Clinical and anamnestic factors in the prediction and diagnosis of fetal growth restriction
Objective: To develop a model for predicting and diagnosing fetal growth restriction based on clinical and anamnestic factors and examination data during pregnancy.Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Amiraslanov E.Yu., Leonova A.A., Soldatova E.E.
Materials and methods: Postnatally, the weight and growth parameters of 473 newborns were assessed according to the INTERGROWTH-21 centile curves, which made it possible to form a study group that included 202 pregnant women with fetal growth restriction. The comparison group included 206 women without fetal growth restriction who delivered at terms corresponding to the terms in the study group. Risk factors, parameters of somatic and gynecological anamnesis, features of the course of pregnancy and delivery, ultrasound and Doppler data, and a comprehensive assessment of the health status of newborns were analyzed. After statistical processing of the parameters, binary logistic regression was used to develop a mathematical model for predicting fetal growth restriction.
Results: The prognostic model based on binary logistic regression, including somatic and gynecological diseases and medical history, had a sensitivity of 55.6% and specificity of 82.3%. When physical examination data during pregnancy, including fetal abdominal circumference as measured by ultrasound, were added, the model had a sensitivity of 96.7% and a specificity of 76.8%.
Conclusion: The models developed using the binary logistic regression method can be proposed for use in practical healthcare to identify risk groups and predict and diagnose fetal growth retardation, which will allow for timely prevention to reduce the incidence of perinatal complications.
Authors' contributions: Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Amiraslanov E.Yu., Leonova A.A., Soldatova E.E. – conception and design of the study, obtaining data for analysis, review of relevant literature, data analysis, drafting of the manuscript, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Amiraslanov E.Yu.,
Leonova A.A., Soldatova E.E. Clinical and anamnestic factors
in the prediction and diagnosis of fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (9): 82-90 (in Russian)
https://dx.doi.org/10.18565/aig.2023.123
Keywords
The issue of fetal growth restriction remains relevant not only within clinical specialties, such as obstetrics and gynecology, neonatology, perinatology, and pediatrics, but also within the public health structure. Addressing this problem is crucial for preventing complications not only during the early neonatal period but also in a range of chronic diseases [1, 2]. Fetal growth restriction is the primary determinant of perinatal and infant mortality/morbidity as well as the development of chronic diseases in adulthood, including neurological, cardiovascular, metabolic, and other disorders [3–5].
Fetal growth restriction is a pathological condition that occurs when the fetus cannot attain genetically determined growth potential. This syndrome is polyetiological, involving factors from both the pregnant woman and fetus, as well as the placenta. Pathological changes in the placenta are the most common cause of this complication [6–9].
According to global statistics, fetal growth restriction occurs in approximately 10% of all pregnancies and is the second leading cause of perinatal mortality, accounting for approximately 30% of all stillbirths. It is also a significant factor in iatrogenic preterm births and intrauterine fetal hypoxia [2, 10–12]. Notably, the incidence of this pathology varies depending on the level of economic development in different countries, reaching up to 25% in low- and middle-income countries [1, 13, 14]. Despite numerous studies, accurately determining the global incidence of fetal growth restriction remains challenging owing to the ongoing lack of specificity in the prediction and diagnostic methods [15–18]. This challenge is exacerbated in developing countries, where the absence of standardized ultrasound examinations, including precise gestational age determination, hampers the quality of initial genetic screening for fetal growth restriction risk assessment [19–21].
According to Crovetto et al. [22], in developed countries, the prediction of fetal growth restriction varies from 12 to 47%, with a false positive rate of approximately 10%, underscoring the significance of this obstetric issue. Gumenyuk E.G. et al. [23] extensively analyzed the history of studying and identifying predictors of fetal growth restriction. The authors highlighted limitations of both classical methods (such as measuring uterine fundal height and ultrasonic fetometry) and more modern techniques (e.g., assessing fetal growth restriction risk through initial genetic screening). Recently, metabolomic studies utilizing artificial intelligence have shown promise in predicting and diagnosing this complication, according to several authors [4, 24, 25].
In this context, it is crucial to conduct research aimed at identifying significant risk factors and developing and optimizing models for predicting and diagnosing fetal growth restriction. These models should be based on clinical, anamnestic, ultrasound, and Doppler parameters that are universally accepted, straightforward, readily available, and applicable at various levels of obstetric care organizations and routinely used in practice.
The present study aimed to develop a model for predicting and diagnosing fetal growth restriction based on clinical and anamnestic factors as well as examination data during pregnancy.
Materials and methods
This retrospective cohort study included 473 pregnant women diagnosed with fetal growth restriction and placental insufficiency at admission, as well as women with a healthy pregnancy, who were observed and delivered at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. The inclusion criteria were the absence of severe somatic and gynecological pathology in pregnant women aged 18–45 years with a singleton pregnancy, without chromosomal pathology and/or congenital malformations in the fetus, and with an antenatal diagnosis of fetal growth restriction (according to the Delphi criteria). The exclusion criteria were multiple pregnancies, chromosomal pathology, fetal malformations, severe extragenital pathology, and pregnancy resulting from assisted reproductive technologies.
Postnatally, weight and growth parameters were assessed in newborns (n=473) according to the INTERGROWTH-21 centile curves, which made it possible to form a study group that included 202 pregnant women with fetal growth restriction. The comparison group included 206 women without fetal growth restriction who delivered at terms corresponding to the terms in the study group.
The baseline evaluation included a history of somatic and gynecological diseases, features of the course of pregnancy and delivery, ultrasound and Doppler findings, and a comprehensive assessment of the health status of newborns at the stage of hospitalization in neonatal departments. Based on the identified statistically significant risk factors, mathematical models were built to predict fetal growth restriction.
Statistical analysis
Statistical analysis was performed using Statistica 12.6, IBM SPSS Statistics 21. The normality of the distribution was tested using the Shapiro–Wilk test (with less than 50 subjects) or by the Kolmogorov-Smirnov test (with more than 50 subjects).
Quantitative variables showing a normal distribution were expressed as mean (M), standard deviation (SD), and 95% confidence interval (95% CI). Normally distributed continuous variables (with equal variances) were compared between the two groups using Student’s t-test and Mann–Whitney U-test for non-parametric data. Variables that did not meet normality assumptions were reported as median (Me) and interquartile range (Q1; Q3). Pearson's chi-square test for 2×2 contingency tables was performed when the expected frequencies were >10), Fisher's exact test when the expected frequencies were <10), and multi-way contingency tables were analyzed using Pearson's chi-square test. Categorical variables are presented as counts and percentages. The critical level of significance when testing the statistical hypotheses was set at p<0.05. To assess the influence of these factors on the formation of fetal pathology, odds ratios (OR) with 95% confidence intervals (CI) were calculated.
Logistic regression was used to construct a predictive model of the probability of a certain outcome. Nigelkirk's coefficient of determination R2 served as a measure of certainty, indicating that part of the variance could be explained by logistic regression. To assess the diagnostic significance of the signs in predicting a certain outcome, ROC curves were used to determine the area under the curve (AUC). The AUC of 0.9–1.0 indicates excellent quality; AUC of 0.8–0.9 indicates very good quality; AUC of 0.7–0.8 indicates good quality; AUC of 0.6–0.7 indicates average; AUC of 0.5–0.6 indicates unsatisfactory quality. The optimal cut-off value was calculated using the Youden index. The predictive value of the constructed models was characterized by their sensitivity (Se) and specificity (Sp).
Results
The clinical and anamnestic characteristics of patients are shown in Table 1. The study groups were comparable in age.
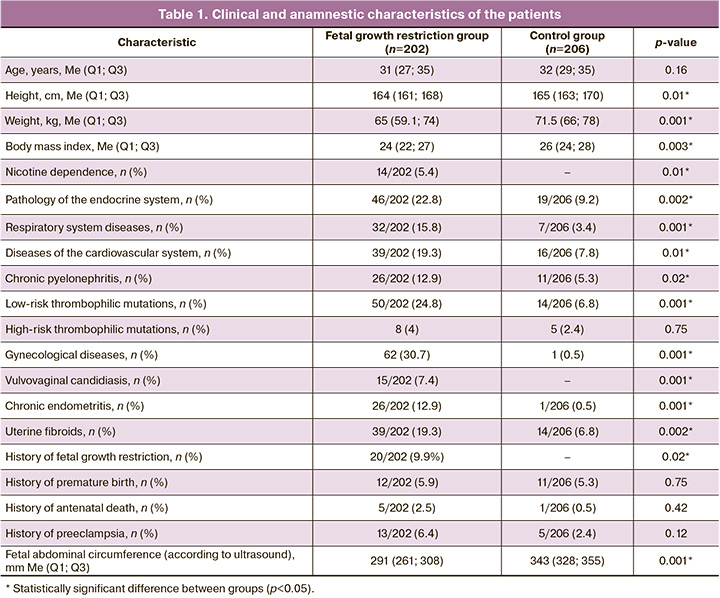
It is noteworthy that pregnant women in the group with fetal growth restriction had a lower body mass index (p=0.003) and were 1.8 times more likely to have a family history of type 2 diabetes mellitus than those in the control group (95% CI:1.04–3.13) (in 59/202 (29.2%) and 38/206 (18.4%) women, respectively).
Considering the influence of chronic nicotine intoxication, alcoholism, and drug addiction as risk factors for the development of fetal growth restriction, an analysis of their frequency of occurrence in the study groups was performed. The women included in the study did not have alcohol or drug addiction. At the same time, chronic nicotine intoxication was statistically significantly more common in the group with fetal growth restriction, 14/202 (5.4%) (p=0.01).
An analysis of comorbidities showed that patients in the study group had a higher incidence of endocrine pathology (2.9 times (95% CI: 1.42–5.79) (46/202 (22.8 %) and 19/206 (9.2%), respectively), respiratory (5.4 times (95% CI: 1.85–15.58) (32/202 (15.8%) and 7/206 ( 3.4%)), cardiovascular systems (by 2.9 times (95% CI: 1.35–6.22) 39/202 (19.3%) and 16/206 (7.8%)) , as well as chronic pyelonephritis (by 2.8 times (95% CI: 1.10–6.95) 26/202 (12.9%) and 11/206 (5.3%), respectively) compared to the comparison group.
Given the importance of hereditary congenital thrombophilias (ICD-10:099.1, D68.2) in the pathogenesis of fetal growth restriction, we analyzed the prevalence of this pathology in the study groups. In the study group, they occurred in every third woman, which was 4.2 times higher than that in the control group (95% CI:2.11–8.39) (61/202 (30.2%) and 19/206 (9.2%), respectively). At the same time, low-risk thrombophilic mutations in the group with fetal growth restriction were detected 7.5 times more often than in the control group (95% CI:2.89–19.37) (50/202 (24.8%) and 14 /206 (6.8%)).
An analysis of gynecological comorbidities showed that compared with controls, pregnant women in the study group were significantly more often diagnosed with gynecological diseases, 62/202 (30.7%) and 1/206 (0.5%) women (95% CI: 7.07–379.39), including vulvovaginal candidiasis,15/202 (7.4%) and 0/206 (0%) (p<0.001), chronic endometritis, 26/202 (12.9%) and 1/206 (0.5 %) (95% CI: 2.31–129.12), and uterine fibroids, 39/202 (19.3%) and 14/206 (6.8%) (95% CI: 1.48–7.31) patients (by groups, respectively).
An analysis of obstetric history showed that every tenth patient in the study group, 20/202 (9.9%) and 0/206 (0%) had a history of the birth of children with fetal growth restriction (p=0.02); antenatal fetal death was five times more common, (5/202 (2.5%) and 1/206 (0.5%) (p=0.42), preeclampsia three times more common, 13/202 (6.4 %) and 5/206 (2.4%) (p=0.12) (by groups, respectively).The history of preterm birth in did not differ between the groups, 12/202 (5.9%) and 11/206 (5 .3%) (p=0.75) (by groups, respectively) (Table 1).
Analysis of the course of pregnancy and childbirth revealed a higher threatened miscarriage rate, 97/202 (48%) and 63/206 (30.6%) (95% CI:1.30–3.39), hypertensive disorders, 57/202 (28.2%), and 18/206 (8.7%) (95% CI:2.07–8.69) with fetal growth restriction (, respectively, by groups). No significant differences were found in the incidence of isthmic-cervical insufficiency, gestational diabetes mellitus, or hypothyroidism (p=0.27, p=0.09, p=0.09).
The median gestational ages at delivery were 264 (247–272) and 274 (269–280) days in the study and control groups, respectively (p<0.001). In the group with fetal growth restriction, very early preterm births occurred five times more often, and early preterm births two times more often; emergency caesarean delivery was 2 times more likely as in the control group, 177/202 (87.6 %) and 103/206 (50%) (p<0.001). The indication for delivery in every second pregnant woman with fetal growth restriction was the deterioration of his condition according to functional diagnostic methods (p<0.0001), in contrast to the comparison group, in which the indications were a uterine scar after a previous cesarean section and/or myomectomy, and in every fifth case, due to the conclusion of related specialists on the exclusion of the straining period (p=0.0003 and p=0.001, respectively). The median duration of pregnancy prolongation from diagnosis to delivery in patients with fetal growth restriction was 34 (range, 15–84) days.
The clinical characteristics of the newborns are shown in Table 2.
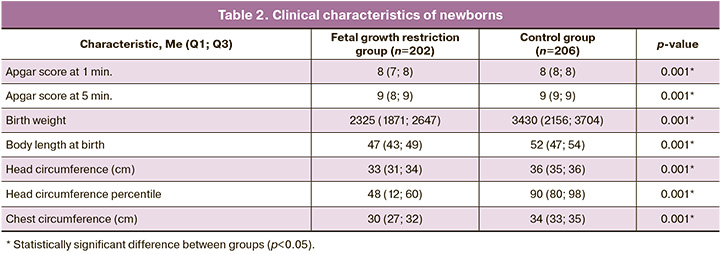
Fetal growth restriction was associated with lower birth weight and body length, smaller head and chest circumferences, and lower Apgar scores at 1 and 5 min (p<0.001).
Of interest in the study was the analysis of the course of the early neonatal period. Newborns in the study group had a statistically significant higher incidence of congenital pneumonia, 62/202 (30.7%) and 30/206 (14.6%) (p=0.0001), respiratory distress syndrome, 53 /202 (26.2%) and 19/206 (9.2%) (p<0.0001), grade 1 intraventricular hemorrhages, 19/202 (9.4%) and 7/206 (3.4%) (p=0.02), DIC, 23/202 (11.4%) and 9/206 (4.4%) (p=0.01), congenital anemia, 25/202 (12.4%) and 7/206 (3.4%) (p=0.002), hyperbilirubinemia of prematurity, 60/202 (29.7%) and 32/206 (15.5%) (p=0.001), neonatal cholestasis, 11/ 202 (5.4%) and 0/206 (0.0%) (p=0.03), gastrointestinal dyskinesia, 28/202 (13.9%) and 12/206 (5.8%) (p=0.01).
According to Verlohren S. et al. [16] and Hoopmann M. et al. [26], the effectiveness of antenatal diagnosis and prediction of fetal growth restriction averages 29.9–32.0%. Sharp A. et al. [20] reported that, despite the routine use of ultrasound during pregnancy, it is possible to detect antenatal from 10 to 36% of newborns with birth weight <10th percentile. At the same time, when conducting audits of perinatal care (ultrasound), inadequate diagnosis of fetal growth restriction is often reported [19, 21, 24, 26, 27].
Given the need to improve the predictive and diagnostic efficiency of fetal growth restriction in the next stage of the study, we developed a prognostic model using binary logistic regression, which included clinical and anamnestic risk factors.
The observed dependence can be described by the following equation:
P=1/(1+e-z),
z=1.068X1+1.139X2+2.562X3+0.935X4+23.203X5-0.043,
where P is the probability of having fetal growth restriction, X1 is chronic pyelonephritis (0 – not present, 1 – present), X2 – low-risk thrombophilic mutations (0 – not present, 1 – present), X3 – chronic endometritis (0 – no, 1 – yes), X4 –uterine fibroids (0 – no, 1 – yes), and X5 – birth of children with a history of fetal growth restriction (0 – no, 1 – yes).
The resulting regression model was statistically significant (p<0.001). Based on the Nigelkirk coefficient of determination, the model explained 24.1% of the observed variance in the presence of fetal growth restriction. This indicator is consistent with the data of Armengaud et al. [28], indicating that fetal growth restriction occurs in 10–15% of all pregnancies.
When assessing the dependence of the probability of developing fetal growth restriction on the value of the logistic function, P, using ROC analysis, the following curve was obtained (Fig. 1).
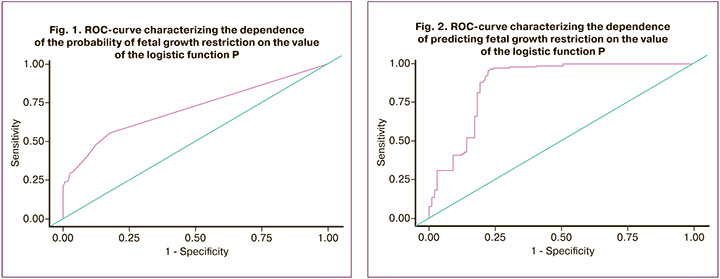
The area under the ROC curve was 0.71 with 95% CI: 0.65–0.77. The resulting model was statistically significant (p<0.001).
The threshold value of the logistic function P at the cutoff point, which corresponded to the highest value of the Youden index, was 0.7. The development of fetal growth restriction was predicted when the value of the logistic function, P, was higher than or equal to this value. The sensitivity and specificity of this model were 55.6 and 82.3% (respectively).
In the next stage of the study, taking into account the need to increase the prediction of fetal growth restriction during pregnancy, a model was created that, in addition to clinical and anamnestic data, included parameters obtained by ultrasound during pregnancy, such as fetal weight (in grams and percentiles), abdominal circumference (mm), and biparietal head size (mm). The model was developed using binary logistic regression. After statistical processing of the parameters, only the ultrasonic indicator "abdominal circumference" was included in the final formula.
The observed dependence is described by the following equation:
P=1/(1+e-z),
z=41.445+0.475X1+0.501X2+3.35X3+0.832X4-0.131X6,
where P is the probability of fetal growth restriction, X1 is chronic pyelonephritis (0 – not present; 1 – present), X2 is low-risk thrombophilic mutations (0 – not present, 1 – present), X3 is chronic endometritis (0 – no, 1 – yes), X4 is uterine fibroids (0 – no, 1 – yes), and X6 is abdominal circumference (mm).
The resulting regression model for predicting and diagnosing fetal growth restriction was statistically significant (p<0.001). Based on the value of the Nigelkirk coefficient of determination, the model explained 80.1% of the observed variance in fetal growth restriction.
When evaluating the dependence of the probability of predicting fetal growth restriction on the value of the logistic function, P, using ROC analysis, the following curve was obtained (Fig. 2).
The area under the ROC curve was 0.87 with 95% CI: 0.83–0.92. The resulting model was statistically significant (p<0.001).
The threshold value of the logistic function P at the cutoff point, which corresponded to the highest value of the Youden index, was 0.5. Fetal growth restriction was diagnosed when the value of the logistic function, P, was higher than or equal to this value. The sensitivity and specificity of this model were 96.7 and 76.8% (respectively).
During the study, a predictive model 1 was obtained with a sensitivity and specificity of 55.6% and 82.3%, respectively. However, to verify fetal growth restriction at different stages of pregnancy, we entered the obtained ultrasound parameters, which made it possible to obtain diagnostic model 2 with high sensitivity and specificity of 96.7 and 76.8%, respectively. It should be noted that abdominal circumference is a fairly routine and easily determined indicator during fetometry, which does not require the use of special ultrasonic formulas for its calculation, which reduces the likelihood of errors during ultrasound. In addition, this parameter is determined everywhere, regardless of the diagnostic sonographer qualification, which will further ensure the routing of pregnant women.
Discussion
Fetal growth restriction is a significant concern for obstetrician-gynecologists and neonatologists worldwide. Its complexity arises from its multifactorial nature, which makes prediction, timely diagnosis, and subsequent treatment challenging. Despite numerous antenatal studies, few have been sufficiently reliable for clinical applications. The diagnosis of fetal growth restriction using routine ultrasound or measurement of uterine fundal height is suboptimal, leading to delayed diagnosis [1, 2, 11, 19].
Notably, Haragan A. et al. [24] acknowledged the limitations of ultrasound in predicting estimated fetal weight. Their study found that ultrasound overestimated the estimated fetal weight in over 75% of newborns, especially those with fetal growth restriction or low birth weight (32% had substantial ultrasound errors, particularly in infants weighing less than 500 g). This error is consistent across different fetal weight estimation formulas, as shown by Hoopmann M. et al. [26].
Currently, various biochemical markers in blood serum, such as pregnancy-associated plasma protein A (PAPP-A), activin, soluble FMS-like tyrosine kinase 1 (sFlt-1), placental growth factor (PlGF), and inhibin A, are proposed as "markers of placental dysfunction." These markers are correlated with adverse pregnancy outcomes and are being incorporated into clinical practice in developed countries. However, their high cost and complexity limit their use in developing nations [16, 29].
An intriguing approach is the use of a fetal growth restriction prediction model combining placental dysfunction markers (sFlt-1/PlGF ratio) with Doppler parameters, such as uterine artery pulsatility index. Studies have shown an improved detection rate of this complication. However, according to Garcia B. et al. [21], universal screening in the second trimester using the uterine artery pulsatility index has sensitivity (60–80%) and specificity (90–95%), but a low positive predictive value (10–20%). Herraiz I. et al. [29] suggested assessing the sFlt-1/PlGF ratio and pulsatility index at the 26th week in high-risk women based on initial screening and risk factors. However, it is unclear whether assessing these markers and the pulsatility index is necessary in low-risk groups or when the index increases during normal pregnancy. Papastefanou I. et al. [18] developed a new competing risk model for predicting stunted and low birth weight fetuses, demonstrating its superiority over traditional methods. However, the use of uterine artery Doppler ultrasound requires specialized training, equipment, and a standardized methodology, posing challenges in both developing and developed countries.
Given these considerations, our study introduced Model 2, which combines clinical and anamnestic risk factors, and ultrasound parameters, specifically abdominal circumference. This model enhances antenatal diagnosis of fetal growth restriction. Notably, it does not rely on invasive markers, such as biochemical parameters (PAPP-A, activin, sFlt-2, PlGF, and inhibin A) and avoids the additional costs and training associated with Doppler-based models. It offers simplicity and the potential for routine clinical use. However, despite its good sensitivity and specificity, further research using advanced approaches, including multi-omics techniques, machine learning, and artificial intelligence, is needed to discover more informative and reliable early markers of fetal growth restriction.
Conclusion
In our study, we developed prognostic model 1 with sensitivity and specificity of 55.6% and 82.3%, respectively. To enhance the verification of fetal growth restriction at various pregnancy stages, we introduced ultrasound parameters, leading to the creation of diagnostic model 2 with high sensitivity (96.7%) and specificity (76.8%). Model 2 explained 80.1% of the observed variance in fetal growth restriction. These binary logistic regression models can be proposed for practical healthcare use to identify at-risk groups and predict and diagnose fetal growth restriction, thereby facilitating timely prevention and reducing perinatal complications.
References
- Melamed N., Baschat A., Yinon Y., Athanasiadis A., Mecacci F., Figueras F. et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021; 152(Suppl. 1): 3-57. https://dx.doi.org/10.1002/ijgo.13522.
- Министерство здравоохранения Российской Федерации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). Клинические рекомендации (протокол лечения). М.; 2022. 71с. [Ministry of Health of the Russian Federation. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Clinical guidelines (treatment protocol). Moscow; 2022. 71p. (in Russian)]. Available at:https://cr.minzdrav.gov.ru/schema/722_1
- Whitehead C.L., McCarthy F.P., Kingdom J. Fetal growth restriction: diagnosis and management. In: Kilby M., Johnson A., Oepkes D., eds. Fetal therapy: Scientific basis and critical appraisal of clinical benefits. 2th ed. Cambridge: Cambridge University Press; 2020: 264-78. https://dx.doi.org/10.1017/9781108564434.025.
- Salmeri N., Carbone I.F., Cavoretto P.I., Farina A., Morano D. Epigenetics beyond fetal growth restriction: a comprehensive overview. Mol. Diagn. Ther. 2022; 26(6): 607-6. https://dx.doi.org/10.1007/s40291-022-00611-4.
- Miller S.L., Huppi P.S., Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016; 594(4): 807-23. https://dx.doi.org/10.1113/JP271402.
- ACOG practice bulletin No. 227: fetal growth restriction. Obstet. Gynecol. 2021; 137(2): 16-28. https://dx.doi.org/10.1097/AOG.0000000000004350.
- Sun C., Groom K.M., Oyston C., Chamley L.W., Clark A.R., James J.L. The placenta in fetal growth restriction: what is going wrong? Placenta. 2020; 96: 10-8. https://dx.doi.org/10.1016/j.placenta.2020.05.003.
- Lackman F., Capewell V., Gagnon R., Richardson B. Fetal umbilical cord oxygen values and birth to placental weight ratio in relation to size at birth. Am. J. Obstet. Gynecol. 2001; 185(3): 674-82. https://dx.doi.org/10.1067/mob.2001.116686.
- Figueras F., Gratacos E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017; 38: 48-58.https://dx.doi.org/10.1016/j.bpobgyn.2016.10.006.
- Nardozza L.M., Caetano A.C., Zamarian A.C., Mazzola J.B., Silva C.P., Marçal V.M. et al. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet. 2017; 295(5): 1061-77. https://dx.doi.org/10.1007/s00404-017-4341-9.
- Suhag A., Berghella V. Intrauterine growth restriction (IUGR): etiology and diagnosis. Curr. Obstet. Gynecol. 2013; 2(2): 102-11.https://dx.doi.org/10.1007/s13669-013-0041-z.
- Damhuis S.E., Ganzevoort W., Gordijn S.J. Abnormal fetal growth: small for gestational age, fetal growth restriction, large for gestational age: definitions and epidemiology. Obstet. Gynecol. Clin. North Am. 2021; 48(2): 267-79.https://dx.doi.org/10.1016/j.ogc.2021.02.002.
- Stacey T., Thompson J.M., Mitchell E.A., Zuccollo J.M., Ekeroma A.J., McCowan L.M. Antenatal care, identification of suboptimal fetal growth and risk of late stillbirth: findings from the Auckland Stillbirth Study. Aust. N. Z. J. Obstet. Gynaecol. 2012; 52(3): 242-7. https://dx.doi.org/10.1111/j.1479-828X.2011.01406.x.
- Froen J.F., Gardosi J.O., Thurmann A., Francis A., Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta Obstet. Gynecol. Scand. 2004; 83(9): 801-7. https://dx.doi.org/10.1111/j.0001-6349.2004.00602.x.
- Ганичкина М.Б., Мантрова Д.А., Кан Н.Е., Тютюнник В.Л., Хачатурян А.А., Зиганшина М.М. Ведение беременности при задержке роста плода. Акушерство и гинекология. 2017; 10: 5-11. [Ganichkina М.B., Mantrova D.A., Kan N.E., Tyutyunnik V.L., Khachaturyan A.A., Ziganshina M.M. Pregnancy management with fetal growth retardation. Obstetrics and Gynecology. 2017; (10): 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.5-11.
- Verlohren S., Herraiz I., Lapaire O., Schlembach D., Zeisler H., Calda P. et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014; 63(2): 346-52. https://dx.doi.org/10.1161/HYPERTENSIONAHA.113.01787.
- Gardosi J., Madurasinghe V., Williams M., Malik A., Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013; 346:108-9.https://dx.doi.org/10.1136/bmj.f108.
- Papastefanou I., Wright D., Syngelaki A., Akolekar R., Nicolaides K.H. Personalized stratification of pregnancy care for small for gestational age neonates from biophysical markers at midgestation. Am. J. Obstet. Gynecol. 2022; 229(1): 57.e1-57.e14. https://dx.doi.org/10.1016/j.ajog.2022.12.318.
- Ярыгина Т.А., Батаева Р.С. Прогнозирование рождения маловесного для гестационного возраста ребенка: оценка эффективности алгоритма Фонда медицины плода (Fetal Medicine Foundation) в первом триместре беременности. Ультразвуковая и функциональная диагностика. 2019; 2: 16-32. [Yarygina T.A., Bataeva R.S. Performance of screening for small-for-gestational age newborn at first trimester using the algorithm proposed by the Fetal Medicine Foundation. Ultrasound and Functional Diagnostics. 2019; (2): 16-32. (in Russian)]. https://dx.doi.org/10.24835/1607-0771-2019-2-16-32.
- Sharp A., Duong C., Agarwal U., Alfirevic Z. Screening and management of the small for gestational age fetus in the UK: A survey of practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018; 231: 220-4. https://dx.doi.org/10.1016/j.ejogrb.2018.10.039.
- García B., Llurba E., Valle L., Gómez-Roig M.D., Juan M., Pérez-Matos C. et al. Do knowledge of uterine artery resistance in the second trimester and targeted surveillance improve maternal and perinatal outcome? UTOPIA study: a randomized controlled trial. Ultrasound Obstet. Gynecol. 2016; 47(6): 680-9. https://dx.doi.org/10.1002/uog.15873.
- Crovetto F., Triunfo S., Crispi F., Rodriguez-Sureda V., Dominguez C., Figueras F., Gratacos E. Differential performance of first-trimester screening in predicting small-for-gestational-age neonate or fetal growth restriction. Ultrasound Obstet. Gynecol. 2017; 49(3): 349-56. https://dx.doi.org/10.1002/uog.15919.
- Гуменюк Е.Г., Ившин А.А., Болдина Ю.С. Поиск предикторов задержки роста плода: от сантиметровой ленты до искусcтвенного интеллекта. Акушерство и гинекология. 2022; 12: 18-24. [Gumenyuk E.G., Ivshin A.A., Boldina Yu.S. Search for predictors of fetal growth retardation: from centimeter tape to artificial intelligence. Obstetrics and Gynecology. 2022; (12): 18-24.(in Russian)]. https://dx.doi.org/10.18565/aig.2022.185.
- Haragan A., Himes K. Accuracy of ultrasound estimated fetal weight in small for gestational age and appropriate for gestational age grown periviable neonates. Am. J. Perinatol. 2018; 35(8): 703-6. https://dx.doi.org/10.1055/s-0037-1617433.
- Bahado-Singh R.O., Yilmaz A., Bisgin H., Turkoglu O., Kumar P., Sherman E. et al. Artificial intelligence and the analysis of multi-platform metabolomics data for the detection of intrauterine growth restriction. PLoS One. 2019; 14(4): e0214121. https://dx.doi.org/10.1371/journal.pone.0214121.
- Hoopmann M., Bernau B., Hart N., Schild R.L., Siemer J. Do specific weight formulas for fetuses < or = 1500 g really improve weight estimation? Ultchall Med. 2010; 31(1): 48-52. https://dx.doi.org/10.1055/s-0028-1109481.
- Ярыгина Т.А., Гус А.И. Задержка (замедление) роста плода: все, что необходимо знать практикующему врачу. Акушерство и гинекология. 2020; 12: 14-24. [Yarygina T.A., Gus A.I. Fetal growth retardation: everything a practitioner needs to know. Obstetrics and Gynecology. 2020; (12): 14-24.(in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.14-24.
- Armengaud J.B., Yzydorczyk C., Siddeek B., Peyter A.C., Simeoni U. Intrauterine growth restriction:clinical consequences on health and disease at adulthood. Reprod. Toxicol. 2021; 99: 168-76. https://dx.doi.org/10.1016/j.reprotox.2020.10.005.
- Herraiz I., Simón E., Gómez-Arriaga P.I., Quezada M.S., García-Burguillo A., López-Jiménez E.A., Galindo A. Clinical implementation of the sFlt-1/PlGF ratio to identify preeclampsia and fetal growth restriction: A prospective cohort study. Pregnancy Hypertens. 2018; 13: 279-85. https://dx.doi.org/10.1016/j.preghy.2018.06.017.
Received 19.05.2023
Accepted 01.09.2023
About the Authors
Maria V. Volochaeva, PhD, Senior Researcher at the Department of Regional Cooperation and Integration, Physician at the 1 Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(919)968-72-98, volochaeva.m@yandex.ru,https://orcid.org/0000-0001-8953-7952, 117997, Russia, Moscow, Ac. Oparina str., 4.
Natalia E. Kan, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900,
Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946, 117997, Russia, Moscow, Ac. Oparina str., 4.
Victor L. Tyutyunnik, Dr. Med. Sci., Professor, Leading Researcher at the Department of Research Administration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015, SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Elrad Yu. Amiraslanov, PhD, Head of the Department of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)549-20-94, e_amiraslanov@oparina4.ru, SPIN-код: 7601-2404, AuthorID: 627756, Scopus Author ID 57009098500,
https://orcid.org/0000-0001-5601-1241, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anastasia A. Leonova, Postgraduate Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(937)453-54-27, nastena27-03@mail.ru, https://orcid.org/0000-0001-6707-3464, 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina E. Soldatova, Postgraduate Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(906)110-51-13, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Maria V. Volochaeva, volochaeva.m@yandex.ru



