Does sexual intercourse during IVF/ICSI cycle affect endometrial thickness in the presence of immunohormonal markers of stress in the seminal plasma?
Men’s stress may alter the concentration of immunological and hormonal factors in seminal plasma, which, on entering the female reproductive tract during sexual intercourse, may affect endometrial development.Nikolaeva M.A., Babayan A.A., Arefieva A.S., Chagovets V.V., Starodubtseva N.L, Frankevich V.E., Kalinina E.A., Krechetova L.V., Sukhikh G.T.
Objective: To investigate the relationship between endometrial thickness in women having intercourse during the proliferative phase of the IVF/ICSI cycle and the content of immunological and endocrine stress markers in the partner's seminal plasma.
Materials and methods: This prospective study included 71 couples with tubal infertility factor having unprotected intercourse during the proliferative phase of IVF/ICSI cycle, supplemented with intravaginal injection of seminal plasma on the day of transvaginal ovarian puncture. The retrospective pilot study included couples with IVF/ICSI success (group 1, n=7) and IVF/ICSI failure (group 2, n=9). They were comparable by clinical, demographic and laboratory parameters, but differed in the content of immunohormonal stress markers in the partner's seminal plasma. The groups were formed based on the content of cytokines IL-18 and IL-1β, steroid hormones, their precursors and seminal plasma metabolites. Endometrial thickness was measured by transvaginal ultrasound on the day of ovulation trigger. The cytokine content in seminal plasma was assessed by flow cytofluorometry and fluorescent microspheres using FlowCytomix technology. The concentration and total amount of steroids in seminal plasma were determined using a combination of high-performance liquid chromatography and tandem mass spectrometry.
Results: Prospective and retrospective studies revealed a reduced endometrial thickness in the group of patients with an increased content of immunoendocrine stress markers in seminal plasma. There was a negative correlation between endometrial thickness and concentration and/or total amount of 17-α-hydroxypregnenolone, testosterone, progesterone, and cytokines IL-18, IL-1β in seminal plasma. The cortisol/DHEA ratio was positively associated with endometrial thickness.
Conclusion: The study's finding suggested that stress-related seminal immunological and hormonal factors in the seminal plasma entering the female reproductive tract during sexual intercourse in the proliferative phase of the IVF/ICSI cycle may negatively impact endometrial development. However, further research is necessary to establish a causal link between the composition of seminal plasma and the endometrial status.
Keywords
Today, in vitro fertilization (IVF) is one of the most successful treatment options for managing infertility. Despite improvements in the embryonic stage of IVF, allowing the selection of high-quality embryos for transfer, the pregnancy rate during embryo transfer remains quite low, averaging 34.6% for IVF and 33.5% for intracytoplasmic sperm injection (ICSI), according to the European IVF Monitoring Consortium in 2017 [1].
Endometrial receptivity as a leading factor in the embryo implantation depends on the morphological and functional characteristics of the endometrium, formed through the dynamic interaction of multiple endocrine and immunological factors [2]. Seminal plasma contains a wide range of steroid hormones, neurotransmitters, cytokines, and growth factors, suggesting its potential role in the regulation of dynamic endometrial remodeling during each menstrual cycle [3]. Numerous in vitro studies reported that human seminal plasma activates a number of transcriptional regulatory pathways underlying decidualization – morphological and functional changes in endometrial cells during the secretory phase of the cycle that play a key role in embryo implantation [4–7].
In this regard, there have been repeated attempts to use seminal plasma in assisted reproductive technology (ART). However, the results obtained using the artificial injection of seminal plasma into a woman's reproductive tract during IVF/ICSI procedures are contradictory. There were reports of both an increase in the implantation rate [8, 9] and a lack of effect [10, 11]. A number of studies have found a statistically nonsignificant trend towards an increase in pregnancy rate [12-14]. An early study reported an increased pregnancy rate with gamete transfer to the fallopian tube [15], but it was not possible to confirm the effect of sexual intercourse in the IVF/ICSI program [16, 17].
The Cochrane review concluded that the quality of evidence of studies claiming that the use of seminal plasma in the IVF/ICSI cycle can increase the pregnancy rate is low [18].
The main reason for the negative results of translational studies may be the different composition of seminal plasma used in experimental studies and in clinical trials. Data on the favorable effect of seminal plasma on embryo implantation have been obtained in animal studies [3] or in vitro culture of human endometrial cells in the presence of seminal plasma from healthy and/or fertile donors [4–7]. At the same time, seminal plasma of infertile patients has been used in ART programs. Obviously, the immunohormonal profile of the seminal plasma of patients enrolled in the IVF/ICSI program can be altered by stress and other adverse factors.
Stress is a state of the body characterized by the activation of adaptive neuroendocrine immune responses to various factors (physical or psychological). Numerous studies have shown that women and their sexual partners who participate in ART programs can experience stress related to infertility and/or infertility treatment [19–23].
The most important stress-releasing systems of the body are the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nerve system. Stimulation of the HPA axis induces the secretion of steroid hormones, including cortisol, dehydroepiandrosterone (DHEA), and its main metabolite DHEA-sulfate (DHEA-S), as well as progesterone by the adrenal cortex [24]. Adrenal steroids are widely used neuroendocrine biomarkers that allow objective measurement of the physiological response to psychosocial stressors [25]. Activation of the sympatheticoadrenal system under stress leads to stimulation of the hypothalamic-pituitary-gonadal axis and increased testosterone production in the testes [26]. Therefore, testosterone and progesterone can also qualify as stress hormones.
The efficiency of stress reactions is provided by the close interaction of the neuroendocrine and immune systems [27]. Psychological stress is an inducer of aseptic inflammatory reactions accompanied by an increase in cytokine levels including interleukin (IL)-18 [28] and IL-1β [29].
We have previously confirmed that the effect of seminal plasma on the efficacy of IVF is determined by the level of stress markers in seminal plasma (30, 31). A favorable effect of seminal plasma on implantation was detected in patients with low levels of stress markers, and the pregnancy rate was 61.2%. The high level of immunohormonal stress markers in seminal plasma, detected in 31% of patients, was associated with a low rate of clinical pregnancy (9.1%).
We hypothesized that one of the most likely mechanisms of the adverse effect of seminal plasma on the female reproductive tract (FRT) may be a change in the morphological and functional characteristics of the endometrium under the influence of exogenous cytokines and steroid hormones contained in elevated amounts in seminal plasma under intimate partner stress.
Formation of the endometrium is controlled by ovarian steroid hormones including estradiol and progesterone and androgens (testosterone, DHEA, and DHEA-S) and by a balanced dynamic cytokine secretion by immunocompetent cells [32]. Therefore, the inflow of excessive concentrations of exogenous hormones and cytokines into the seminal plasma in FRT can create a state of “local hyperandrogenism, hyperprogesteronism, and hypercytokinemia” and disrupt intercellular interactions involved in endometrial repair.
Recently, it has been convincingly demonstrated that the molecular signatures of proliferative-phase endometrium are associated with embryo implantation failures [33]. Therefore, the effect of seminal plasma on IVF efficacy can be realized by unprotected sexual intercourse during the proliferative phase of the IVF/ICSI cycle.
Adequate endometrial structure and thickness, both during the introduction of the ovulation trigger and during the implantation window, is considered to be one of the significant factors determining the outcomes of ART [34, 35].
Therefore, this study aimed to investigate the association between the thickness of endometrium formed at sexual contacts during the proliferative phase of the IVF/ICSI cycle and the content of immunological and endocrine stress markers in the seminal plasma of sexual partners.
Materials and methods
The prospective study enrolled married infertile couples with tubal factor (n=71) who were treated at the Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia. All patients were examined in accordance with the order of the Russian Ministry of Health №107n of August 30, 2012 "On the order of use of assisted reproductive technologies, contraindications and limitations for their use". All manipulations related to the implementation of IVF/ICSI protocols were performed by specialists of the Professor B.V. Leonov Department of Assisted Technologies in the Treatment of Infertility, the V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia according to the protocol previously approved by the Research Ethics Committee.
Inclusion criteria were woman age ≤41 years, tubal infertility factor, preserved ovarian reserve, regular menstrual cycle, normal karyotype, normal uterine anatomy, no marked extragenital pathology, absence of urogenital infections, chronic inflammatory pelvic disease, and systemic autoimmune diseases, no more than two failed IVF/ICSI attempts, regular sexual life without contraception.
Inclusion criteria for men were age ≤49 years, normal karyotype, no obvious extragenital pathology, no obvious pathozoospermia, no antisperm antibodies (ΜΑR≤10%), no use of drugs with spermotoxic effect, no chemotherapy and radiation, no urogenital infections, chronic inflammatory pelvic diseases and systemic autoimmune diseases, regular sexual life without contraception.
Criteria for women exclusion were somatic diseases contraindicated for carrying pregnancy and childbirth, endocrine, immunological, uterine infertility, polycystic ovarian syndrome, extragenital and internal endometriosis stages III and IV, interstitial or sub-serosal uterine fibroids over 3 cm, use of donor egg cells or surrogacy. Non-inclusion criteria for men were grade III–IV pathozoospermia, presence of anti-sperm antibodies (MAR>10%), erectile dysfunction. Exclusion criteria were endometrial thickness on the day of ovulation trigger administration ≤8 mm, ovarian hyperstimulation syndrome in female patients, leukocyte count in ejaculate >1 million/ml.
Patients included in the study were treated by controlled ovarian hyperstimulation with a gonadotropin-releasing hormone (GnRH) antagonist. Ovarian function was stimulated by recombinant FSH and/or human menopausal gonadotropins. The ovulatory trigger was administered as soon as ≥3 follicle reached 14-15 mm in diameter. When leading follicles reached pre-ovulatory size (17–18 mm), the ovulation trigger chorionic gonadotropin (5000–10000 units) was administered. Transvaginal ovarian puncture (TVP) was performed 35–36 h after trigger injection. Injection of 0.5 ml of the partner's seminal plasma into the posterior vaginal fornix was performed immediately after TVP. Depending on the parameters of the sperm, the fertilization of the obtained oocytes was carried out by IVF or ICSI. Transfer of an embryo of good quality according to the classification of D. Gardner and W. Schoolcraft [36] was carried out on day 5 after TVP. Micronized progesterone was used to support the luteal phase of the induced cycle. The patients had unprotected sexual intercourse from days 4–5 to days 11–12 of the menstrual cycle, then abstained from intercourse until the blood test for pregnancy was completed. Clinical pregnancy was recorded when the fetus was visualized in the uterine cavity 21 days after embryo transfer. Live birth was defined as the birth of at least one child who was born alive and lived more than 1 month.
Endometrial thickness was assessed using a transvaginal ultrasound in a sagittal section perpendicular to the midline between the endometrial and myometrial layers on the day of ovulation triggering. All transvaginal ultrasound examinations were performed using a BK Medical Flex Focus 500 ultrasound device.
Semen samples were obtained on the day of TVP by masturbation after a 3–5-day period of sexual abstinence. Standard semen analysis was performed according to the World Health Organization (WHO) protocol [37]. Flow cytofluorometry was used to estimate the leukocyte content in the ejaculate [38]. Mixed agglutination reaction (MAR test) was performed using SpermMar kits (FertiPro, Beernem, Belgium) according to the WHO protocol [37]. After centrifugation of the ejaculate, 0.5 ml of seminal plasma was used for intravaginal injection. The remaining seminal plasma volume was aliquoted and then stored at -80°C for subsequent detection of cytokines and steroids.
Based on the outcomes of the prospective study, 2 groups of patients with low and high levels of total IL-18 in the seminal plasma of the sexual partners were formed (≤1432, 4 pg, n=48 and >1432.4 pg, n=23). Low and high levels of IL-18 were determined based on the cut-off point determined by ROC analysis of IL-18 levels in seminal plasma to predict pregnancy, as described previously [30].
The retrospective study included two groups of patients categorized by IVF/ICSI outcomes, clinical, demographic, and laboratory parameters, data on the content of IL-18 and IL-1β cytokines in seminal plasma, steroid hormones, and their precursors and metabolites identified in the prospective study. Immunoendocrine markers of stress were higher in Group 1 with an onset of pregnancy (n=7) compared with Group 2 with no pregnancy (n=9), and the groups were comparable in clinical, demographic, and laboratory parameters. Inclusion criteria for women were age ≤ 41 years, for men age ≤ 43 years. The inclusion criteria for all study participants were 18 kg/m2 body mass index (BMI) ≤ 28 kg/m2 and absence of unhealthy habits.
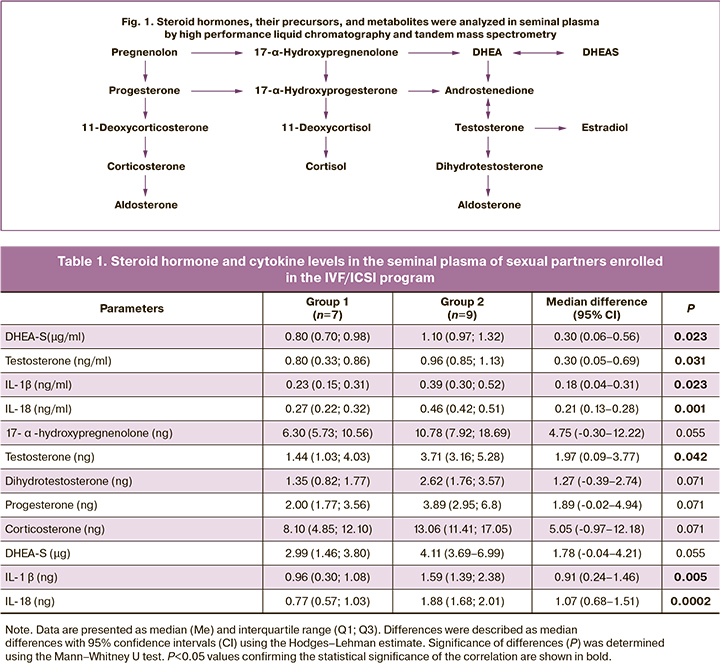
Seminal plasma cytokine status was assessed by flow cytofluorometry and fluorescent microspheres using FlowCytomix technology and IL-18 detection kits (Human IL-18 and IL-1β Simplex Kits, Bender MedSystems, Austria). Flow cytofluorimeter FACSCalibur (BD Biosciences, USA) and BD CellQuest Pro software version 5.2.1 (BD Biosciences, USA) were used [30, 31]. A combination of high-performance liquid chromatography and tandem mass spectrometry was used to assess the concentration of steroid hormones, their precursors, and metabolites in seminal plasma (Fig. 1) [31]. The total contents of cytokines and steroids in ejaculate (calculated as the concentration of analytes × volume of ejaculate) were also determined.
Statistical analysis
Statistical analysis was performed using MedCalc 19.2.5. The normality of the distribution of the data included in the retrospective study was tested by the Shapiro-Wilk test. The endometrial thickness in the prospective and retrospective study had a normal distribution in each of the two compared groups (P>0.20); therefore, the endometrial thickness was compared using the Student's t-test. The numerical variables (Tables 1, 2, and 3) of the 2 groups included in the retrospective study were not normally distributed (P<0.05) and compared using the Mann–Whitney U test. Quantitative variables that showed normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with the interquartile range (Q1; Q3) was reported. The effect size was described as the difference between means with 95% confidence interval (CI) and the difference between medians with 95% CI using the Hodges–Lehman estimate. Differences between categorical variables were analyzed using the χ2 test. Pearson’s correlation coefficients (r) were calculated to determine the correlation between endometrial thickness and sperm immunohormonal parameters after logarithmic transformation of the data. The significance threshold was defined as P<0.05.
Results and discussion
The findings of the prospective study showed that endometrial thickness was associated with the total IL-18 content in the seminal plasma of the sexual partner (Fig. 2). The endometrial thickness in patients with high levels of IL-18 in seminal plasma was 9.7 (1.0) mm and was significantly different from the endometrial thickness in the low IL-18 group (P=0.018), which was 10.2 (0.7) mm. The difference between means with 95% CI was 0.5 (-0.9–-0.1) mm.
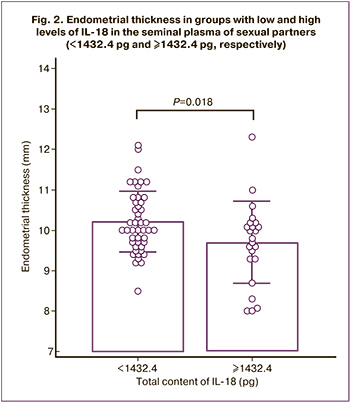
Small differences could be due to the presence of a number of confounders determining the decrease in endometrial thickness. The heterogeneity of the study sample, which included a group of patients without pregnancy and no stress markers in seminal plasma (26.8%) [30], may also have attenuated the association between endometrial thickness and the level of immunohormonal markers in seminal plasma.
To obtain more meaningful evidence of the effect of intimate partner stress on FRT, a retrospective pilot study was conducted that included selectively chosen groups of patients formed according to the outcomes of IVF/ICSI, clinical, demographic and laboratory parameters of intimate partners, and the level of immunohormonal stress markers in seminal plasma. The group of patients with an absence of pregnancy not associated with the presence of stress markers in seminal plasma was excluded from the study. It should be noted that increased levels of proinflammatory cytokines in the seminal plasma may also be associated with smoking [39] and obesity [40]. For this reason, smoking patients and patients with an elevated BMI were excluded from the present study.
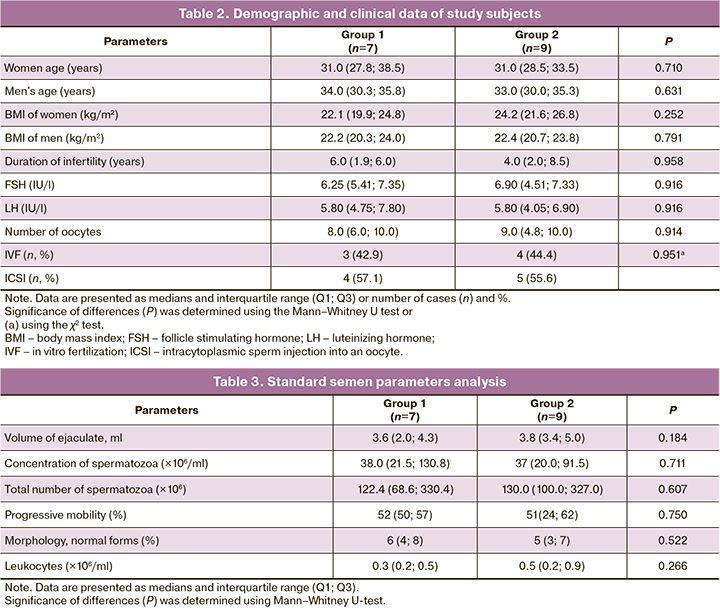
Table 1 summarizes the results of the comparison of the levels of immunoendocrine stress markers in the seminal plasma of the sex partners in the two groups. Group 2 had increased concentrations of DHEA-S, testosterone, and cytokines IL-18 and IL-1β in seminal plasma and total testosterone and cytokines IL-18 and IL-1β in seminal plasma. The increase in total 17-α-hydroxypregnenolone, dihydrotestosterone, progesterone, corticosterone and DHEA-S in the seminal plasma of patients in group 2 was close to statistical significance (P<0.071). No differences were found in the content of 17-α-hydroxyprogesterone, cortisol, DHEA, androsterone, 11-deoxycorticosterone and aldosterone (P>0.1; data not shown). Concentrations of pregnenolone, estradiol, and 11-deoxycortisol in seminal plasma were below the lower limit of quantification.
There were no differences between sperm parameters (Table 2) and between the clinical profile and laboratory parameters in the sexual partners (Table 3). Age and BMI did not differ between the two groups for both women (P=0.711 and P=0.252, respectively) and men (P=0.633 and P=0.791, respectively).
Endometrial thickness was lower in group 2 patients compared to group 1 (P=0.027), being 9.6 (0.9) mm and 10.6 (0.7) mm, respectively. The difference in the means with 95% CI was -1.0 (-1.9–-0.1) mm.
Differences between groups in endometrial thickness were more pronounced in the retrospective study compared to the prospective study (the mean difference was -0.5 mm and -1.0 mm, respectively).
Thus, we formed groups of patients that differed in IVF/ICSI outcomes, levels of stress markers in seminal plasma, and endometrial thickness with the same level of clinical and laboratory parameters in men and women.
Assuming that cytokines and hormones entering FRT during sexual intercourse can influence endometrial formation, we evaluated the association between endometrial thickness and the content of 16 steroid hormones and cytokines IL-18 and IL-1β, immunohormonal stress markers in seminal plasma (Table 4). There was a negative correlation between endometrial thickness and the concentration of 17-α-hydroxypregnenolone, testosterone, progesterone, IL-18, and IL-1β in seminal plasma. A negative correlation was also found between endometrial thickness and total 17-α-hydroxypregnenolone and progesterone in seminal plasma. A positive correlation was found between endometrial thickness and the cortisol/DHEA ratio.
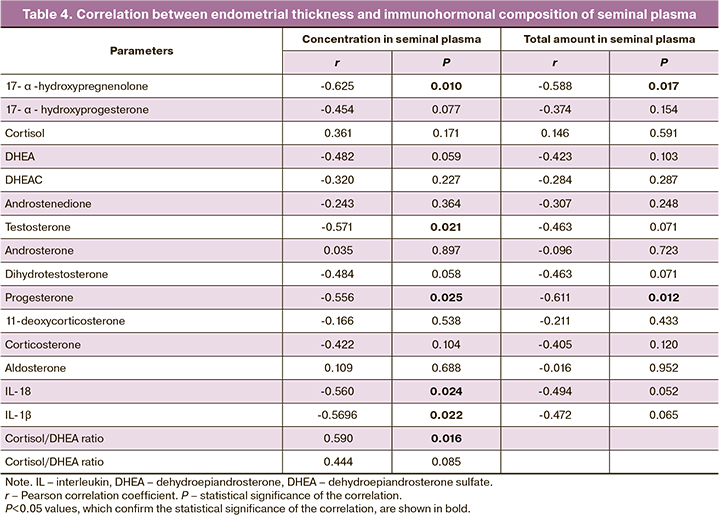
Our findings on the negative association between the content of testosterone and its precursor 17-α-hydroxypregnenolone in seminal plasma are consistent with the previously revealed adverse effects of increased concentration of endogenous and exogenous androgens (testosterone, DHEA and DHEA-S) on endometrial morpho-functional state.
The human endometrium is a multicellular target tissue for steroids that undergoes dynamic remodeling during each menstrual cycle. The main factors that underlie the increase in endometrial thickness are endometrial cell proliferation and angiogenesis, which reach their maximum activity in the middle proliferative phase [41]. Both these processes are controlled by the ovarian steroid hormones estradiol and progesterone. Androgens and their receptors, which are expressed in the glands, surface epithelium, and endometrial stroma, also play an important role in endometrial structural and functional remodeling [42, 43]. Androgens have been established to have a direct effect on both epithelial and stromal endometrial cells, demonstrating an antiproliferative effect in the normal endometrium [44] and inhibiting the progression of hyperplasia in estrogen-dependent malignant neoplasms [45].
At the same time, when endogenous and exogenous androgens are in excess, their antiproliferative effect can be reflected in the endometrium. Exogenous administration in female to male transsexual subjects leads to endometrial atrophy [46]. Increased blood concentrations of DHEA, DHEA-S, and testosterone in women is a biochemical diagnostic marker of hyperandrogenism, one of the common causes of reproductive failure in women [47]. A close correlation between the biochemical form of hyperandrogenemia and impaired endometrial morphology and function has been revealed [48].
Thus, the association between increased androgen content in seminal plasma and decreased endometrial thickness revealed in this study is consistent with the impairment of endometrial morphology and functions in androgen excess established in numerous studies.
We revealed the association between endometrial thickness and both concentration and total amount of immunohormonal components in seminal plasma. Seminal components during intravaginal administration of seminal plasma are delivered to the uterus using a unique rapid transport system by diffusion of molecules from the lymphatic and venous vessels of the vagina into the uterine arterial system [49]. This type of delivery ensures that most of the biologically active molecules contained in the seminal plasma are transported to the uterus. Thus, during sexual activity in the IVF cycle, both the concentration and the total content of immunohormonal factors in seminal plasma can influence the endometrial state.
For example, a pronounced decrease in the endometrial thickness was found when the total amount of progesterone in the seminal plasma was increased. We would like to note that during the proliferative phase of the menstrual cycle, progesterone is produced in small amounts and its concentration in the blood serum usually does not exceed 1.5 ng/ml; at the same time, the total amount of progesterone determined in the seminal plasma of patients in Group 2 ranged from 2.0 ng to 9.3 ng. Thus, it is very likely that a significant increase in progesterone intake during sexual intercourse may result in a local excess of progesterone in the endometrium.
The antiproliferative effects of progesterone have been described in numerous studies. Progesterone is known to limit estrogen-induced proliferation, as evidenced by the reduced incidence of endometrial cancer in patients on hormone replacement therapy, synthetic analogues of progesterone [50]. Increased levels of progesterone in the early luteal phase lead to suppression of estrogen receptor expression and attenuation of estrogen-mediated proliferation of the endometrial epithelium, which is a prerequisite for successful implantation [51]. At the same time, the antiproliferative effects of progesterone in the follicular phase can lead to the inhibition of regenerative processes and, accordingly, to a decrease in endometrial thickness.
It should be noted that the pronounced effects of hormone supply to FRT can be caused not only by their increased content in seminal plasma, but also by the high level of androgen and progesterone receptor expression that reaches its maximum in the proliferative phase [52, 53], and also by the enhancing effect of androgens on progesterone receptor expression in the proliferation phase [54].
The findings suggest that the increase in the content of pro-inflammatory cytokines IL-18 and IL-1β in seminal plasma can also adversely affect the endometrial state, altering the balance of local immune reactions [32, 55].
Endometrial thickness can be a marker of the functional state of the endometrium altered under the influence of seminal plasma components. Animal studies have shown that testosterone is a negative regulator of granulocyte-macrophage colony-stimulating factor production, a key immunoregulator of embryo implantation, by endometrial cells [56]. In in vitro studies, testosterone was found to inhibit the expression of HOXA10, a transcription factor required to control the development, differentiation, and formation of endometrial receptivity in patients with polycystic ovarian syndrome [57]. DHEA has also been shown to inhibit the pentose-phosphate pathway of glucose metabolism in stromal endometrial cells, both human and mouse, which prevents the process of decidualization and implantation [58].
When interpreting the results of the current study, a number of limitations should be taken into account. The first one is the small number of patients, which can lead to type II statistical error. This is a consequence of the selective choice of patients using strict inclusion and exclusion criteria.
The limited number of observations does not allow a multivariate regression analysis to evaluate the each of the studied parameters contribution to the relationship of endometrial thickness and seminal plasma composition.
It should also be noted that the study does not allow the establishment of a causal relationship between seminal plasma stress-dependent factors and endometrial thickness, since a significant confounder may be stress in the woman associated with stress in the sexual partner [59].
Obviously, the identified patterns confirmed for the unique group of sexual partners demonstrating high levels of stress should not be extrapolated to the entire IVF/ICSI patient population. The results of this study confirm that the absence of any marked effect of seminal plasma on pregnancy rates during IVF/ICSI treatment may be due to the heterogeneity of the male population participating in IVF programs with respect to the degree and intensity of stress responses determined by genetic and epigenetic factors [60].
Conclusion
The expression of stress markers in seminal plasma may be a key pathogenetic factor in impaired embryo implantation in women during intercourse with stressed partners during IVF cycles. At the same time, contacts with stress-resistant partners whose seminal plasma is characterized by a balanced composition of cytokines and hormones may lead to a significant increase in the pregnancy rate. If the effect of stress-related seminal factors on the endometrial morpho-functional state is confirmed, a personalized sexual regimen that depends on the stress level of the sexual partner could potentially increase the pregnancy rate by recommending to engage in or abstain from unprotected intercourse in the IVF/ICSI cycle.
Clearly, more research is needed to confirm the patterns found in this study and to understand the physiological mechanism underlying the relationship between male neuroendocrine function and the female reproductive system.
References
- Wyns C., De Geyter C., Calhaz-Jorge C., Kupka M.S., Motrenko T., Smeenk J. et al.; European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2017: results generated from European registries by ESHRE. Hum. Reprod. Open. 2021; 2021(3): hoab026. https://dx.doi.org/10.1093/hropen/hoab026.
- Kayisli U.A., Guzeloglu-Kayisli O., Arici A. Endocrine-immune interactions in human endometrium. Ann. N. Y. Acad. Sci. 2004; 1034: 50-63. https://dx.doi.org/10.1196/annals.1335.005.
- Robertson S.A., Sharkey D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016; 106(3): 511-9. https://dx.doi.org/10.1016/j.fertnstert.2016.07.1101.
- Chen J.C., Johnson B.A., Erikson D.W., Piltonen T.T., Barragan F., Chu S. et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Hum. Reprod. 2014; 29(6): 1255-70. https://dx.doi.org/10.1093/humrep/deu047.
- Rodriguez-Caro H., Dragovic R., Shen M., Dombi E., Mounce G., Field K. et al. In vitro decidualisation of human endometrial stromal cells is enhanced by seminal fluid extracellular vesicles. J. Extracell. Vesicles. 2019; 8(1): 1565262.https://dx.doi.org/10.1080/20013078.2019.1565262.
- George A.F., Jang K.S., Nyegaard M., Neidleman J., Spitzer T.L., Xie G. et al. Seminal plasma promotes decidualization of endometrial stromal fibroblasts in vitro from women with and without inflammatory disorders in a manner dependent on interleukin-11 signaling. Hum. Reprod. 2020; 35(3): 617-40. https://dx.doi.org/10.1093/humrep/deaa015.
- Moharrami T., Ai J., Ebrahimi-Barough S., Nouri M., Ziadi M., Pashaiefar H., Yazarlou F. et al. Influence of follicular fluid and seminal plasma on the expression of endometrial receptivity genes in endometrial cells. Cell J. 2021; 22(4): 457-66. https://dx.doi.org/10.22074/cellj.2021.6851.
- Bellinge B.S., Copeland C.M., Thomas T.D., Mazzucchelli R.E., O'Neil G., Cohen M.J. The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertil. Steril. 1986; 46(2): 252-6. https://dx.doi.org/10.1016/s0015-0282(16)49521-x.
- Chicea R., Ispasoiu F., Focsa M. Seminal plasma insemination during ovum-pickup-a method to increase pregnancy rate in IVF/ICSI procedure. A pilot randomized trial. J. Assist. Reprod. Genet. 2013; 30(4): 569-74.https://dx.doi.org/10.1007/s10815-013-9955-7.
- Fishel S., Webster J., Jackson P., Faratian B. Evaluation of high vaginal insemination at oocyte recovery in patients undergoing in vitro fertilization. Fertil. Steril. 1989; 51(1): 135-8. https://dx.doi.org/10.1016/s0015-0282(16)60442-9.
- Jafarabadi M., Sasani A., Ramezanzadeh F., Zandieh Z., Shariat M., Haghollahi F. Intracervical application of seminal plasma at the time of oocyte pickup during in vitro fertilization. Acta Medica Mediterranea. 2016; 32: 2085-90.
- Coulam C.B., Stern J.J. Effect of seminal plasma on implantation rates. Early Pregnancy. 1995; 1(1): 33-6.
- von Wolff M., Rösner S., Thöne C., Pinheiro R.M., Jauckus J., Bruckner T. et al. Intravaginal and intracervical application of seminal plasma in in vitro fertilization or intracytoplasmic sperm injection treatment cycles – a double-blind, placebo-controlled, randomized pilot study. Fertil. Steril. 2009; 91(1): 167-72. https://dx.doi.org/10.1016/j.fertnstert.2007.11.036.
- Friedler S., Ben-Ami I., Gidoni Y., Strassburger D., Kasterstein E., Maslansky B. et al. Effect of seminal plasma application to the vaginal vault in in vitro fertilization or intracytoplasmic sperm injection treatment cycles-a double-blind, placebo-controlled, randomized study. J. Assist. Reprod. Genet. 2013; 30(7): 907-11. https://dx.doi.org/10.1007/s10815-013-0033-y.
- Marconi G., Auge L., Oses R., Quintana R., Raffo F., Young E. Does sexual intercourse improve pregnancy rates in gamete intrafallopian transfer? Fertil. Steril. 1989; 51(2): 357-9. https://dx.doi.org/10.1016/s0015-0282(16)60507-1.
- Tremellen K.P., Valbuena D., Landeras J., Ballesteros A., Martinez J., Mendoza S. et al. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum. Reprod. 2000; 15(12): 2653-8. https://dx.doi.org/10.1093/humrep/15.12.2653.
- Aflatoonian A., Ghandi S., Tabibnejad N. The effect of intercourse around embryo transfer on pregnancy rate in assisted reproductive technology cycles. Int. J. Fertil. Steril. 2009; 2(4): 169-72.
- Ata B., Abou-Setta A.M., Seyhan A., Buckett W. Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer). Cochrane Database Syst. Rev. 2018; (2): CD011809. https://dx.doi.org/10.1002/14651858.CD011809.pub2.
- Ilacqua A., Izzo G., Emerenziani G.P., Baldari C., Aversa A. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018; 16(1): 115. https://dx.doi.org/10.1186/s12958-018-0436-9.
- Zaidouni A., Fatima O., Amal B., Siham A., Houyam H., Jalal K. et al. Predictors of infertility stress among couples diagnosed in a public center for assisted reproductive technology. J. Hum. Reprod. Sci. 2018; 11(4): 376-83.https://dx.doi.org/10.4103/jhrs.JHRS_93_18.
- Pedro J., Vassard D., Malling G.M.H., Hougaard C.Ø., Schmidt L., Martins M.V. Infertility-related stress and the risk of antidepressants prescription in women: a 10-year register study. Hum. Reprod. 2019; 34(8): 1505-13. https://dx.doi.org/10.1093/humrep/dez110.
- Sejbaek C.S., Pinborg A., Hageman I., Sørensen A.M., Koert E., Forman J.L. et al. Depression among men in ART treatment: a register-based national cohort study. Hum. Reprod. Open. 2020; 2020(3): hoaa019. https://dx.doi.org/10.1093/hropen/hoaa019.
- Roozitalab S., Rahimzadeh M., Mirmajidi S.R., Ataee M., Esmaelzadeh Saeieh S. The relationship between infertility, stress, and quality of life with posttraumatic stress disorder in infertile women. J. Reprod. Infertil. 2021; 22(4): 282-8. https://dx.doi.org/10.18502/jri.v22i4.7654.
- Nguyen A.D., Conley A.J. Adrenal androgens in humans and nonhuman primates:production, zonation and regulation. Endocr. Dev. 2008; 13: 33-54. https://dx.doi.org/10.1159/000134765.
- Sze Y., Brunton P.J. Sex, stress and steroids. Eur. J. Neurosci. 2020; 52(1):2487-2515. https://dx.doi.org/10.1111/ejn.14615.
- Chichinadze K., Chichinadze N. Stress-induced increase of testosterone: contributions of social status and sympathetic reactivity. Physiol. Behav. 2008; 94(4): 595-603. https://dx.doi.org/10.1016/j.physbeh.2008.03.020.
- Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000; 52(4): 595-638.
- Sekiyama A., Ueda H., Kashiwamura S., Nishida K., Kawai K., Teshima-Kondo S. et al. IL-18; a cytokine translates a stress into medical science. J. Med. Invest. 2005; 52(Suppl.): 236-9. https://dx.doi.org/10.2152/jmi.52.236.
- Goshen I., Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front. Neuroendocrinol. 2009; 30(1): 30-45. https://dx.doi.org/10.1016/j.yfrne.2008.10.001.
- Nikolaeva M.A., Babayan A.A., Stepanova E.O., Smolnikova V.Y., Kalinina E.A., Fernández N., Krechetova L.V., Vanko L.V., Sukhikh G.T. The relationship of seminal transforming growth factor-β1 and interleukin-18 with reproductive success in women exposed to seminal plasma during IVF/ICSI treatment. J. Reprod. Immunol. 2016; 117: 45-51. https://dx.doi.org/10.1016/j.jri.2016.03.006.
- Nikolaeva M., Arefieva A., Babayan A., Chagovets V., Kitsilovskaya N., Starodubtseva N., Frankevich V., Kalinina E., Krechetova L., Sukhikh G. Markers of stress in seminal plasma at IVF/ICSI failure: a preliminary study. Reprod. Sci. 2021; 28(1): 144-58. https://dx.doi.org/10.1007/s43032-020-00253-z.
- Pantos K., Grigoriadis S., Maziotis E., Pistola K., Xystra P., Pantou A. et al. The role of interleukins in recurrent implantation failure: a comprehensive review of the literature. Int. J. Mol. Sci. 2022; 23(4): 2198. https://dx.doi.org/10.3390/ijms23042198.
- Mackens S., Santos-Ribeiro S., Racca A., Daneels D., Koch A., Essahib W. et al. The proliferative phase endometrium in IVF/ICSI: an in-cycle molecular analysis predictive of the outcome following fresh embryo transfer. Hum. Reprod. 2020; 35(1): 130-44. https://dx.doi.org/10.1093/humrep/dez218.
- Багдасарян Л.А., Корнеева И.Е. Толщина эндометрия: предиктор эффективности программ ЭКО/ICSI (обзор литературы). Гинекология. 2018; 20(1): 113-6. [Bagdasaryan L.A., Korneeva I.E. Thickness of endometrium: predictor of the effectiveness of IVF/ICSI programs (literature review). Gynecology. 2018; 20 (1): 113-6. (in Russian)].
- Краснопольская К.В., Оразов М.Р., Ершова И.Ю., Федоров А.А. Тонкий эндометрий и бесплодие. М.: ГЭОТАР-Медиа; 2022. 208с. [Krasnopolskaya K.V., Orazov M.R., Ershova I.Yu., Fedorov A.A. Thin endometrium and infertility. Moscow: GEOTAR-Media, 2022; 208 p. (in Russian)].
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO; 2010.
- Hacker-Klom U.B., Göhde W., Nieschlag E., Behre H.M. DNA flow cytometry of human semen. Hum. Reprod. 1999; 14(10): 2506-12. https://dx.doi.org/10.1093/humrep/14.10.2506.
- Antoniassi M.P., Intasqui P., Camargo M., Zylbersztejn D.S., Carvalho V.M., Cardozo K.H. et al. Analysis of the functional aspects and seminal plasma proteomic profile of sperm from smokers. BJU Int. 2016; 118(5): 814-22. https://dx.doi.org/10.1111/bju.13539.
- Leisegang K., Henkel R., Agarwal A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am. J. Reprod. Immunol. 2019; 82(5): e13178. https://dx.doi.org/10.1111/aji.13178.
- Petracco R.G., Kong A., Grechukhina O., Krikun G., Taylor H.S. Global gene expression profiling of proliferative phase endometrium reveals distinct functional subdivisions. Reprod. Sci. 2012; 19(10): 1138-45. https://dx.doi.org/10.1177/1933719112443877.
- Цховребова Л.Т., Шевцова М.А., Аксененко А.А., Дуринян Э.Р., Гависова А.А. Андрогенные рецепторы и их уникальность. Акушерство и гинекология. 2020; 12: 62-6. https://dx.doi.org/10.18565/aig.2020.12.62-66. [Tskhovrebova L.T., Shevtsova M.A., Aksenenko A.A., Durinyan E.R., Gavisova A.A. Androgen receptors and their uniqueness. Obstetrics and Gynecology. 2020; 12: 62-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.62-66.
- Jiang N.X., Li X.L. The disorders of endometrial receptivity in PCOS and its mechanisms. Reprod. Sci. 2022; 29(9): 2465-76. https://dx.doi.org/10.1007/s43032-021-00629-9.
- Simitsidellis I., Saunders P.T.K., Gibson D.A. Androgens and endometrium: New insights and new targets. Mol. Cell. Endocrinol. 2018; 465: 48-60.https://dx.doi.org/10.1016/j.mce.2017.09.022.
- Gibson D.A., Simitsidellis I., Collins F., Saunders P.T. Evidence of androgen action in endometrial and ovarian cancers. Endocr. Relat. Cancer. 2014; 21(4): T203-18.
- Perrone A.M., Cerpolini S., Maria Salfi N.C., Ceccarelli C., De Giorgi L.B., Formelli G. et al. Effect of long-term testosterone administration on the endometrium of female-to-male (FtM) transsexuals. J. Sex. Med. 2009; 6(11): 3193-200. https://dx.doi.org/10.1111/j.1743-6109.2009.01380.x.
- Palomba S., Piltonen T.T., Giudice L.C. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum. Reprod. Update. 2021; 27(3): 584-618. https://dx.doi.org/10.1093/humupd/dmaa051.
- Semeniuk L.M., Likhachov V.K., Yuzvenko T.Y., Dobrovolska L.М., Makarov O.G. Risk markers of reproductive loss in women with hyperandrogenism. Wiad. Lek. 2018; 71(8): 1550-3.
- Cicinelli E., de Ziegler D. Transvaginal progesterone: evidence for a new functional 'portal system' flowing from the vagina to the uterus. Hum. Reprod. Update. 1999; 5(4): 365-72. https://dx.doi.org/10.1093/humupd/5.4.365.
- Beral V., Bull D., Reeves G.; Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the million women study. Lancet. 2005; 365(9470): 1543-51. https://dx.doi.org/10.1016/S0140-6736(05)66455-0.
- Halasz M., Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J. Reprod. Immunol. 2013; 97(1): 43-50. https://dx.doi.org/10.1016/j.jri.2012.10.011.
- Brenner R.M., Slayden O.D. Progesterone receptor antagonists and the endometrial antiproliferative effect. Semin. Reprod. Med. 2005; 23(1): 74-81. https://dx.doi.org/10.1055/s-2005-864035.
- Critchley H.O., Saunders P.T. Hormone receptor dynamics in a receptive human endometrium. Reprod. Sci. 2009; 16(2): 191-9. https://dx.doi.org/10.1177/1933719108331121.
- Babayev S.N., Park C.W., Keller P.W., Carr B.R., Word R.A., Bukulmez O. Androgens upregulate endometrial epithelial progesterone receptor expression: potential implications for endometriosis. Reprod. Sci. 2017; 24(10): 1454-61. https://dx.doi.org/10.1177/1933719117691145.
- Nikolaeva M., Babayan A., Stepanova E., Arefieva A., Dontsova T., Smolnikova V., Kalinina E., Krechetova L., Pavlovich S., Sukhikh G. The link between seminal cytokine interleukin 18, female circulating regulatory T cells, and IVF/ICSI success. Reprod. Sci. 2019; 26(8): 1034-44. https://dx.doi.org/10.1177/1933719118804404.
- Robertson S.A., Mau V.J., Tremellen K.P., Seamark R.F. Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J. Reprod. Fertil. 1996; 107(2): 265-77. https://dx.doi.org/10.1530/jrf.0.1070265.
- Cermik D., Selam B., Taylor H.S. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003; 88(1): 238-43. https://dx.doi.org/10.1210/jc.2002-021072.
- Frolova A.I., O'Neill K., Moley K.H. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol. Endocrinol. 2011; 25(8): 1444-55. https://dx.doi.org/10.1210/me.2011-0026.
- Quant H.S., Zapantis A., Nihsen M., Bevilacqua K., Jindal S., Pal L. Reproductive implications of psychological distress for couples undergoing IVF. J. Assist. Reprod. Genet. 2013; 30(11): 1451-8. https://dx.doi.org/10.1007/s10815-013-0098-7.
- Ryan M., Ryznar R. The molecular basis of resilience: a narrative review. Front. Psychiatry. 2022; 13: 856998. https://dx.doi.org/10.3389/fpsyt.2022.856998.
Received 29.07.2022
Accepted 19.08.2022
About the Authors
Marina A. Nikolaeva, Dr. Bio. Sci., Leading Researcher at the Laboratory of Clinical Imunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation, MNikolaeva@oparina4.ru, https://orcid.org/0000-0002-1251-6755, 4, Oparina str., Moscow, 117997, Russian Federation.Alina A. Babayan, PhD, Researcher at the IVF Department named after Prof. B.V. Leonov, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation, a_babayan@oparina4.ru, https://orcid.org/0000-0003-0963-6382, 4, Oparina str., Moscow, 117997, Russian Federation.
Alla S. Arefieva, Researcher at the Laboratory of Clinical Imunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation, a_arefyeva@oparina4.ru, https://orcid.org/0000-0001-9046-3196, 4, Oparina str., Moscow, 117997, Russian Federation.
Vitaliy V. Chagovets, PhD, Head of the laboratory of Metabolomics and Bioinformatics of the Department of Systems Biology in Reproductive Medicine of the Institute
of Translational Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation, v_chagovets@oparina4.ru, https://orcid.org/0000-0002-5120-376X,
4, Oparina str., Moscow, 117997, Russian Federation.
Natalia L. Starodubtseva, PhD, Head of the laboratory of Clinical Proteomics of the Department of Systems Biology in Reproductive Medicine of the Institute of Translational Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation, aurum19@mail.ru, https://orcid.org/0000-0001-6650-5915,
4, Oparina str., Moscow, 117997, Russian Federation.
Vladimir E. Frankevich, Dr. Sci. (Physics and Mathematics), Deputy Director for Research, Head of the Department of Systems Biology in Reproduction, V.I. Kulakov
NMRC for OG&P, Ministry of Health of the Russian Federation, v_frankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579,
4, Oparina str., Moscow, 117997, Russian Federation.
Elena A. Kalinina, Dr. Med. Sci., Head of the IVF Department named after Prof. B.V. Leonov, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation, e_kalinina@oparina4.ru, https://orcid.org/0000-0002-8922-2878, 4, Oparina str., Moscow, 117997, Russian Federation.
Lyubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Imunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation,
l_krechetova@oparina4.ru, https://orcid.org/0000-0001-5023-3476, 4, Oparina str., Moscow, 117997, Russian Federation.
Gennady T. Sukhikh, Academician of the RAS, Dr. Med. Sci., Professor, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation,
g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260, 4, Oparina str., Moscow, 117997, Russian Federation.
Corresponding author: Marina A. Nikolaeva, MNikolaeva@oparina4.ru
Authors' contributions: Nikolaeva M.A., Arefieva A.S., Babayan A.A., Sukhikh G.T. – conception and design of the study; Nikolaeva M.A. – statistical analysis, manuscript drafting; Babayan A.A. – collection and analysis of clinical material, manuscript editing; Arefieva A.S. – laboratory tests, data analysis and interpretation; Chagovets V.V. – instrumental (HPLC-MS) analysis, data interpretation; Starodubtseva N.L., Frankevich V.E. – organization of processing and instrumental analysis of clinical material, manuscript editing; Kalinina E.A. – conception and design of the study, organization of clinical material collection; Krechetova L.V. – organization of laboratory testing of clinical material, manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the state order under the theme "Solving the problem of infertility in modern conditions by developing a clinical and diagnostic model of infertile marriage and using innovative technologies in assisted reproductive programs", registration number 22-A21-121040600410-7.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Nikolaeva M.A., Babayan A.A., Arefieva A.S., Chagovets V.V., Starodubtseva N.L., Frankevich V.E., Kalinina E.A., Krechetova L.V., Sukhikh G.T.
Does sexual intercourse during IVF/ICSI cycle affect endometrial thickness
in the presence of immunohormonal markers of stress in the seminal plasma?
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 10: 103-114 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.103-114



