Influence of perinatal factors on neonatal outcomes in Rh(D)‑mediated hemolytic disease of the fetus and newborn treated with intrauterine transfusion
Uretskaya E.V., Lenyushkina A.A., Krogh-Jensen O.A., Tetruashvili N.K., Kostyukov K.V., Uvarenkova P.A., Zubkov V.V., Degtyarev D.N.
Background: In recent decades, intrauterine transfusion (IUT) of donor red blood cells has become a key medical technology to prevent HDFN's adverse outcomes. However, the factors affecting neonatal outcomes in HDFN patients need further research.
Objective: To explore neonatal outcomes amongst patients with Rh(D)-mediated HDFN treated with IUTs and evaluate the effect of perinatal factors on neonatal morbidity and mortality.
Materials and methods: A single-center 16‐year cohort study included all neonates (n=132) treated with IUTs for Rh(D)-mediated HDFN at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology from January 2008 to December 2023. Most mothers were transferred from remote country regions with low anti-D Rh immunization coverage. Primary neonatal outcomes were death and potentially disabling brain injury (PVL/IVH grade III/perinatal stroke). The main perinatal factors were the presence and severity of hydrops fetalis, number of IUTs, gestational age (GA) at 1st IUT, and dynamics of
pre-IUT fetal hemoglobin (Hb).
Results: Median GA at birth was 34 weeks (IQR 32–34.4). The median number of IUTs was 2 (IQR 1–3). Hydrops fetalis reversed in 30 out of 53 patients. Lethal outcomes were observed in 8/132 neonates (6%). The need for exchange transfusion was 62/132 (47%). No cases of bilirubin encephalopathy were registered. Hydrops fetalis was associated with higher risk of death (RR=10.2; 95% CI: 1.3–82.4) and brain injury (RR=8.9; 95% CI: 2.1–38.4). Most cases (12/14) of severe brain injury developed antenatally. Among neonates with ≥2 IUTs (n=95), the absence of increment in pre-IUT fetal Hb was associated with postnatal signs of hydrops (OR=111; 95% CI: 3.6–1268), lethal outcome (OR=67.7; 95% CI: 3.6–1268), and severe brain injury (OR=19.6; 95% CI: 4.6–84).
Conclusion: Hydrops fetalis continues to be a leading perinatal factor negatively influencing neonatal survival and neurologic outcomes. The absence of positive dynamics of pre-IUT fetal Hb reflects a significantly higher risk of hydrops, severe perinatal brain injury, and lethal outcomes.
Authors' contributions: Uretskaya E.V. – study managing, data collection, review of publications on the topic of the article, text writing; Lenyushkina A.A., Tetruashvili N.K., Kostyukov K.V., Zubkov V.V., Degtyarev D.N. – editing; Krog-Jensen O.A. – data and statistical analysis, editing; Uvarenkova P.A. – literature review.
Conflict of interest: Authors declare no conflict of interest.
Funding: The investigation has not been sponsored.
Ethical Approval: The research project was approved by the local Ethics Committee, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: Mothers/legal representatives of newborns provided informed consent for the publication of the data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Uretskaya E.V., Lenyushkina A.A., Krogh-Jensen O.A., Tetruashvili N.K., Kostyukov K.V., Uvarenkova P.A., Zubkov V.V., Degtyarev D.N. Influence of perinatal factors on neonatal outcomes in
Rh(D)-mediated hemolytic disease of the fetus and newborn treated with intrauterine transfusion.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (9): 116-130 (in Russian)
https://dx.doi.org/10.18565/aig.2024.164
Keywords
In developed countries, the implementation of key strategies over the past 25 years has resulted in significant improvements in neonatal outcomes in severe cases of Rh immunization. These strategies include high coverage of specific prophylaxis immunization against the D antigen of the Rh system, introduction of routine screening for alloantibodies in the first trimester of pregnancy, organization of centralized care for pregnant women with alloimmunization, and timely administration of intrauterine therapy [1]. In the first years after the introduction of intrauterine transfusion (IUT) of donor erythrocytes, the majority of clinical cases at the time of the start of intrauterine treatment were still represented by edematous forms of hemolytic disease, and the survival rate of fetuses with severe edema syndrome remained significantly lower than that of fetuses with anemia but without fetal hydrops [2–5]. The long-term presence of severe fetal hydrops is associated with perinatal hypoxic-hemorrhagic damage to the central nervous system (CNS). Therefore, preventing the development of fetal edema syndrome is of paramount importance not only for survival but also for reducing the incidence of disability in children. The experience of managing pregnancies with severe Rh immunization in developed countries has made it possible to significantly reduce the incidence of severe fetal hydrops owing to the timely detection of severe anemia and its intrauterine correction [6, 7]. In cases where fetal hydrops was identified at the outset of the intrauterine correction process, the majority of cases demonstrated complete reversal of the condition [1]. As a result, the survival rate of newborns with severe forms of Rh immunization (irrespective of the presence of fetal hydrops at the antenatal stage) is 95% [1, 8]. In most cases, the gestational age of children at the time of birth exceeds 32 weeks. Additionally, it is noteworthy that the hemoglobin levels at birth were higher in these infants. As of 2018, the results of more than 3,500 IUTs in patients with severe forms of alloimmunization to erythrocyte antigens have been published worldwide. Of these, approximately one-third were conducted at the reference center of Leiden University Hospital (Netherlands). The experts at this center currently represent the world's foremost authorities in the treatment of hemolytic disease of the fetus and newborn (HDFN). They have achieved a remarkably low incidence of complications, with a survival rate of 97% [8].
Domestic studies in this area have been conducted in small groups of patients. Infants with hemolytic disease caused by the Rh factor and IUT are born in a critical condition and require intensive care in the initial hours of life. The trajectory of the neonatal period and the severity of the newborn's condition are directly contingent upon the timing of delivery and the level of fetal hemoglobin at the commencement of intrauterine treatment [9].
The V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia (the Center) has a multidisciplinary team of specialists who provide consulting, routing, and specialized care to patients who need intrauterine correction of fetal anemia. Most of these patients came from different regions of the Russian Federation after telemedicine consultations. Over 16 years, the Center has accumulated extensive experience in managing severe forms of Rh immunization, including in patients with an extremely complicated history of hemolytic disease of the fetus (HDF) (lack of anti-D prophylaxis, high parity, and repeated perinatal losses). The present article offers an analysis of the impact of perinatal factors on neonatal outcomes of hemolytic disease by the Rh factor during treatment with IUT.
This study aimed to evaluate neonatal outcomes in patients with Rh hemolytic disease and a history of IUT, and to assess the impact of perinatal factors on neonatal morbidity and mortality.
Materials and methods
Study design
This single-center retrospective-prospective cohort observational study included 132 newborns with hemolytic disease by the Rh factor, born in the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia from 2008 to 2023, whose mothers received IUT. This institution is a multidisciplinary national reference center that provides specialized high-tech medical care for pregnant women and newborns. Data on patients and IUT procedures were obtained from a retrospectively and prospectively filled electronic database (retrospective stage of the study, from January 2008 to July 2017; prospective stage, from August 2017 to December 2023). The absolute majority were patients with erythrocyte alloimmunization mainly by the D antigen; In one case, there was immunization against rare antigens of the Rh system, and in three cases, combined immunization was diagnosed (against D-antigen of the Rh system and A-antigen of the AB0 system – in two cases, against D-antigen of the Rh system and S-antigen of the MNS system – in one). Among the patients included in the study, there were three twins: in two cases, intrauterine treatment was performed for both fetuses; in one case, only one fetus received IUT, and the other was Rh-negative. Newborns with multiple malformations and nonimmune hydrops were excluded.
The indication for IUT was severe fetal anemia diagnosed using the peak blood flow velocity in the middle cerebral artery (MCA) of the fetus (1.5-fold increase in the median, typical for a given gestational age according to Doppler sonography). Other ultrasound signs of severe fetal anemia were cardiomegaly and hydrops.
Parameters under investigation
Maternal history, pregnancy characteristics, antenatal treatment details, and HDF severity (fetal hemoglobin (Hb) level and presence of hydrops before the first IUT) were recorded (Table 1). Newborns were followed up until they reached 3 months of age.
Neonatal outcomes included the presence of potentially disabling CNS lesions, death, survival without potentially disabling CNS lesions, and the presence of postnatal hydrops (birth of a child with the edematous form of hemolytic disease of the newborn (HDN)). Hydrops was considered moderate if the patient had only mild ascites, and severe if ascites was pronounced and/or fluid was found in other cavities (hydrothorax and hydropericardium), as well as signs of subcutaneous fat edema [1, 10, 11]. Potentially disabling neurological outcomes were considered severe hypoxic-hemorrhagic lesions of the CNS in newborns (ischemic stroke, periventricular leukomalacia (PVL) [12], grade 3 intraventricular hemorrhage (IVH) [13], parenchymal and subarachnoid hemorrhages, venous hemorrhagic infarctions, and hemorrhages in the cerebellum and posterior cranial fossa). Depending on the symptom complex at birth, the following forms of HDN were distinguished: edematous (hemolytic anemia with fetal hydrops), icteric (hemolytic anemia with jaundice), and anemic (hemolytic anemia without jaundice or fetal hydrops). Secondary assessed neonatal outcomes were bronchopulmonary dysplasia (BPD) [14, 15], retinopathy ≥ stage III. [16], disseminated intravascular coagulation syndrome [17], necrotizing enterocolitis (NEC) [18, 19], respiratory distress syndrome (RDS) [20], congenital pneumonia [21], transient tachypnea of the newborn (TTN) [22].
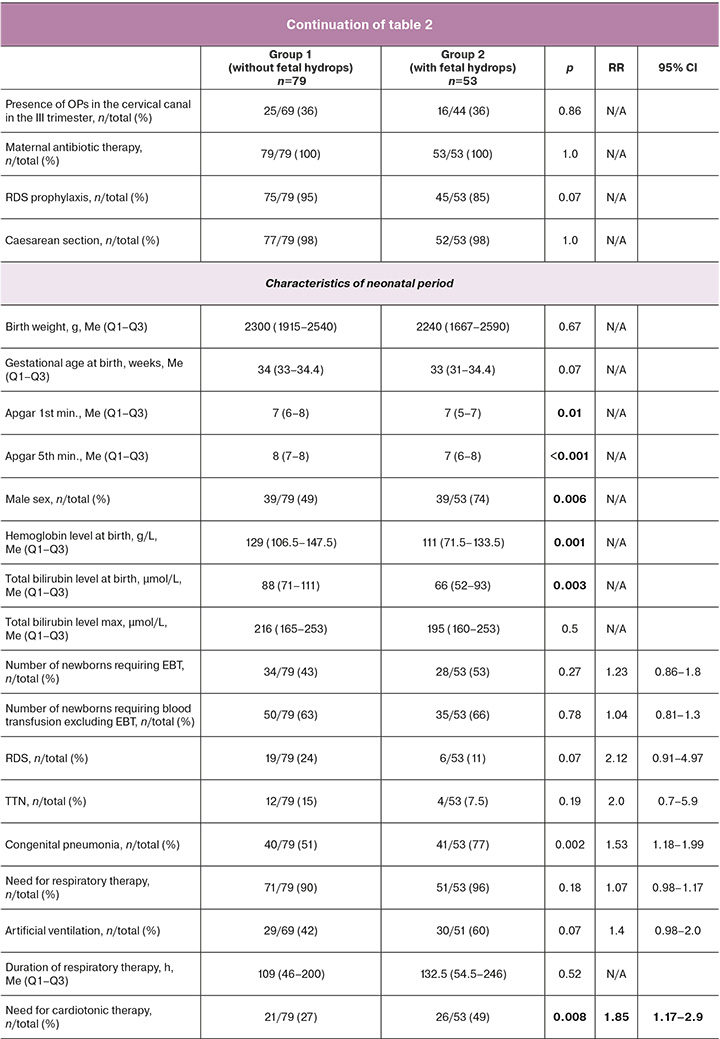
All newborns in the study were divided into two groups depending on the presence or absence of a history of hydrops: in Group 1 (n=79), signs of hydrops were absent throughout the intrauterine period and at birth; in Group 2 (n=53), signs of hydrops were determined at the antenatal stage and/or at birth.
Neonatal outcomes were assessed separately in the subgroups according to the following criteria:
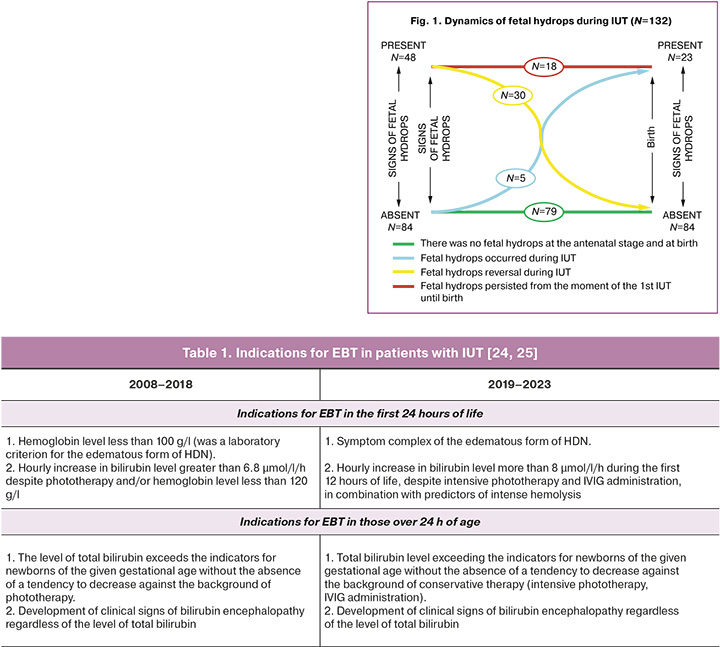
1). dynamics of edema syndrome against the background of intrauterine treatment. Patients in the 2nd group (n=79) were divided into two subgroups: 2a, patients with intrauterine hydrops reversion (children with icteric or anemic form of HDN) and 2b, patients with hydrops persistence or its appearance against the background of IUT (children with edematous form of HDN) (Fig. 1). Hydrops reversion is the complete disappearance of edema syndrome in the fetus against the background of intrauterine correction of severe anemia [11, 23].
2) the severity of edema syndrome. Patients in the 2nd group (n=79) were divided into two subgroups: 2c, moderate fetal hydrops and 2d, severe fetal hydrops (according to the maximum degree of hydrops that occurred).
3) the severity of hydrops at birth (among patients with postnatal signs). Hydrops was considered moderate if the newborn had only slight ascites in the absence of pericardial effusion, and severe if ascites was pronounced and/or fluid was found in other cavities (hydrothorax and hydropericardium), as well as signs of subcutaneous fat edema [11].
4) dynamics of hemoglobin level during intrauterine treatment in patients who received two or more IUTs. Fetal Hb values were assessed before each IUT. The dynamics were considered positive in the case of a stable trend towards an increase in Hb level with each subsequent IUT. The dynamics were considered negative if the Hb level decreased, remained critically low throughout the intrauterine period (less than 60 g / l), or the upward trend was unstable (initially, the Hb level increased but decreased again before the last IUT).
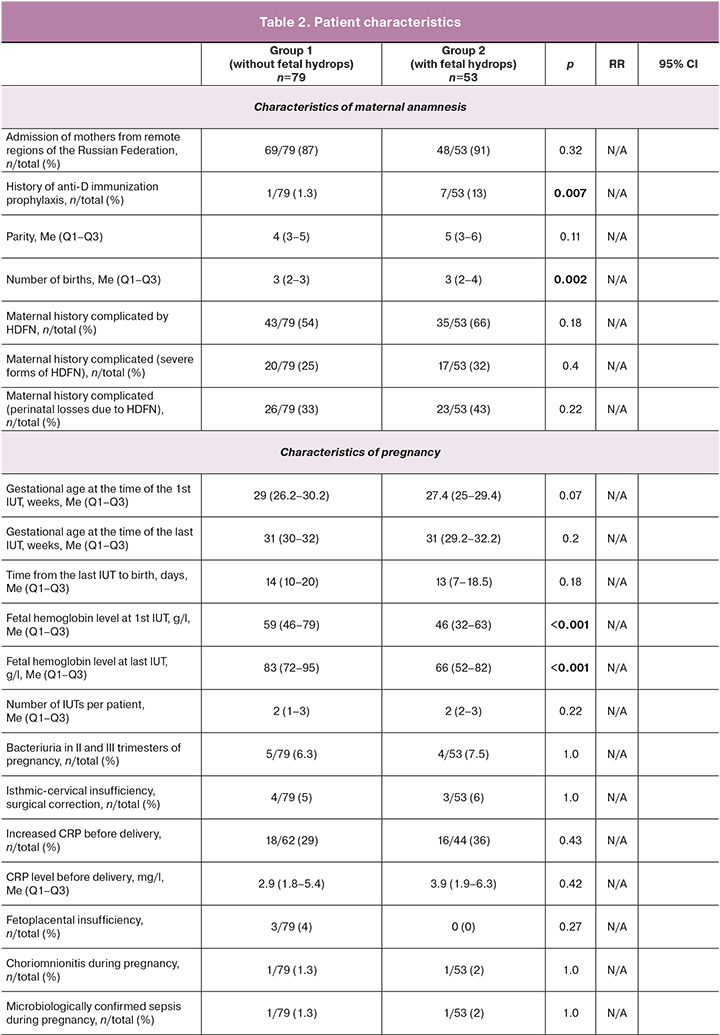
Diagnostics and treatment at the neonatal stage were performed in accordance with clinical guidelines [24, 25]. Indications for exchange blood transfusion (EBT) are provided in the Table 1.
Statistical analysis
Statistical analysis was performed using standard methods of variation using SPSS version 23 (IBM, USA). The normality of the distribution was evaluated through the application of several statistical tests, including the Kolmogorov–Smirnov test with Lilliefors correction, Shapiro–Wilk test, and assessment of excess and asymmetry indicators. A distribution that differed from the normal distribution was observed in all groups. To ascertain the statistical significance of the differences in quantitative data, the nonparametric Mann–Whitney U test was used to compare the two groups. The results are presented as median (Me) and interquartile range of the 25th–75th percentiles (Q1–Q3). Differences were considered statistically significant at a significance level of p<0.05. When analyzing nominal variables (frequency), the Pearson’s χ2 test with Yates' correction and Fisher's exact test for small numbers of observations were used.
To assess the strength of the relationship between the binary variables, the relative risk (RR) with a 95% confidence interval (CI) was calculated. In the event of an analysis conducted within a single subgroup (as opposed to the entire cohort of subjects), the odds ratio (OR) and 95% confidence interval (CI) were employed to quantify the strength of the relationship.
ROC analysis was used to evaluate the correlation between quantitative and binary variables. The threshold level of fetal hemoglobin at the last IUT was associated with an increased risk of giving birth to a child with the edematous form of HDN. The quality of the model was evaluated using the area under the receiver operating characteristic curve in Se (sensitivity) and 1-Sp (specificity) coordinates. The area under the curve (AUC) value was presented with a 95% confidence interval. To calculate the performance characteristics, the threshold level was selected in accordance with the requirements for the maximum combined sensitivity and specificity. A 95% confidence interval (CI) for the performance characteristics was calculated based on the chi-squared distribution. An area under the curve coefficient within the range of 0.9–1.0 was deemed an indicator of the highest informativeness (accuracy) of the test, within the range of 0.8–0.9 – good informativeness, within the range of 0.7–0.8 – satisfactory, within the range of 0.6–0.7 – mediocre informativeness, and below – useless classification.
Results
The maternal history, pregnancy course, and clinical characteristics of the patients are presented in Table 1, along with the corresponding neonatal outcomes. The majority of mothers in both groups were referred to the Center from regions of the Russian Federation (87 and 91%, respectively). They had an extremely complicated history of HDFN (54 and 66%, respectively) due to previous perinatal losses (33 and 43%) and the birth of children with severe forms of HDN (25 and 32%), mainly without a history of anti-D immunization prophylaxis. The parity of mothers was higher in the group with signs of hydrops (mean=5 versus 4), and the number of births in history (mean=3) was comparable in both groups. The median gestational age of the fetus at the time of the woman's admission to the Center for intrauterine treatment was 29 (26.2–30.2) and 27.4 (25–29.4) weeks, respectively. The mean gestational age of the fetuses at the time of the last IUT was 31 weeks, with no significant difference observed between the two groups. Similarly, the time elapsed from the last IUT to birth did not differ significantly, with averages of 14 and 13 days, respectively.
The mean number of IUTs performed per patient was two in both groups. It is noteworthy that the Hb levels in fetuses with hydrops were lower, both in the first (46 vs. 59 g/L) and last (66 vs. 83 g/L) IUTs. No additional statistically significant differences were identified at the antenatal stage between patients in the two groups. Notably, approximately one-third of the mothers exhibited an elevation in C-reactive protein (CRP) prior to delivery. Additionally, microbiological examination of cervical canal discharge revealed an increase in opportunistic microorganisms (OPM) during the third trimester in 36% of the patients in each group. Two patients (one from each group) were diagnosed with positive blood culture results and chorioamnionitis. All patients were administered a broad-spectrum antibiotic (protected aminopenicillins and cephalosporins) both before and after intrauterine intervention. The frequency of fetal RDS prevention was 95% and 85%, respectively, and 98% of deliveries were performed by caesarean section.
In all cases, delivery was precipitated by an increase in the severity of anemia and a decline in fetal condition. All pregnant women underwent cesarean delivery because of premature gestational age and an unripe cervix. The median gestational age at delivery in the group with an intrauterine diagnosis of hydrops was one week lower (33 weeks compared to 34 weeks). This is due to the greater severity of anemia in fetuses with hydrops, worse tolerance of IUT due to heart failure, and, as a result, a rapid repeated increase in anemia.
Prior to 2021, deliveries of pregnant women following IUT were conducted between the 31st and 34th weeks of gestation, with a median gestational age of 33 weeks. Later, according to new clinical guidelines, the gestational age was increased to 35–36 weeks. The prolongation of pregnancy was associated with improvement of the IUT technique (carried out after the 32nd week, with a fetal weight of over 2000 g) and improved fetal observation and monitoring capabilities.
The majority of pregnant women (98%, 129/132) underwent planned cesarean delivery following a consultative discussion. The main reasons for early delivery were increasing severity of anemia at pregnancy >33 weeks (72%), fetal distress (deterioration on cardiotocography (CTG) parameters, and blood flow in the umbilical cord vessels) in 19% of cases. The less common causes of premature delivery were onset of regular labor (3%), chorioamnionitis (2%), critical decrease in fetoplacental blood flow ("zero" diastolic blood flow in the umbilical artery) (2%), and incompetent uterine scar after previous operative deliveries (2%). In isolated cases, indications for delivery were premature rupture of membranes, increasing the severity of preeclampsia in the mother, placenta previa, and placenta accreta. Most newborns (99%, 130/132) were born prematurely and all children were admitted to the intensive care unit.
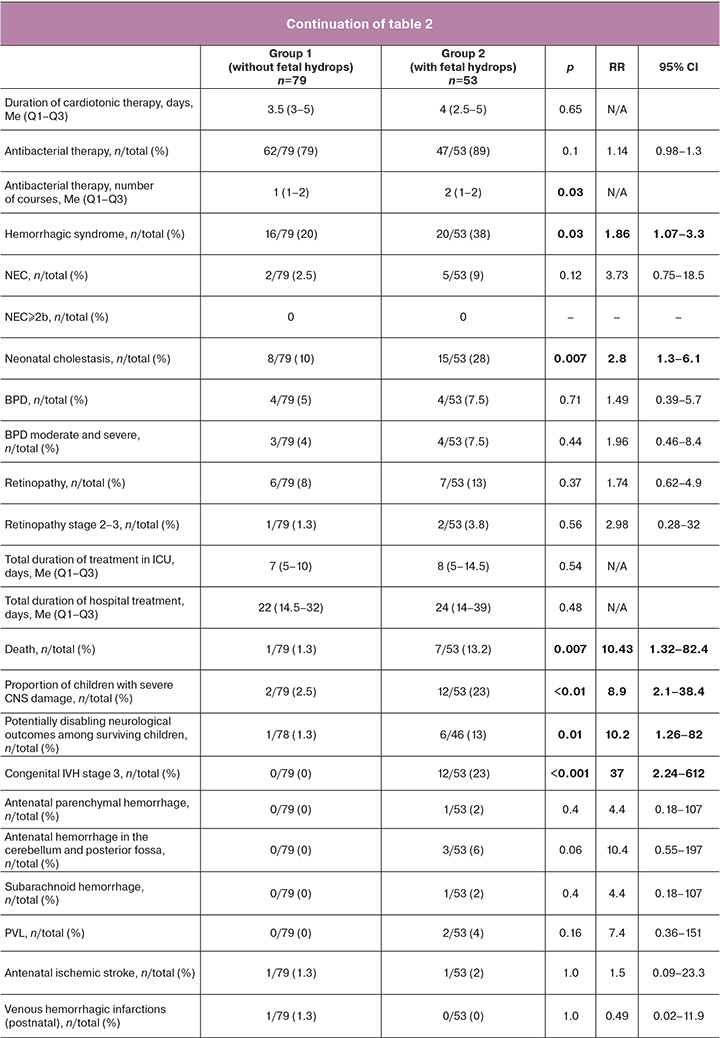
The median birth weights were 2300 and 2240 g, respectively. The median Apgar scores were 7/8 and 7/7, respectively, and statistically significant differences were observed between the groups. The median Hb and total bilirubin levels at birth were significantly higher in the group of patients without hydrops (129 vs. 111 g/L and 88 vs. 66 μmol/L, respectively). None of the newborns had nuclear jaundice. Approximately half of the patients in each group required EBT after birth. In terms of respiratory diagnoses, congenital pneumonia was the most common in both groups (51 and 77%, respectively), followed by RDS (24 and 11%, respectively), and TTN (15 and 7.5%, respectively). Most patients (>90%) required respiratory therapy. The need for invasive mechanical ventilation (MV) was observed in 42 and 60% of patients, respectively. The need for cardiotonic therapy was significantly higher in the group of patients with hydrops (49% vs. 27%). Severe hemorrhagic syndrome after birth developed in patients in the hydrops group almost 2 times more often (38% versus 20%). Most of the children (79 and 89%, respectively) received antibacterial therapy. In each group, there were cases of moderate and severe BPD and stage 2–3 retinopathy in extremely premature patients; however, all of them were discharged without the need for additional oxygen, and none of them required retinal surgery. Isolated cases of NEC were limited to stages 1–2a, and none of the patients underwent surgery. The total duration of treatment in the intensive care unit (ICU) and hospital in both the groups was comparable (Table 2).
In the group of patients with a history of hydrops, 7 had fatal outcomes (13.2%). In the group of patients without hydrops, the only fatal outcome was recorded in the late neonatal period due to the development of severe multiple organ failure due to severe blood transfusion complications after repeated EBT. Autopsy of the same patient revealed venous hemorrhagic cerebral infarctions that developed in the 2nd week of life. Another case of severe CNS damage in patients without hydrops was antenatal ischemic stroke; however, the child did not show any pathological neurological symptoms during the neonatal period. Thus, among the survivors in the group of patients without hydrops, only one patient had a potentially disabling neurological outcome at the time of discharge from the hospital (Table 2).
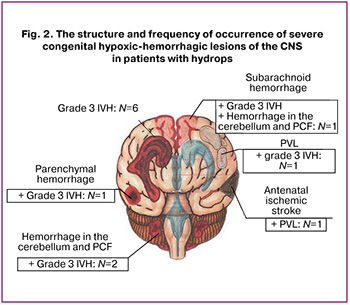
All severe hypoxic-hemorrhagic CNS lesions in patients with hydrops that developed at the antenatal stage were detected in 12/53 children (23%). Their structure deserves special attention: antenatal ischemic stroke combined with congenital PVL was detected in one patient, isolated congenital IVH stage 3 occurred in six patients, in four IVH stage 3 was combined with hemorrhage in other brain structures, and in one with PVL (Fig. 2).
Neonatal outcomes according to the dynamics of edema syndrome in patients with HDFN
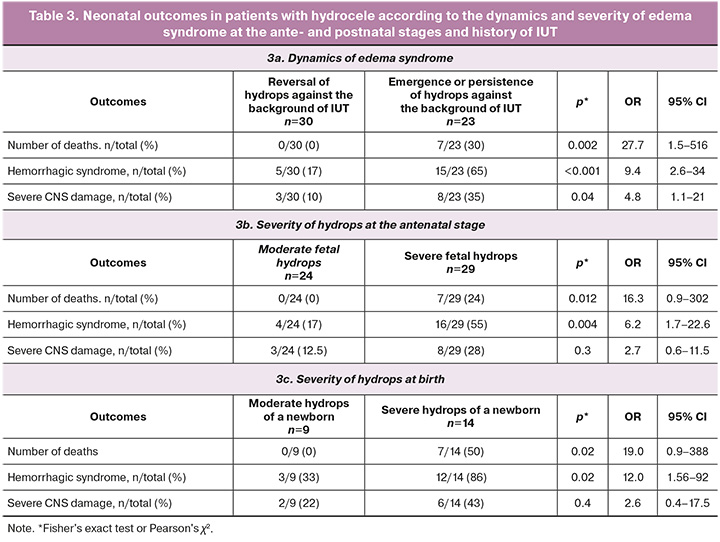
Considering the statistically significant differences in the outcomes between the study groups, patients with hydrops were further divided into two subgroups according to the dynamics of edema syndrome: reversion and persistence of hydrops (Table 3, Section 2a). High mortality was noted in the group of patients with persistent hydrops (7/23, 30%), and there were no fatal outcomes in the group with reversion of hydrops. Additionally, a significantly higher incidence of severe hemorrhagic syndrome (65% versus 17%) and severe central nervous system (CNS) damage (35% versus 10%) was observed in patients with persistent hydrops (Table 3, Section 2a)
Neonatal outcomes according to the severity of hydrops at the antenatal stage
Neonatal outcomes also varied according to the severity of hydrops during the antenatal stage (Table 3, Section 2b). There were no fatal outcomes in patients with moderate hydrops, whereas in patients with severe hydrops, the mortality rate was 24% (7/29). Severe hemorrhagic syndrome after birth was also more common (55% vs. 17%). No statistically significant differences in the neurological outcomes were observed.
Neonatal outcomes according to the severity of hydrops at birth
Of 132 neonates, 23 were diagnosed with the edematous form of HDN at birth. Depending on the severity of edema syndrome, the patients were divided into two groups: moderate (n=9) and severe (n=14) hydrops at birth [11] (Table 3, Section 2c). Neonatal outcomes varied depending on the severity of hydrops at birth (Table 3, Section 2c). There were no fatal outcomes in cases of moderate hydrops of the newborn, and in severe hydrops, the mortality rate was 50% (7/14). Severe hemorrhagic syndrome after birth was also more common (86% vs. 33%). No statistically significant differences in the neurological outcomes were observed.
Neonatal outcomes according to the dynamics of fetal Hb levels during intrauterine treatment
The dynamics of fetal Hb levels during intrauterine treatment were assessed as indicators of HDFN severity. This indicator can only be assessed in patients with repeated IUTs (≥ 2) in cases of successful blood sampling during cordocentesis before transfusion. The indicator was assessed in 95 newborns who received two to seven IUTs. Fetal Hb levels were assessed before each IUT. The dynamics were positive in 75 patients and negative in 20 (Fig. 3).
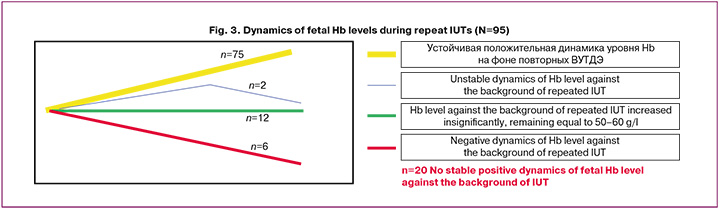
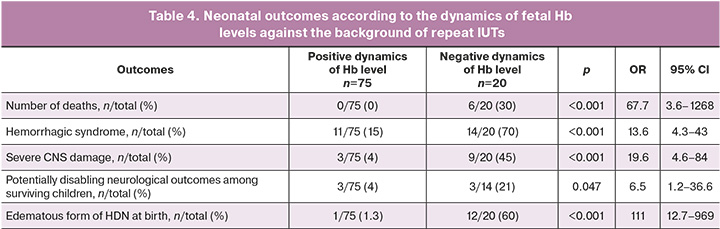
There were no fatal outcomes in patients with persistently elevated Hb levels during IUTs (n=75, Fig. 3), whereas the mortality rate was 30% (6/20) in patients with negative dynamics of fetal Hb levels (Table 4). Only one patient with positive dynamics of Hb levels during IUTs had the edematous form of HDN at birth; the hydrocele was moderate. In the group of patients with negative dynamics of Hb levels at birth, the edematous form of HDN was predominant (60%, of 12/20). Almost half of the children had severe CNS damage (45%) and 79% (11/14) survived without disabling neurological outcomes. Among patients with positive dynamics of Hb levels, three had severe CNS damage at birth (which was determined at the antenatal stage at the time of the first IUT) and a potentially disabling outcome. Thus, the survival rate without disabling neurological outcomes in this group was 96% (Table 4).
According to our study, the initial fetal Hb level did not affect neonatal outcomes, and the fetal Hb level before the last IUT was significantly lower in patients with postnatal signs of the edematous form of HDFN than in newborns with the absence or reversion of hydrops (Me=48 g/l, Q1–Q3=39–57 vs Me=80 g/l, Q1–Q3=68–95, p<0.001). The ROC analysis determined that the critical Hb level before the last IUT was ≤58 g/l, which indicates a high probability of giving birth to a child with the edematous form of HDN (AUC=0.875, 95% CI 0.77–1.0; Se=76.9%, Sp=91.7%, p<0.001). It should also be noted that by 2023, 28 newborns with HDN by Rh factor and a history of IUT were treated in NICU No. 2, which is the maximum number of patients per year since the start of using this method of intrauterine treatment of severe forms of HDF in the Center. At the same time, the mortality rate was 0%, including among newborns with the edematous form of HDN, and severe perinatal hypoxic-hemorrhagic CNS lesions did not develop at either the intrauterine or postnatal stages. Thus, survival without disabling outcomes in neonates with HDN by Rh factor and a history of IUT was 100% for the first time despite an increase in the number of patients with severe forms of HDFN.
Discussion
At V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, women with the most severe forms of Rh immunization are referred from various regions of the Russian Federation. Many of these women experience repeated perinatal losses due to HDFN. In our study, we observed that patients with fetal hydrops were admitted earlier than those with fetal anemia without hydrops. This difference is likely associated with an earlier onset of transplacental transmission of maternal anti-erythrocyte antibodies and subsequent hemolysis of fetal erythrocytes. Additionally, the degree of antibody aggressiveness and/or combined immunization is likely to play a significant role. This is further evidenced by the lower hemoglobin levels in fetuses with hydrops at both the first and last IUT. Furthermore, the lower Hb and bilirubin levels at birth in patients with hydrops also suggest that hemolysis peaked during the antenatal stage.
Severe indirect hyperbilirubinemia in children with HDN and a history of IUT is extremely rare, occurring primarily in cases of combined alloimmunization with erythrocyte antigens and/or when fetal erythrocytes are predominately their own rather than donor-derived. It can also arise in cases of liver failure due to various factors, including prematurity, congenital infection, and severe hepatomegaly. Consequently, bilirubin encephalopathy as a cause of unfavorable neurological outcomes in children with HDN and IUT is losing its significance, while severe antenatal hypoxic-hemorrhagic lesions of the CNS in the studied group of children have become more prominent among the factors influencing prognosis. Our study provides the first detailed account of the localization of perinatal hypoxic-hemorrhagic lesions in the CNS of patients with HDFN and IUT. Simultaneously, hydrops fetalis and neonatal hydrops have been extensively studied as conditions that exhibit distinct dynamics and severity during both the ante- and postnatal stages.
The introduction of the IUT method, along with measures for nonspecific and specific prevention of alloimmunization by erythrocyte antigens, has significantly reduced the incidence of severe forms of HDFN due to the Rh factor. In recent years, according to the reference center of Leiden University Hospital [26], this has led to the almost complete disappearance of its edematous form in Western European countries. In the latest cohort, which included patients from 2011 to 2016, only 6% (7/115) of the patients exhibited signs of hydrops, with severe hydrops detected in only one fetus.
A study conducted in Belgium found that severe hydrops fetalis occurred in 5% of patients who received erythrocyte immunization [27]. However, in countries where routine screening for red cell antibodies is less common or absent, the incidence of hydrops is significantly higher. A group of researchers from Brazil [28] reported that the incidence of the edematous form of HDF was 34% at the time of presentation. In the largest tertiary perinatal center in India, the prevalence of hydrops in fetuses with HDF at the time of the first IUT was 22% [29].
A 30-year cohort study from the Leiden University Hospital Reference Center [26] identified and detailed the factors contributing to the gradual and nearly complete disappearance of severe hydrops in fetuses alloimmunized with red cell antigens. This positive experience could and should be applied to the Russian Federation. Non-specific prevention of HDFN due to the Rh factor includes informing patients with Rh-negative blood regarding the importance of maintaining any pregnancy. In cases of Rh isoimmunization, patients should be made aware of the possibility of selecting and transferring embryos with a Rh-negative genotype chosen through preimplantation genetic testing in assisted reproductive technology programs. Specific antenatal and postnatal prevention involves administering human immunoglobulin anti-Rhesus [D] to pregnant and postpartum women with Rh-negative blood and a negative level of anti-Rhesus antibodies, when the fetus or newborn has Rh-positive blood, in accordance with current clinical guidelines. To prevent immunization by minor antigens of the Rh system, such as C and E, and other antigen systems expressed on the erythrocyte membrane (e.g., Kell and Duffy), only non-specific prevention measures are utilized. This primarily concerns blood transfusions for women of reproductive age, which must be performed in strict accordance with the antigen profile.
In addition to preventive measures, the positive experience of the Netherlands was due to the introduction of a unified national guideline for managing pregnancies with alloimmunization. This guideline includes screening, laboratory and clinical monitoring, and routing pregnant women to the Fetal Therapy Center. Since 2011, screening has been expanded to identify pregnant women with Rhesus antigen. If the screening results are positive, the antigenic status of the fetus in the first trimester is determined by studying paternal antigens or cell-free DNA from the maternal plasma (available for D, c/C, E, and K antigens). With a positive antigenic status of the fetus, an antibody-dependent cellular cytotoxicity (ADCC) test was performed on maternal blood every two weeks. This indicator, which is unfortunately not used in Russia, is the only informative test for reliable assessment of the aggressiveness of antibodies and for predicting the severity of hemolysis to determine the risk group for intensive monitoring. Successful management and prolongation of pregnancy in recent years, as well as improvements in neonatal outcomes, were also facilitated by advancements in techniques and a reduction in the number of complications associated with the IUT procedure. Unified approaches to the diagnosis and treatment of pregnant women and newborns in large centers have also significantly impacted the quality of medical care (Fig. 4).
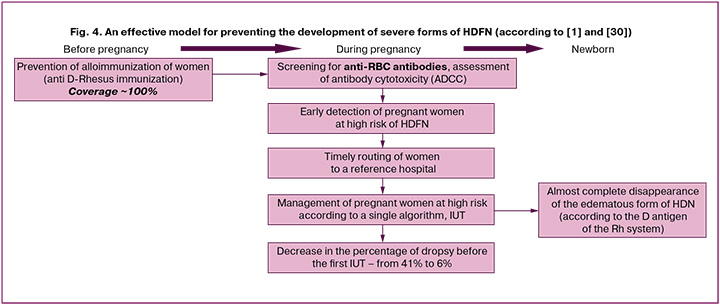
In our study, conducted on a population of patients with an extremely low level of anti-D immunization prophylaxis and a heavily complicated maternal history of HDFN, we found that, at the time of routing to the Center, the proportion of fetuses showing signs of hydrocephalus remains high. On average, at the start of IUT, approximately 40% of patients exhibit signs of hydrocephalus, with approximately half of the fetuses having severe hydrocephalus. However, there is a clear trend toward a decrease in the percentage of fetal hydrocephalus at the time of admission, starting in 2016, when most women began to be routed through the telemedicine consultation channel by specialists from the regional health institutions' Center. Thus, in 2023, the proportion of fetal hydrops at the time of admission was 33%; however, only one-third of patients had severe hydrops, and in 67% of cases, complete reversal was achieved with treatment.
The overall survival rate of newborns with hemolytic disease and a history of IUT over a 16-year observation period was 94 %, with 87% for patients with signs of hydrops and 99% for those without hydrops. Despite a constant increase in the number of newborns in the NICU with this severe condition, 100% survival was achieved in 2022 and 2023, a rate comparable to that of European reference centers. For instance, in the Netherlands, the survival rate of patients without hydrops increased from 91% to 96% between 1987 and 2016, whereas the survival rate for those with hydrops increased from 63% to 95%. In the largest tertiary perinatal center in India, the survival rates for patients with and without edema syndrome were also comparable at 90% and 94%, respectively [29].
We identified fetal hydrops as one of the most informative perinatal factors related to the outcomes. According to our data, regardless of the severity and dynamics of edema syndrome, it worsened the neurological prognosis by tenfold (Table 1). This finding aligns with a Dutch study, which reported that fetal hydrops was associated with an 11-fold increase in the risk of neurological deficit. Persistent or emergent fetal hydrops during treatment is linked to a 28-fold increase in mortality, a 9-fold increase in the risk of developing severe hemorrhagic syndrome, and a nearly 5-fold increase in the proportion of children with perinatal hypoxic-hemorrhagic CNS damage. Severe hydrops at the antenatal stage, in comparison to moderate hydrops, increased mortality by 16 times and the risk of developing severe hemorrhagic syndrome in the early neonatal period by six times. Severe hydrops in newborns, relative to moderate hydrops, is associated with a 19-fold increase in mortality and a 12-fold increase in the risk of developing severe hemorrhagic syndrome after birth. Although we have not found any publications examining the effect of hydrops and its dynamics on neonatal outcomes in such detail, it is evident that preventing the development of edema syndrome in the fetus is crucial not only for survival but also for reducing long-term disability in children.
In our study, the prognostic value of the presence, absence, and severity of hydrops in the development of perinatal hypoxic-hemorrhagic CNS lesions in surviving children was insufficient. This has prompted the search for a more sensitive perinatal factor. We considered several potential perinatal factors: maternal history of HDFN, gestational age at first and last IUT, number of IUTs, fetal hemoglobin level at first and last IUT, duration from the last IUT to birth, and gestational age at birth. However, none of these factors have demonstrated prognostic significance for disabling neurological outcomes.
An increase in the risk of neurological developmental delay with an increasing number of IUTs, as described by Dutch researchers [26], was not detected in this study because of differing endpoints. Our study focused solely on the presence of potentially disabling neurological outcomes at the time of patient discharge from the hospital, determined through instrumental methods (neurosonography, magnetic resonance imaging of the brain, and electroencephalogram), and neurological symptoms. In a Dutch study, neurological development was assessed in children aged two years using a specific scale. Additionally, the effect of a lower hemoglobin level before the first IUT on neurological outcomes, as reported by Dutch authors [26], was not observed in our study due to the differing endpoints. It is evident that deterioration of long-term outcomes can result not only from organic damage to the CNS but also from severe anemia, which negatively impacts the neuropsychological functions of the developing fetal brain.
The dynamics of fetal Hb levels in the context of repeated IUTs have been identified as perinatal factors with a stable relationship with neonatal outcomes. The lack of stable positive dynamics in fetal Hb levels during IUT increases the likelihood of giving birth to a child with the edematous form of HDN by 111 times, the risk of a lethal outcome by 68 times, the development of severe hemorrhagic syndrome in the early neonatal period by 13.6 times, and perinatal hypoxic-hemorrhagic lesions of the CNS in surviving newborns by 20 times. Thus, this parameter can be proposed as a predictor of adverse neonatal outcomes and as a criterion for assessing the effectiveness of intrauterine treatment for severe forms of HDF. The use of this parameter has not been described in either foreign or domestic studies.
The limitations of this study include the absence of a unified protocol for managing patients at ante- and postnatal stages until 2017.
Conclusion
In the population of patients with high parity and multiple births at the reference center, there was an extremely low rate of anti-D immunization prophylaxis and a significant history of hemolytic disease of the newborn. Many patients were admitted for observation at the V.I. Kulakov NMRC for OG&P already displaying signs of severe fetal hydrops. Despite treatment at the antenatal stage, the majority of children were born prematurely and exhibited clinical manifestations of HDN of varying severities. The incidence of the edematous form of HDN at birth was 17.4% (23 out of 132). Severe HDN with persistent fetal hydrops has been identified as the main cause of death in newborns and perinatal hypoxic-hemorrhagic CNS damage. Preventing the development of edema syndrome in the fetus is crucial to increasing survival rates and reducing disability in newborns. The dynamics of fetal hemoglobin levels in the context of IUT serves as the most significant perinatal factor, reliably indicating the risk of delivering a child with the edematous form of HDN and the likelihood of severe perinatal hypoxic-hemorrhagic CNS damage.
References
- Zwiers C., Oepkes D., Lopriore E., Klumper F.J., de Haas M., van Kamp I. The near disappearance of fetal hydrops in relation to current state-of-the-art management of red cell alloimmunization. Prenat. Diagn. 2018; 38(12): 943-50. https://dx.doi.org/10.1002/pd.5355.
- Liley A.W. Intrauterine transfusion of foetus in haemolytic disease. Br. Med. J. 1963; 2(5365): 1107-9. https://dx.doi.org/10.1136/bmj.2.5365.1107.
- Rodeck C.H., Kemp J.R., Holman C.A., Whitmore D.N., Karnicki J., Austin M.A. Direct intravascular fetal blood transfusion by fetoscopy in severe rhesus isoimmunization. Lancet. 1981; 1(8221): 625-7. https://dx.doi.org/10.1016/s0140-6736(81)91549-x.
- Altunyurt S., Okyay E., Saatli B., Canbahishov T., Demir N., Ozkan H. Neonatal outcome of fetuses receiving intrauterine transfusion for severe hydrops complicated by Rhesus hemolytic disease. Int. J. Gynecol. Obstet. 2012; 117(2): 153-6. https://dx.doi.org/10.1016/j.ijgo.2011.12.013.
- Van Kamp I.L., Klumper F.J., Oepkes D., Meerman R.H., Scherjon S.A., Vandenbussche F.P. et al. Complications of intrauterine intravascular transfusion for fetal anemia due to maternal red-cell alloimmunization. Am. J. Obstet. Gynecol. 2005; 192(1): 171-7. https://dx.doi.org/10.1016/j.ajog.2004.06.063.
- Pasman S.A., Claes L., Lewi L., Van Schoubroeck D., Debeer A., Emonds M. et al. Intrauterine transfusion for fetal anemia due to red blood cell alloimmunization: 14 years experience in Leuven. Facts Views Vis. Obgyn. 2015;7(2): 129-36.
- Ree I.M.C., Smits-Wintjens V.E.H.J., van der Bom J.G., van Klink J.M.M., Oepkes D., Lopriore D. et al. Neonatal management and outcome in alloimmune hemolytic disease. Expert Rev. Hematol. 2017; 10(7): 607-16. https://dx.doi.org/10.1080/17474086.2017.1331124.
- Zwiers C., van Kamp I., Oepkes D., Lopriore E. Intrauterine transfusion and non-invasive treatment options for hemolytic disease of the fetus and newborn – review on current management and outcome. Expert Rev. Hematol. 2017; 10(4): 337-44. https://dx.doi.org/10.1080/17474086.2017.1305265.
- Иванова А.В., Захарова С.Ю. Особенности гематологических показателей у детей, перенесших внутриутробное внутрисосудистое переливание крови, по поводу гемолитической болезни плода по резус-фактору. Российский вестник перинатологии и педиатрии. 2015; 60(4): 157-8. [Ivanova A.V., Zaharova S.Yu. Features of hematological parameters in children undergoing intrauterine intravascular blood transfusion, about fetal hemolytic disease Rh. Russian Bulletin of Perinatology and Pediatrics. 2015; 60(4): 157-8. (in Russian)].
- Савельева Г.М., ред. Резус-сенсибилизация. Гемолитическая болезнь плода: диагностика, лечение, профилактика. Пути снижения младенческой заболеваемости и смертности. Методические рекомендации. М.; 2019. 40 c. [Savel'yeva G.M., ed. Rh-sensitization. Hemolytic disease of the fetus: diagnosis, treatment, prevention. Ways to reduce infant morbidity and mortality: guidelines. Moscow; 2019. 40 p. (in Russian)].
- Van Kamp I.L., Klumper F.J., Bakkum R.S., Oepkes D., Meerman R.H., Scherjon S.A. et al. The severity of immune fetal hydrops is predictive of fetal outcome after intrauterine treatment. Am. J. Obstet. Gynecol. 2001; 185(3): 668-73. https://dx.doi.org/10.1067/mob.2001.116690.
- Volpe J.J., ed. Volpe’s neurology of the newborn. 6th ed. Philadelphia, PA: Elsevier; 2018: 389-457 (ch. 14-16).
- Inder T.E., Perlman J.M., Volpe J.J. Preterm intraventricular hemorrage/posthemorrhagic hydrocephalus. In: Volpe J.J., ed. Volpe’s neurology of the newborn. 6th ed. Philadelphia, PA: Elsevier; 2018: 637-700.
- Jobe A.H., Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001; 163(7): 1723-9. https://dx.doi.org/10.1164/ajrccm.163.7.2011060.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Бронхолегочная дисплазия. 2024. [Ministry of Health of the Russian Federation. Сlinical Guidelines. Bronchopulmonary Dysplasia. 2024. (in Russian)].
- Chiang M.F., Quinn G.E., Fielder A.R., Ostmo S.R., Paul Chan R.V., Berrocal A. et al. International classification of retinopathy of prematurity, Third edition. Ophthalmology. 2021; 128(10): e51-e68. https://dx.doi.org/10.1016/j.ophtha.2021.05.031.
- Veldman A., Fischer D., Nold M.F., Wong F.Y. Disseminated intravascular coagulation in term and preterm neonates. Semin. Thromb. Hemost. 2010; 36(4): 419-28. https://dx.doi.org/10.1055/s-0030-1254050.
- Bell M.J., Ternberg J.L., Feigin R.D., Keating J.P., Marshall R., Barton L. et al. Neonatal necrotizing enterocolitis. therapeutic decisions based upon clinical staging. Ann. Surg. 1978; 187(1): 1-7. https://dx.doi.org/10.1097/00000658-197801000-00001.
- Walsh M.C., Kliegman R.M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. North Am. 1986; 33(1): 179-201. https://dx.doi.org/10.1016/s0031-3955(16)34975-6.
- Sweet D.G., Carnielli V.P., Greisen G., Hallman M., Klebermass-Schrehof K., Ozek E. et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023; 120(1): 3-23. https://dx.doi.org/10.1159/000528914.
- Gleason C.A., Sawyer T., eds. Avery’s disease of the newborn. 11th ed. Philadelphia, PA: Elsevier; 2023: 607.
- Gleason C.A., Sawyer T., eds. Avery’s disease of the newborn. 11th ed. Philadelphia, PA: Elsevier; 2023: 608-9.
- Grannum P.A., Copel J.A., Moya F.R., Scioscia A.L., Robert J.A., Winn H.N. et al. The reversal of hydrops fetalis by intravascular intrauterine transfusion in severe isoimmune fetal anemia. Am. J. Obstet. Gynecol. 1988; 158(4): 914-9. https://dx.doi.org/10.1016/0002-9378(88)90094-4.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Гемолитическая болезнь плода и новорожденного (ГБН). 2017. [Ministry of Health of the Russian Federation. Сlinical Guidelines. Hemolytic disease of the fetus and newborn. 2017. (in Russian)].
- Антонов А.Г., Дегтярев Д.Н., Нароган М.В., Карпова А.Л., Сенькевич О.А., Сафаров А.А., Сон Е.Д., Малютина Л.В. Гемолитическая болезнь плода и новорожденного. Клинические рекомендации. Неонатология: новости, мнения, обучение. 2018; 6(2): 131-42. [Antonov A.G., Degtyarev D.N., Narogan M.V., Karpova A.L., Senkevich O.A., Safarov A.A., Son E.D., Malyutina L.V. Hemolytic disease of the fetus and newborn. Clinical guidelines. Neonatology: News, Opinions, Training. 2018; 6(2): 131-42. (in Russian)].
- Lindenburg I.T., Smits-Wintjens V.E., van Klink J.M., Verduin E., van Kamp I.L., Walther F.J. et al.; LOTUS study group. Long-term neurodevelopmental outcome after intrauterine transfusion for hemolytic disease of the fetus/newborn: The LOTUS study. Am. J. Obstet. Gynecol. 2012; 206(2): 141.e1-8. https://dx.doi.org/10.1016/j.ajog.2011.09.024.
- Osanan G.C., Silveira Reis Z.N., Apocalypse I.G., Lopes A.P., Pereira A.K., da Silva Ribeiro O.M. et al. Predictive factors of perinatal mortality in transfused fetuses due to maternal alloimmunization: what really matters? J. Matern. Fetal Neonatal. Med. 2012; 25(8): 1333-7. https://dx.doi.org/10.3109/14767058.2011.633668.
- Deka D., Dadhwal V., Sharma A.K., Shende U., Agarwal S., Agarwal R. et al. Perinatal survival and procedure-related complications after intrauterine transfusion for red cell alloimmunization. Arch. Gynecol. Obstet. 2016; 293(5): 967-73. https://dx.doi.org/10.1007/s00404-015-3915-7.
- Pahuja S., Gupta S.K., Pujani M., Jain M. The prevalence of irregular erythrocyte antibodies among antenatal women in Delhi. Blood Transfus. 2011; 9(4): 388-93. https://dx.doi.org/10.2450/2011.0050-10.
- van 't Oever R.M., Zwiers C., de Winter D., de Haas M., Oepkes D., Lopriore E. et al. Identification and management of fetal anemia due to hemolytic disease. Expert Rev. Hematol. 2022; 15(11): 987-98. https://dx.doi.org/10.1080/17474086.2022.2138853.
Received 11.07.2024
Accepted 05.09.2024
About the Authors
Evgenia V. Uretskaya, Anesthesiologist-Intensivist, Researcher at the Neonatal Intensive Care Unit No. 2 of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(920)894-77-36, evuretskaya@mail.ru, https://orcid.org/0000-0002-8961-3000Anna A. Lenyushkina, PhD, Head of the Neonatal Intensive Care Unit No. 2, Associate Professor at the Neonatology Department of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)531-44-44 ex. 2700, 2697, a-lenushkina@yandex.ru, https://orcid.org/0000-0001-8929-2991
Olga A. Krogh-Jensen, PhD, Neonatologist at the Neonatal Intensive Care Unit No. 2 of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Associate Professor at the Neonatal Department of the Institute of Children's Health, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University),
19991, Russia, Moscow, Trubetskaya str., 8/2, +7(926)014-01-35, o_krogh@oparina4.ru, olgaborisevich@gmail.com, https://orcid.org/0000-0002-5178-5659
Nana K. Tetruashvili, PhD, Нead of the Obstetric Department of Pregnancy Pathology No. 2, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-14-77, tetrauly@mail.ru, n_tetruashvili@oparina4.ru, https://orcid.org/0000-0002-9201-2281
Polina A. Uvarenkova, Neonatologist at the Neonatal Patology Unit No. 2 of the the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(930)825-08-84, uvarenkova1997polina@yandex.ru, https://orcid.org/0000-0001-6699-7315
Kirill V. Kostyukov, Dr. Med. Sci., Head of the Department of the Ultrasound and Functional Diagnosis, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(926) 214-97-84, kostyukov_k@yahoo.com,
https://orcid.org/0000-0003-3094-4013
Viktor V. Zubkov, Dr. Med. Sci., Director of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Professor, Department of Neonatology of the N.F. Filatov Clinical Institute of Children's Health, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow,
Trubetskaya str., 8/2, v_zubkov@oparina4.ru, https://orcid.org/0000-0001-8366-5208
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Head of the Department of Neonatology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8/2, d_degtiarev@oparina4.ru,
https://orcid.org/0000-0001-8975-2425
Corresponding author: Evgenia V. Uretskaya, evuretskaya@mail.ru



