome was an increase in endometrial thickness more than 7 mm on the day of embryo transfer and the secondary outcome was the pregnancy rate.
Statistical analysis
Statistical analysis was performed using STATISTICA software package (StatSoft Inc.). Constant variables that have a normal distribution were described using the arithmetic mean and standard deviation, M (SD). They were compared in the groups with a normal distribution and equality of variances by means of Student's t-test. Discrete variables were compared using the Chi-square criterion, their results are presented as percentages. A p value <0.05 was evaluated as statistically significant. For binary outcomes, the effect size was calculated as the odds ratio (OR) with a 95% confidence interval (CI), and for continuous data it was calculated as the difference in average values with 95% CI.
Results and Discussion
The characteristics of the patients are presented in Table 1.
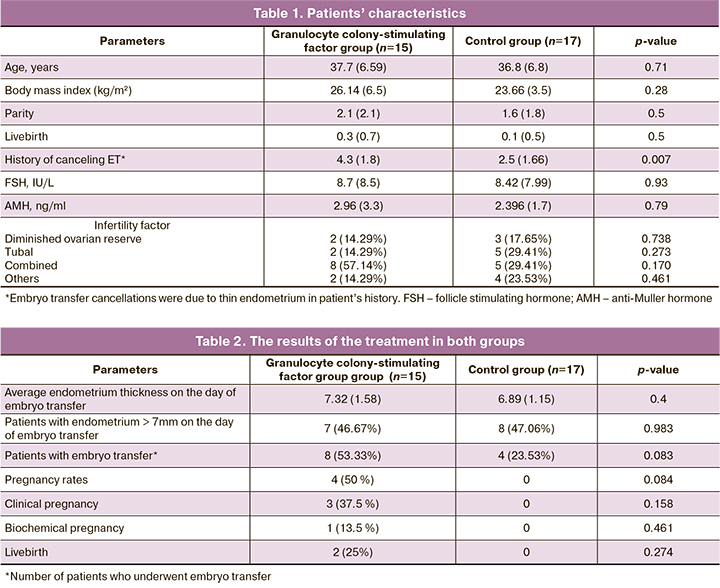
Endometrial thickness more than 7 mm was observed in 7 patients (46.67%) in the main group, and in 8 (47.06%) patients in the control group, the difference was not statistically significant (p=0.983, OR 1.633 with 95% CI). The average increase in endometrial thickness in G-CSF group compared to the previous cycle was 0.6 mm. The average endometrial thickness on the day of embryo transfer was 7.32 mm in G-CSF group. In control group, the average endometrial thickness was 6.89 mm, the difference was not statistically significant (p=0.4, the difference between the average values is 0.43 with 95% CI [-0.5594, 1.4194]). Embryo transfer was performed in 8 (53.33%) patients in G-CSF group and in 4 patients in the control group (23.53%), the difference was not statistically significant (p=0.083, OR 3.714 with 95% CI). In G-CSF group, pregnancy occurred in 4 (50%) patients, clinical pregnancy was achieved in 3 (37.5%) cases, one patient had a biochemical pregnancy. In the control group there no pregnancy was achieved by any of the patients; the values were not statistically significant (p=0.84). At the time of publication of the article there were two live births (25%) in G-CSF group and one ongoing pregnancy. The results of the treatment are presented in Table 2.
In our study, no side effects were noted after the administration of G-CSF.
Conclusion
Considering that the average number of canceling transfer cycles in patients was 4.3 (1.8), in our study, embryo transfer was performed in 8 (57.14%) patients, and pregnancy occurred in 4 (50%) patients, we can say that, despite the statistically insignificant results, there is a tendency to increase the endometrial thickness and pregnancy rates in G-CSF group. Also, there is a tendency to decrease embryo transfer cycle cancellations.
Nevertheless, it is necessary to conduct a study on a larger sample of patients for more reliable data and the possibility of introducing G-CSF into the recommendations for the treatment of patients with thin endometrium in IVF programs.
References
- Khalifa G., Brzyski R.G., Oehninger S., Acosta AA, Muasher SJ. Sonographic appearance of the endometrium: the predictive value for the outcome of in vitro fertilization in stimulated cycles. Hum. Reprod. 1992; 7(5): 677-80. https://dx.doi.org/10.1093/oxfordjournals.humrep.a137718.
- Zhang X., Chen C.H., Confino E., Barnes R., Milad M., Kazer R.R. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil. Steril. 2005; 83(2): 336-40. https://dx.doi.org/10.1016/j.fertnstert.2004.09.020.
- Абдурахманова Н.Ф., Гвоздева А.Д., Зиганшина М.М., Долгушина Н.В. Результаты программ вспомогательных репродуктивных технологий у пациенток с «тонким» эндометрием. Гинекология. 2019; 21(1): 23-7. [Abdurakhmanova N.F., Gvozdeva A.D., Ziganshina M.M., Dolgushina N.V. The results of assisted reproductive technology programs in patients with “thin” endometrium. Gynecology. 2019; 21(1): 23-7. (in Russian)]. https://dx.doi.org/10.26442/20795696.2019.1.190232.
- Kasius A., Smit J.G., Torrance H.L., Eijkemans M.J., Mol B.W., Opmeer B.C., Broekmans F.J. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum. Reprod. Update. 2014; 20(4): 530-41.https://dx.doi.org/10.1093/humupd/dmu011.
- Боярский К.Ю., Гайдуков С.Н., Пальченко Н.А. Современный взгляд на проблему рецептивности и тонкого эндометрия в программах ВРТ (обзор литературы). Проблемы репродукции. 2013; 19(4): 51-60. [Boyarsky K.Yu., Gaidukov S.N., Palchenko N.A. Modern view on the problem of receptivity and thin endometrium in assisted reproductive technology programs (literature review). Problems of reproduction. 2013; 19(4): 51-60.(in Russian)].
- Корсак В.С., Каменецкий Б.А., Михайлов А.В. Значимость толщины и ультразвуковой структуры эндометрия в программе ЭКО. Проблемы репродукции 2001; 7(3): 36-9. [Korsak V.S., Kamenetsky B.A.,Mikhailov A.V. Significance of endometrial thickness and ultrasound structure in the IVF program. Problems of reproduction 2001; 7(3): 36-9.(in Russian)].
- Sher G., Fisch J.D. Vaginal sildenafil (Viagra): a preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum. Reprod. 2000; 15(4): 806-9. https://dx.doi.org/10.1093/humrep/15.4.806.
- Gleicher N., Vidali A., Barad D.H. Successful treatment of unresponsive thin endometrium. Fertil. Steril. 2011; 95(6): 2123. e13-7.https://dx.doi.org/10.1016/j.fertnstert.2011.01.143.
- Barash A., Dekel N., Fieldust S., Segal I., Schechtman E., Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 2003; 79(6): 1317-22. https://dx.doi.org/10.1016/s0015-0282(03)00345-5.
- Thomas J., Liu F., Link D.C. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Curr. Opin. Hematol. 2002; 9(3): 183-9. https://dx.doi.org/10.1097/00062752-200205000-00002.
- Dale D.C., Cottle T.E., Fier C.J., Bolyard A.A., Bonilla M.A., Boxer L.A. et al. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am. J. Hematol. 2003; 72(2): 82-93. https://dx.doi.org/10.1002/ajh.10255.
- Gomez R.C., Pinto M.A., Gonzalez B.M. Colony-stimulating factors: clinical evidence for treatment and prophylaxis of chemotherapy-induced febrile neutropenia. Clin. Transl. Oncol 2006; 8(10): 729-34. https://dx.doi.org/10.1007/s12094-006-0119-4.
- Gleicher N., Kim A., Michaeli T., Lee H.-J., Shohat-Tal A., Lazzaroni E.,Barad D.H. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum. Reprod. 2013; 28(1):172-7. https://dx.doi.org/10.1093/humrep/des370.
- Kunicki M., Łukaszuk K., Liss J., Skowrońska P., Szczyptańska J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst. Biol. Reprod. Med. 2016; 63(1): 49-57. https://dx.doi.org/10.1080/19396368.2016.1251505.
- Bin X., Qiong Z., Jie H., Dabao X., Yanping L. Two protocols to treat thin endometrium with granulocyte colony-stimulating factor during frozen embryo transfer cycles. Reprod. Biomed. Online. 2015; 30(4): 349-58. https://dx.doi.org/10.1016/j.rbmo.2014.12.006.
- Barad D.H., Yu Y., Kushnir V.A., Shohat-Tal A., Lazzaroni E., Lee H.J.,Gleicher N. A randomized clinical trial of endometrial perfusion with granulocyte colony-stimulating factor in in vitro fertilization cycles:impact on endometrial thickness and clinical pregnancy rates. Fertil. Steril. 2014; 101(3): 710-5. https://dx.doi.org/10.1016/j.fertnstert.2013.12.016.
- Tehraninejad E., Tanha F.D., Asadi E., Kamali K., Aziminikoo E., Rezayof E. G-CSF intrauterine for thin endometrium, and pregnancy outcome. J. Family Reprod. Health. 2015; 9(3):107-12.
- Li Yu, Pan P., Chen X., Li Lin, Li Yi, Yang D. Granulocyte colony-stimulating factor administration for infertile women with thin endometrium in frozen embryo transfer program. Reprod. Sci. 2014; 21(3): 381-5. https://dx.doi.org/10.1177/1933719113497286.
- Eftekhar M., Sayadi M., Arabjahvani F. Transvaginal perfusion of G-CSF for infertile women with thin endometrium in frozen ET program: A nonrandomized clinical trial. Iran. J. Reprod. Med. 2014; 12(10): 661-6.
- Mishra V.V., Choudhary S., Sharma U., Aggarwal R., Agarwal R., Gandhi K., Goraniya N. Effects of granulocyte colony-stimulating factor (GCSF) on persistent thin endometrium in frozen embryo transfer (FET) cycles. J. Obstet. Gynecol. India. 2016; 66(Suppl.1): S407-11.
Received 17.12.2019
Accepted 07.02.2020
About the Authors
Lana G. Dzhincharadze, graduate student of 1st Gynecology department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(926)073-77-73. E-mail: lanachka@list.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Aydar N. Abubakirov, PhD, Head of 1st Gynecology department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(495)438-26-22. E-mail: nondoc555@yahoo.com. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nona G. Mishieva, PhD, Senior researcher of 1st Gynecology department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named
after Academician V.I. Kulakov Ministry of Health of Russia. Tel.: +7(495)438-26-22. E-mail: nondoc555@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Dzhincharadze L.G., Abubakirov A.N., Mishieva N.G. Intrauterine use of granulocyte colony-stimulating factor in patients with thin endometrium in frozen embryo transfer programs.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 8: 106-110 (in Russian)
https://dx.doi.org/10.18565/aig.2020.8.106-110



