Severe pre-eclampsia and fetal growth restriction: longterm projections for mother and offspring
Objective. To determine the impact of severe preeclampsia on peri- and neonatal outcomes, long-term prognosis for mother and offspring. Materials and methods. A retrospective cohort study including 123 women who had preeclampsia during a current pregnancy. 83 women had severe preeclampsia (PE) and 31 women of them had fetal growth restriction (FGR), the other 40 women had mild PE. The study also included their children who was born between 2012 and 2019. The women were followed for 5–7 years and their infants – for the first year of life. Results.The analysis of neonatal complications revealed statistically significant differences between the groups with severe and mild PE: respiratory distress syndrome (RDS) in newborns – 57.8% and 5%; congenital infectious and inflammatory diseases – 60.2% and 10% including congenital pneumonia in 59.03% and 7.5%. respectively; asphyxia – 18.1% and 7.5%; intraventricular hemorrhage –27.7% and 5%; cutaneous hemorrhagic syndrome –31% and 7.5%, respectively (p<0.05). In the first year of life, children from the group of severe PE had the most negative outcomes. Thus, cerebral palsy (CP) (OR=1,7), speech delay (OR=1,5) and neurotic reactions (OR=1,7), visual disorders (OR=3,2) were most common in this group. Conclusion.Women who had severe early preeclampsia during pregnancy had a threefold risk of developing hypertension and a twofold risk of metabolic syndrome compared to the control group 5–7 years after delivery. Morbidity and mortality of children were significantly higher in the group with severe early preeclampsia compared to mild PE.Dolgopolova E.L., Lomova N.A., Karavaeva A.L., Zubkov V.V., Shmakov R.G.
Keywords
Currently, preeclampsia (PE) remains one of the serious complications of pregnancy, affecting 2 to 5% of all pregnancies and can lead to adverse fetal outcomes, both in the short and long term.
In women with a history of PE, the risk of chronic arterial hypertension increases 2–4 times, mortality from cardiovascular complications increases 2 times, and the risk of stroke increases 1.5 times [1]. The fetus is subject to the following antenatal risks: fetal growth restriction (FGR), preterm birth (usually iatrogenic), oligohydramnios, placental abruption, distress syndrome, and intrauterine death [2–4]. It is also believed that prenatal hypertensive disorders in a pregnant woman can lead to significant cardiovascular consequences in the offspring, for example, early hypertension and an increased risk of coronary heart disease and stroke [5]. Early manifestation of PE (before 34 weeks of gestation) occurs only in 5–20%, but it is mostly unfavorable for both the mother and the fetus, always proceeds in a severe form and is accompanied by intrauterine IGR. Suprisingly, women with early PE are more likely to have a family history of PE and a disease may repeate in subsequent pregnancies [6].
The main feature of early-onset PE is pathological development of the placenta. Placentation in PE is usually characterized by abnormal vascular remodeling of the spiral arteries beginning in the first trimester of pregnancy, several weeks before the onset of clinical manifestation. Local hypoperfusion leads to release of various factors, including inflammatory cytokines and anti-angiogenic proteins, increasing the ratio between soluble fms-like tyrosine kinase-1 and pro-angiogenic placental growth factor, which can contribute to the systemic endothelial response, clinically showing with PE and IGR.
Women whose pregnancy is complicated by late onset of PE, usually have only minimal or complete absence of abnormal vascular remodeling of the spiral arteries and there is no restriction of intrauterine growth of the fetus.
At the moment, delivery is the only effective treatment for PE. Therefore, today it is relevant to search for ways to prolong pregnancy and stabilize the condition of both the mother and the fetus. Thus, a promising trend in the treatment of early severe PE is a therapy based on the creation of constant positive airway pressure, which reduces the drug load, and improves the prognosis for the newborn [7]. As a rule, the outcome for children born to mothers with severe PE, especially with an early manifestation, is the most unfavorable. These babies are usually preterm, with an extremely low or low birth weight, require respiratory support and have many complications.
Objective: to determine the effect of severe PE on peri- and neonatal outcomes, long-term prognosis for mother and offspring.
Materials and methods
A retrospective cohort study was conducted between 2012 and 2019 including 123 women who underwent PE during a current pregnancy and their newborn babies, observed in the FSBI «National Medical Research Center for Obsterics, Gynecology and Perinatology named after Academian V.I. Kulakov» of the Ministry of Health of the Russian Federation. Eighty-three pregnant women (group I) were diagnosed with severe PE with IGR, and the remaining 40 pregnant women (group II) were diagnosed with moderate PE.
The anamnestic data of women included in the study, childbirth and newborn histories were analyzed, the condition of children at birth, during hospitalization and their further state of health during follow-up observation during the first year of life were assessed.
The exclusion criteria were: chronic arterial hypertension, diabetes mellitus, impaired renal function, cancer and inflammatory diseases during pregnancy. All mothers were questioned by telephone; as a result, a cohort of 75 women was formed, whose children was observed in the Outpatient Pediatric Department of the Center.
Clinical data on the mother's pregnancy was obtained from archive birth history records; data about the children – from the archive records of the newborns. The assessment of the condition of the newborn was carried out with Apgar scale at 1 and 5 minutes, the assessment of physical development was based on centile tables for full-term and premature infants. Complications of the neonatal period (asphyxia at birth, hemorrhagic complications, neurological and respiratory disorders) were taken into account. According to the developed algorithm, children were examined during the first year of life at the Outpatient Pediatric Department of the Center.
Statistical analysis
The research data was entered into spreadsheets (MSExcel), further processed using the statistical formulae of this program and the STATISTICA software package. Quantitative indicators were assessed for compliance with the normal distribution, for this, the Kolmogorov–Smirnov test was used. Further, the equality of variances in the compared groups was checked using the Leuven test. When both conditions were met, parametric methods of statistical analysis were used. In the case of describing quantitative indicators, the data obtained was combined into a series of variations, in which the arithmetic mean values (M) and standard deviations (SD) were calculated. When comparing the mean values in normally distributed populations of quantitative data, the Student's t-test was calculated. The obtained values of the Student's t-test were assessed by comparison with critical values. Differences in indicators were considered statistically significant at a significance level of p<0.05. With the distribution of signs that differ from normal, the obtained data was described as a median (ME) and quartiles (Q1; Q3). The Mann–Whitney U-test was used to compare independent populations in cases where there were no signs of normal data distribution.
When comparing relative indicators, we used the relative risk indicator (RR), which reflects how many times the risk of an outcome in the presence of a risk factor is higher than the risk of an outcome in the absence of a risk factor. In order to project the obtained RR values onto the general population, we calculated the boundaries of the 95% confidence interval (95% CI). Based on the data obtained, the significance of the relationship between the outcome and the factor was considered proven if the confidence interval was outside the no-effect boundary, accepted as 1.
Results
200 birth histories were analyzed in the period from 2012 to 2019, with the diagnosis of PE established on the basis of the International Classification of Diseases of the 10th revision (ICD-10). After patient selection according to the inclusion and exclusion criteria, 123 women and their newborn babies remained in the study.
The age of patients ranged from 24 to 45 years, with an average of 32.62 (5.9) years; in group I the average age was 32.92 (6.04) years, in group II – 32 (5.49) years. In group I, 24 women (28.9%) had a history of PE in the previous pregnancy, in group II – 15 women (37.5%). 37 pregnant women (44.5%) from group I were primagravida, as well as 11 women from group II (27.5%). In the group with severe PE, arterial hypertension occurred at 28.49 (6.41) weeks of pregnancy, proteinuria joined at 31.23 (4.3) weeks of gestation, and the group with moderate observed PE – at 31.14 (5.4 ) weeks of pregnancy (p = 0.004) and 32.09 (7.39) weeks, respectively (p = 0.6) (Table 1).
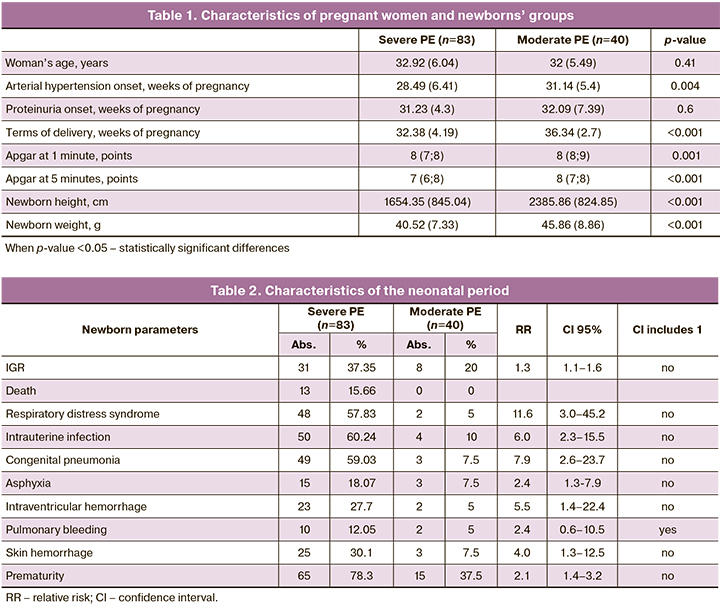
The IGR syndrome was observed more often in the group with severe PE – in 37.35% of cases (31 patients), in 20% (8 patients) – in the group with moderate PE (Table 2). 4 women in the severe PE group developed HELLP syndrome. All women in group I were delivered by caesarean section at a gestational age of 26 to 37 weeks. The terms of delivery in the group with moderate PE were significantly higher – on average 36.34 (2.7) weeks, than in the group with severe PE – 32.38 (4.19) weeks. In the structure of the causes of premature delivery in the group with moderate PE, one of the main was the deterioration of the fetus wellbeing according to functional assessment methods (ultrasound, Doppler, cardiotocography) – in 45% of cases; in the group with severe PE, these were: an increase in the severity of PE in 39%, deterioration of the fetus – in 38%, the lack of effect from the therapy – in 10%, and the development of HELLP syndrome – in 5% of cases.
All children were born alive. In the group of severe PE, 78.3% of children were born prematurely, in the group of moderate PE – 37.5% of premature babies (RR=2.1; CI 1.4–3.2). When analyzing data from newborns, anthropometric indicators were lower in the group with severe PE (1654.35 (845.04) g; 40.52 (7.33) cm) than in the group with moderate PE (2385.86 (824.85) g; 45.86 (8.86) cm), p <0.001. Moreover, in the group with severe PE, 32.5% of children were born with extremely low body weight (<1000 g). The Apgar score was also higher in the group with moderate PE: at 1 minute – 8 (7; 8) points, at 5 minutes – 9 (8; 9) points, in the group with severe PE at 1 minute – 7 (6; 8) points, p=0.001, at the 5th minute – 8 (7; 8) points, p <0.001. Severe birth asphyxia was observed exclusively in newborns in the group with severe PE (n=4).
77.5% of healthy children were born in the group with moderate PE. When analyzing the neonatal period in the group with severe PE, complications more often occurred there: respiratory distress syndrome (RR=11.6; 95% CI 3.0–45.2), congenital infectious and inflammatory diseases (RR=6.0; 95% CI 2.3-15.5), including congenital pneumonia (RR=7.9; CI 2.6-23.7), asphyxia (RR=2.4; 95% CI 1.3-7.9) , intraventricular hemorrhage (RR=5.5; 95% CI 1.4–22.4), hemorrhagic skin syndrome (RR=4.0; 95% CI 1.3–12.5) (Table 2).
Afterwards a prospective analysis of the health status of children of the first year of life in mothers with severe and moderate PE was carried out. In the group with severe PE mortality rate was 13.2% (11 children). Early neonatal mortality occurred in 54.5% (6 children); late neonatal mortality – in 27.3% (3 children) and in 2 cases (18.2%) – infant mortality. The causes of death in the neonatal period were: the development of disseminated intravascular coagulation and multiple organ failure, a severe course of the infectious process, including congenital neonatal sepsis (in 5 cases) against the background of prematurity.
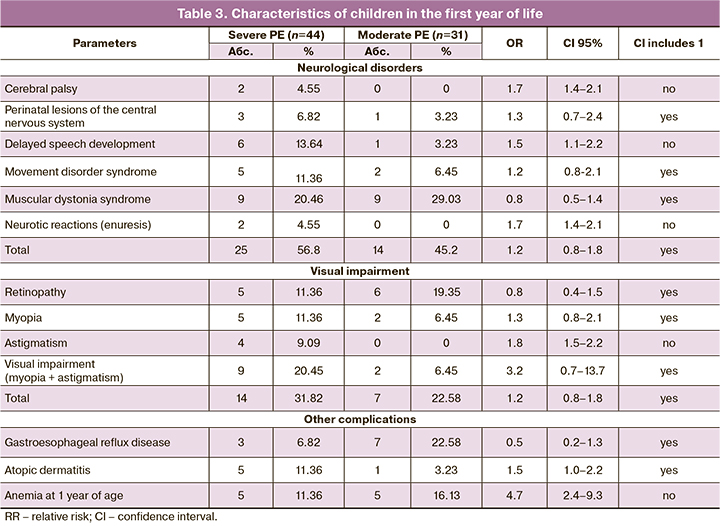
75 out of 123 children were observed at the Outpatient Department of the Center, while the rest were observed at home. Among children observed in Scientific Advisory Pediatric Department of the Center 26 have no deviations in health and developmental disorders – 12 (27.3%) from the group of severe PE and 14 (45.2%) from group II. The rest of the children had complications requiring the supervision of specialized specialists. Thus, 39 children were observed by a neurologist, 21 children – by an ophthalmologist, 10 children – by a gastroenterologist, 6 children – by an allergist. When analyzing individual nosologies among children of the first year of life, observed by a neurologist, there were: infantile cerebral palsy (CP) 1.7 times more often (95% CI 1.4–2.1), delayed speech development 1.5 times more often (95% CI 1.1–2.2) and neurotic reactions 1.7 times more often (95% CI 1.4–2.1) in the group with severe PE than in the group with moderate PE (Table 3). Children with a diagnosis of cerebral palsy have pronounced disorders of motor and speech development, severe spastic paresis and paralysis. Despite the fact that there were no significant differences in the incidence of muscular dystonia syndrome in the study groups, more severe forms of the disease were observed in the group with severe PE. Visual impairment (myopia, myopia in combination with astigmatis, premyopia) requiring correction at the 1st year of life was observed more often in newborns in the group with severe PE (RR=3.2; 95% CI 0.7–13.7). Retinopathy was mainly observed in premature infants: in group I – 1–2 degree of the disease, in group II – only 1 degree or preretinopathy. A gastroenterologist observed children mainly from group II due to the development of functional disorders of the gastrointestinal tract. Infants from group I were more often observed with allergic reactions (Table 3).
Additionally, health state of women after suffering PE was studied. Already 5–7 years after pregnancy complicated by PE, these women had a higher mean systolic and diastolic pressure – 127/86 mm Hg, than in women without this complication of pregnancy – 119/79 mm Hg. PE during pregnancy increased the risk of arterial hypertension 3.5 times (95% CI 2.5–5.2). The RR of metabolic syndrome in women after PE was 2.18 (95% CI 1.3–3.5) compared to women without a history of PE.
Discussion
Despite numerous studies aimed at finding methods for predicting, preventing and treating such a severe complication of pregnancy as PE, its frequency remains unchanged (2–8%); PE has a negative impact on health of the newborn and the further health of women. With the early manifestation of the disease (up to 34 weeks of gestation), the disease is always severe and aggravates the condition of the fetus.
Due to two conflicting interests of mother and child, timing of delivery is one of the major challenges in clinical practice, especially in women with early PE. Women with PE are at risk of developing acute renal or hepatic failure, liver rupture, pulmonary edema, cerebral hemorrhage, disseminated intravascular coagulation, and eclampsia, while the risk of placental abruption, IUU, and intrauterine fetal death is increased compared with women without PE. On the other hand, prolongation of pregnancy for an intrauterine patient due to the consequences of prematurity is an important task, although maternal complications and the risk of decompensation of placental insufficiency may jeopardize this advantage [8]. If PE is diagnosed after 37 weeks of gestation, induction of labor is an established tactic of choice for both mother and newborn [9]. If PE or pregnancy-induced hypertension occurs at 34–37 weeks, anticipated monitoring prior to clinical deterioration is warranted, as emergency delivery significantly increases the risk of respiratory distress syndrome in the newborn, and adverse maternal outcomes have not been clinically proven [8]. Childbirth is indicated, regardless of gestational age, if there are signs of critical PE or eclampsia.
The body weight at birth in children from mothers with early onset of PE is on average 23% lower than normal for a given gestational age [10]. In accordance with this, the fetal mortality rate increases: 5.2 per 1000 fetal deaths versus 3.6 per 1000, compared to uncomplicated pregnancies. In women with early PE, the risk of stillbirth is even seven times higher than in normal pregnancies [11]. So, in our study, mortality was observed only in the group with severe PE and amounted to 13.2% (11 children), of which 5 (45.5%) were diagnosed with sepsis. Early neonatal mortality occurred in 54.5% of cases; late neonatal mortality – in 27.3% of cases and in 18.2% of cases there was infant mortality.
Due to abnormal placentation, the intrauterine environment can permanently change the structure of organs and the function of the body's biofeedback systems and increase the individual's susceptibility to diseases at a later age [12,13]. Recently Pinheiro et al. (2016) reported conflicting results within offsprings with cardiovascular disease after intrauterine exposure to PE in a meta-analysis. Most studies have found that children born to mothers with PE between the ages of 9 and 17 have higher systolic blood pressure, others have higher diastolic blood pressure, and some have both [12]. Kajantie et al. (2009) found an increased risk of hypertension in adults whose mothers suffered from severe PE [14].
The association between PE and asthma in children aged 3 years has not been proven after analysis of comorbid factors, including family history. Eight studies have described the intellectual development of children from mothers who underwent PE and found negative effects on cognitive function throughout life, compared to population data [12, 15]. For example, Van Wassenaer et al. (2011) found that in a prospective cohort of 4–5-year-olds born to mothers with severe PE, the mean IQ was 8 points lower than in the normal population [16]. The discrepancy in long-term outcomes can be explained by a large number of confounding factors, including the severity of PE, birth weight, fetal sex, antenatal therapy (eg, corticosteroids) and neonatal treatment (eg, ventilation, antibiotic use), and genetic aspects.
In our study, we assessed the effect of PE on the health of children in the first year of life. Children born to mothers with severe PE, both at birth and thereafter, suffered from more severe diseases than those with moderate PE. So, when analyzing individual nosologies among children of the first year of life, observed by a neurologist, there were: cerebral palsy – 1.7 times more often, delayed speech development – 1.5 times more often and neurotic reactions – 1.7 times more often in group with severe HE than in the moderate HE group.
The long-term prognosis of children with regard to cardiovascular disease remains an important issue. In 2015, a large 20-year follow-up of children born to women with PE was published. 30% of subjects had high blood pressure by the age of 20. Children born prematurely to mothers with PE have a 3-fold increased risk of developing hypertension by the age of 20 [17].
In addition to direct life-threatening complications during pregnancy, it has become apparent that women are at increased risk of developing cardiovascular disease after PE.
So, in this study, already 5–7 years later, women after pregnancy complicated by PE showed a higher mean systolic and diastolic pressure – 127/86 mm Hg., than women without this complication of pregnancy – 119/79 mm Hg. The transferred PE during pregnancy increased the risk of arterial hypertension by 3.5 times, metabolic syndrome – 2.18 times compared with women without a history of PE.
The Danish register-based cohort study examined 700,000 women with an average follow-up of 14.6 years [18]. After severe PE, there was a 6-fold (range: 5.45– 6.77) increase in arterial hypertension, a 1.7-fold (range: 1.22–2.40) increase in coronary heart disease, 1.9-fold (range : 1.35–2.70) increase in thromboembolism and 4-fold (range: 3.04–4.46) increase in type 2 diabetes. Bellamy et al. (2007), conducted a systematic review with meta-analyzes, examining more than three million women 10–15 years after pregnancy, obtained similar results: women with a history of PE have a 3.7-fold increased risk of hypertension, the risk of coronary heart disease – 2.2 times, the risk of stroke – 1.8 times and the risk of thromboembolism – 1.19 times [19]. Even shortly after pregnancy (two years after delivery), 30% of women who had gestational hypertension or PE had hypertension, and 25% suffered from metabolic syndrome [20]. The severity of PE is associated with the severity of cardiovascular disease later in life. Among women with early PE, 45% had hypertension 3 months to 5 years after pregnancy, compared with 25% with late onset of PE [21]. In women with early PE, the risk of death from cardiovascular diseases increased 9–10 times, while in women with late PE – 2 times [22]. In addition, the risk of cardiovascular diseases increases, as well as the time of the onset of hypertension at a younger age: 7.7 years earlier in women with hypertensive disorder during pregnancy than in women without a history of pregnancy complications [23]. In a longitudinal Canadian cohort with a 25-year follow-up of 1,000,000 women, recurrent PE was significantly associated with cardiovascular outcomes compared with a single PE [24]. Thus, PE that occurs during pregnancy can be considered as a marker of the development of cardiovascular diseases at a relatively young age.
Conclusion
Thus, PE is a common pregnancy-specific disease that has a serious impact on long-term outcome for both women and their children. Women with a history of PE are more prone to cardiovascular disease. This implies the possibility of developing and evaluating prevention programs at a relatively young age, immediately after pregnancy complicated by PE. The morbidity and mortality of children from women with PE depends on the severity and timing of the onset of the disease, the timing of delivery, and the presence of IGR syndrome. Further research is required to understand the possibility of therapy and prolongation of pregnancy to reduce morbidity.
References
- NICE. National Institute for Health and Care Excellence. NICE guideline. Hypertension in pregnancy: diagnosis and management (NG133). 2019. Available at: www.nice.org.uk/guidance/ng133
- Madazli R., Yuksel M.A., Immamoglu M., Tuten A., Oncul M., Aydin B. et al. Comparison of clinical and perinatal outcomes in early- and late-onset preeclampsia. Arch. Gynecol. Obstet. 2014; 290(1): 53-7. https://dx.doi.org/10.1007/s00404-014-3176-x.
- Haddad B., Deis S., Goffinet F., Paniel B.J., Cabrol D., Siba B.M. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am. J. Obstet. Gynecol. 2004; 190(6): 1590-5; discussion 1595-7. https://dx.doi.org/10.1016/j.ajog.2004.03.050.
- Rezk M., Gamal A., Emara M. Maternal and fetal outcome in de novo preeclampsia in comparison to superimposed preeclampsia: a two-year observational study. Hypertens. Pregnancy. 2015; 34(2): 137-44. https://dx.doi.org/10.3109/10641955.2014.982329.
- Davis E.F., Lazdam M., Lewandowski A.J., Worton S.A., Kelly B., Kenworthy Y. et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012; 129(6): e1552-61. https://dx.doi.org/10.1542/peds.2011-3093.
- Hernández-Díaz S., Toh S., Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009; 338: b2255. https://dx.doi.org/10.1136/bmj.b2255.
- Сковородина Т.В., Вишнякова П.А., Цвиркун Д.В., Шмаков Р.Г., Высоких М.Ю., Калачин К.А., Пырегов А.В. Влияние неинвазивной респираторной терапии у беременных с ранней тяжелой преэклампсией на уровни маркеров преэклампсии и митохондриальных DAMPs. Акушерство и гинекология. 2018; 10: 52-8. [Skovorodina T.V., Vishnyakova P.A., Tsvirkun D.V., Shmakov R.G., Vysokikh M.Yu., Kalachin K.A., Pyregov A.V. The impact of non-invasive respiratory therapy in pregnant women with early severe preeclampsia on the levels of preeclampsia markers and mitochondrial DAMPs. Obstetrics and Gynekology. 2018; 10: 52-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.10.52-58
- Broekhuijsen K., van Baaren G.J., van Pampus M.G., Ganzevoort W., Sikkema J.M., Woiski M.D. et al. Immediate delivery versus expectant monitoring for hypertensive disorders of pregnancy between 34 and 37 weeks of gestation (HYPITAT-II): an open-label, randomised controlled trial. Lancet. 2015; 385(9986): 2492-501. https://dx.doi.org/10.1016/S0140-6736(14)61998-X.
- Koopmans C.M., Bijlenda D., Groen H., Mc Vijgen S., Aarnoudse J.G., Bekedam D.J. et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009; 374(9694): 979-88. https://dx.doi.org/10.1016/S0140-6736(09)60736-4.
- Odegard R.A., Vatten L.J., Nilsen S.T., Salvesen K.A., Austgulen R. Preeclampsia and fetal growth. Obstet. Gynecol. 2000; 96(6): 950-5.
- Harmon Q.E., Huang L., Umbach D.M., Klungsøyr K., Engel S.M., Magnus P. et al. Risk of fetal death with preeclampsia. Obstet. Gynecol. 2015; 125(3): 628-35. https://dx.doi.org/ 10.1097/AOG.0000000000000696.
- Pinheiro T.V., Brunetto S., Ramos J.G.L., Bernardi J.R., Goldani M.Z. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J. Dev. Orig. Health Dis. 2016; 7(4): 391-407. https://dx.doi.org/10.1017/S2040174416000209.
- von Ehr J., von Versen-Höynck F. Implications of maternal conditions and pregnancy course on offspring’s medical problems in adult life. Arch. Gynecol. Obstet. 2016; 294(4): 673-9. https://dx.doi.org/10.1007/s00404-016-4178-7.
- Kajantie E., Eriksson J.G., Osmond C., Thornburg K., Barker J.P. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009; 40(4): 1176-80. https://dx.doi.org/ 10.1161/STROKEAHA.108.538025.
- Cheng S.W., Chou H.C., Tsou K.I., Fang L.J., Tsao P.N. Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Hum. Dev. 2004; 76(1): 39-46. https://dx.doi.org/ 10.1016/j.earlhumdev.2003.10.004.
- van Wassenaer A.G., Westera J., van Schie P.E.M., Houtzager B.A., Cranendonk A., de Groot L. et al. Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2011; 204(6): 510. e1-9. https://dx.doi.org/ 10.1016/j.ajog.2011.02.032.
- Davis E.F., Lewandowski A.J., Aye C., Williamson W., Boardman H., Huang R.C. et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015; 5(6): e008136. https://dx.doi.org/10.1136/bmjopen-2015-008136.
- Lykke J.A., Landorf-Roos J., Sibai B.M., Funai E.F., Triche E.W., Paidas M.J. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009; 53(6): 944-51. https://dx.doi.org/10.1161/HYPERTENSIONAHA.109.130765.
- Bellamy L., Casas J.P., Hingorani A.D., Wikkiams D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007; 335(7627): 974. https://dx.doi.org/10.1136/bmj.39335.385301.BE.
- Hermes W., Franx A., van Pampus M.G., Bloemenkamp K.W.M., Bots M.L., van der Post J.A. et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: a cohort study. Am. J. Obstet. Gynecol. 2013; 208(6): 474. e1-8. https://dx.doi.org/10.1016/j.ajog.2013.02.016.
- Veerbeek J.H.W., Hermes W., Breimer A.Y., van Rijn B.B., Koenen S.V., Mol B.W. et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015; 65(3): 600-6. https://dx.doi.org/10.1161/HYPERTENSIONAHA.114.04850.
- Mongraw-Chaffin M.L., Cirillo P.M., Cohn B.A. Preeclamsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010; 56(1): 166-71. https://dx.doi.org/10.1161/HYPERTENSIONAHA.110.150078.
- Heida K.Y., Franx A., van Rijn B.B., Eijkemans M.J.C., Boer J.M.A., Verschuren M.W.M. et al. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. 2015; 66(6): 1116-22. https://dx.doi.org/10.1161/HYPERTENSIONAHA.115.06005.
- Auger N., Fraser W.D., Schnitzer M., Leduc L., Healy-Profitos J., Paradis G. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart. 2017; 103(3): 235-43. https://dx.doi.org/10.1136/heartjnl-2016-309671.
Received 21.10.2020
Accepted 02.12.2020
About the Authors
Elena. L. Dolgopolova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: dolgopolovae93@mail.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.Natalia A. Lomova, PhD, Researcher of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: natasha-lomova@yandex.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna L. Karavaeva, Head clinician of the Neonatal Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: a_karavaeva@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Viktor V. Zubkov, MD, Director of the Institute of Neonatology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: v_zubkov@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Roman G. Shmakov, MD, Professor, Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. E-mail: r_shmakov@oparina4.ru. ORCID: 0000-0002-2206-1002. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Dolgopolova E.L., Lomova N.A., Karavaeva A.L., Zubkov V.V., Shmakov R.G. Severe preeclampsia and fetal growth retardation: long-term projections for mother and offspring.
Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2020; 12: 100-107 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.100-107



