Окислительный стресс (ОС) определяют как дисбаланс между продукцией активных форм кислорода (АФК) и азота (АФА) и их утилизацией системой антиоксидантной защиты. Основным источником продукции АФК и АФА в клетке являются митохондрии. Митохондрии играют важную роль в регуляции энергетического метаболизма клетки, поддержании внутриклеточного кальциевого гомеостаза, запуске процессов апоптоза, а также в продукции АФК и АФА, выполняющих сигнальную функцию [1]. Во время беременности функциональная активность митохондрий повышается, так как к функции обеспечения энергией организма матери, добавляется функция обеспечения трансплацентарного переноса от матери к развивающемуся плоду питательных веществ и продуктов метаболизма в обратную сторону. Одновременно с повышением продукции энергии митохондриями во время беременности, повышается продукция АФК и АФА митохондриями, что сопровождается компенсаторным повышением активности антиоксидантной защиты. Такое состояние сбалансированного изменения окислительно-восстановительного статуса организма характерно для физиологического течения беременности и необходимо для нормальной плацентации и дальнейшего формирования плода [2]. Однако митохондриальные дисфункции различной природы приводят к неконтролируемому ОС, повреждающему клетки и ткани, что рассматривается как один из ведущих факторов патогенеза таких осложнений беременности, как преждевременные роды (ПР), преэклампсия (ПЭ) и синдром задержки роста плода (СЗРП) [3–8].
 Митохондрии содержат в своем составе несколько копий собственной митохондриальной ДНК (мтДНК). Хотя количество мтДНК положительно коррелирует с количеством и размером митохондрий, оно также может изменяться не только при смене энергетических потребностей клетки, но и при усилении ОС и под действием других патологических факторов [9, 10]. В отличие от ядерной ДНК – мтДНК более подвержена повреждениям, в том числе в результате повышенной продукции АФК, поскольку имеет кольцевую структуру, не содержит интроны и другие некодирующие последовательности (в ядерной ДНК около 90% последовательности не несет информацию) и не защищена гистонам; кроме того система репарации мтДНК значительно менее эффективна. Накапливающиеся нарушение в структуре мтДНК провоцирует первичную митохондриальную дисфункцию, приводящую к снижению уровня АТФ в клетке и еще большему увеличению продукции АФК и АФА, обуславливая, тем самым, нарушение работы целых тканей и органов [11–17]. В некоторых экспериментальных работах и клинических исследованиях было показано, что изменение содержания мтДНК в крови положительно коррелирует с таковым в других тканях и может в определенной степени отражать процессы, происходящие в других тканях организма [18]. На основании того, что беременность связана с возрастающим ОС даже в случае физиологического ее течения, возникло предположение, что изменение количества копий мтДНК в разных тканях и в крови беременной женщины в том числе может служить биологическим маркером степени индукции ОС и повышения риска возникновения того или иного осложнения гестации. Так, Qiu с соавт. (2012) в ретроспективном исследовании показали, что риск развития ПЭ положительно коррелирует с количеством копий мтДНК в крови матери [19].
Митохондрии содержат в своем составе несколько копий собственной митохондриальной ДНК (мтДНК). Хотя количество мтДНК положительно коррелирует с количеством и размером митохондрий, оно также может изменяться не только при смене энергетических потребностей клетки, но и при усилении ОС и под действием других патологических факторов [9, 10]. В отличие от ядерной ДНК – мтДНК более подвержена повреждениям, в том числе в результате повышенной продукции АФК, поскольку имеет кольцевую структуру, не содержит интроны и другие некодирующие последовательности (в ядерной ДНК около 90% последовательности не несет информацию) и не защищена гистонам; кроме того система репарации мтДНК значительно менее эффективна. Накапливающиеся нарушение в структуре мтДНК провоцирует первичную митохондриальную дисфункцию, приводящую к снижению уровня АТФ в клетке и еще большему увеличению продукции АФК и АФА, обуславливая, тем самым, нарушение работы целых тканей и органов [11–17]. В некоторых экспериментальных работах и клинических исследованиях было показано, что изменение содержания мтДНК в крови положительно коррелирует с таковым в других тканях и может в определенной степени отражать процессы, происходящие в других тканях организма [18]. На основании того, что беременность связана с возрастающим ОС даже в случае физиологического ее течения, возникло предположение, что изменение количества копий мтДНК в разных тканях и в крови беременной женщины в том числе может служить биологическим маркером степени индукции ОС и повышения риска возникновения того или иного осложнения гестации. Так, Qiu с соавт. (2012) в ретроспективном исследовании показали, что риск развития ПЭ положительно коррелирует с количеством копий мтДНК в крови матери [19].
Поскольку вопрос раннего прогнозирования и, как следствие, своевременной коррекции осложнений гестации по-прежнему является очень актуальным в повседневной акушерской практике, более подробное исследование изменений количества мтДНК в крови беременных женщин представляет несомненный интерес.
Целью исследования стало определение числа копий мтДНК в плазме крови женщин при физиологическом и осложненном течении беременности.
Материал и методы исследования
Проанализировано течение беременности и исходы родов у 142 женщин, наблюдавшихся в женской консультации и обследованных в I триместре. В зависимости от результатов анализа характера течения беременности и исхода родов сформировано 4 группы: 1-я – женщины с физиологическим течением беременности (ФБ; отсутствие осложнений беременности, родоразрешение в сроке от 37 до 40 недель гестации); 2-я – женщины с ПР (родоразрешение в сроке от 22 до 37 недель гестации); 3-я – женщины c беременностью, осложненной ПЭ (согласно критериям ВОЗ развитие ПЭ на сроке гестации >20 недель); 4-я – женщины с беременностью, осложненной СЗРП (отставание размеров плода от гестационного срока, масса плода при рождении ниже десятого процентиля для данного срока гестации).

Общие критерии включения в исследование: наблюдение и обследование в I триместре; одноплодная беременность, наступившая самопроизвольно без использования методов вспомогательных репродуктивных технологий (ВРТ); информированное согласие пациентки на вступление в исследование.
Критерии исключения из исследования: отказ пациентки от участия в исследовании или добровольное желание прекратить его; женщины, обратившиеся в женскую консультацию во II или III триместре и не обследованные в I триместре; многоплодная беременность; беременность, наступившая с использованием методов ВРТ; прерывание беременности в сроке до 22 недель или антенатальная гибель плода; тяжелая психосоматическая патология.
Содержание мтДНК измеряли в плазме крови. Общую ДНК, содержащуюся в образце крови, экстрагировали с использованием набора реактивов ПРОБА-НК (ДНК-технологии, Россия), согласно инструкции фирмы-производителя. Количество выделенной ДНК в каждой пробе измеряли на спектрофотометре DS-11 (Novex, США). Количество мтДНК определяли в плазме крови методом полимеразной цепной реакции (ПЦР) в реальном времени. Количество ПЦР-продуктов целевого гена – участка D-петли мтДНК (mtDNA D-loop), нормировали на количество ПЦР-продукта ядерного гена – β2-микроглобулина. В реакцию брали 100 нг общей ДНК, каждую пробу анализировали в трех повторностях. Количество ПЦР-продуктов в реакции оценивали по значению Ct, определяемому как n-й цикл реакции, при котором флюоресценция достигает установленного порогового значения. Значения относительной экспрессии, отражающей копийность мтДНК, выражали в условных единицах 2(-ΔС) [12, 20–22].
Статистическая обработка данных выполнена с использованием пакета прикладных программ SPSS Statistics 22.0. При расчетах использовали Т-критерий для независимых выборок, однофакторный дисперсионный анализ, критерий U Манна–Уитни для независимых выборок. Связь между изучаемыми показателями оценивали по результатам корреляционного анализа с использованием вычисления коэффициента корреляции Пирсона. Для оценки чувствительности и специфичности проводили ROC-анализ. Статистически значимыми считались отличия при р<0,05.
Результаты и обсуждение
По результатам анализа характера течения беременности и ее исхода группу с ФБ составили 105 женщин, с ПР – 14 женщин, с ПЭ – 12 женщин, с СЗРП – 11 женщин.
Возраст беременных в группах варьировал от 17 до 43 лет (средний возраст – 28,4±5,0 года). Средний возраст женщин в группе с ФБ составил 28,6±5,2 года, в группе с ПР – 27,4±4,7 года, в группе с ПЭ – 28,5±5,2 года, в группе с СЗРП – 27,5±4,1 года. Средняя масса тела пациенток в группе с ФБ – 63,4±10,5 кг, в группе с ПР – 69,2±2,8 кг, в группе с ПЭ – 81,2±21,4 кг, в группе с СЗРП – 58,1±5,1 кг.
Результаты определения относительного числа копий мтДНК в крови пациенток в I триместре беременности представлены в таблице и на рис. 1.
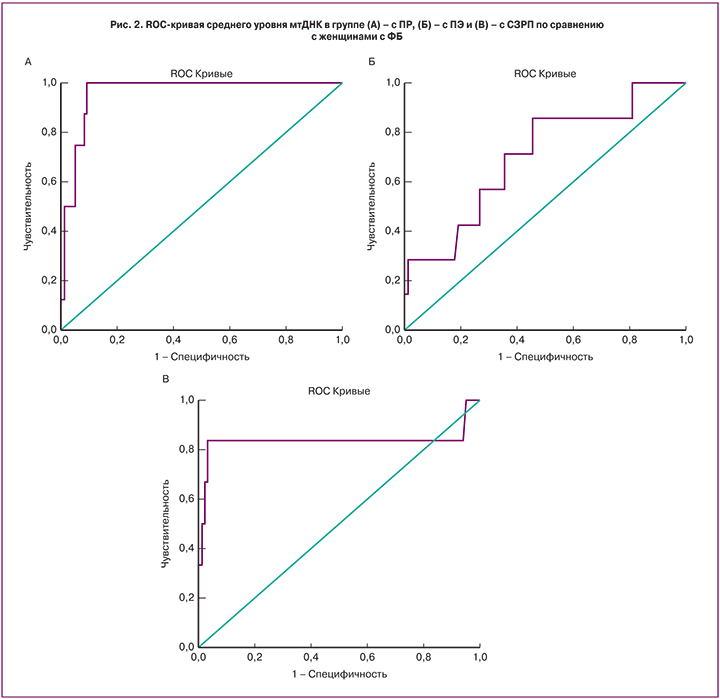
При сравнении групп с ФБ и ПР отмечено значимое увеличение (на 48%) содержания мтДНК в группе с ПР (р<0,01). Результаты ROC-анализа свидетельствуют о том, что увеличение среднего показателя копийности мтДНК 2(-ΔС)≥209 в крови женщин в I триместре беременности свидетельствует о повышенном риске ПР (рис. 2А). Площадь под кривой (AUC) равна 0,961, чувствительность метода составляет 100%, специфичность – 91%.
При сравнении групп ПР и ПЭ также выявлено значимое повышение (на 30%) уровня мтДНК в группе с ПР (р<0,05, рис. 1). При сравнении групп ПР и СЗРП не выявлено статистически значимых различий между группами по количеству мтДНК (р>0,05, рис. 1).
При сравнении группы ПЭ с ФБ обнаружено значимое увеличение уровня мтДНК на 25% (р<0,05). По результатам ROC-анализа пороговым значением повышения риска развития ПЭ является мтДНК 2(-ΔС)≥158. Чувствительность метода – 71%, специфичность – 64%, площадь под кривой равна 0,702 (рис. 2Б). Полученные нами результаты согласуются с работой Qiu с соавт., где они показали, что у беременных женщин с ПЭ уровень мтДНК в цельной крови был выше (средний показатель копийности составил 271,5) по сравнению с группой контроля (средний показатель копийности – 239,3) [19].
Ту же направленность изменений, что и в группах ПР и ПЭ, наблюдали при сравнении групп СЗРП и ФБ – достоверное повышение (на 49%) количества мтДНК в крови в группе СЗРП (р<0,01). По результатам ROC-анализа пороговое значение количества мтДНК – 2(-ΔС)≥221, чувствительность метода – 83%, специфичность – 90%, AUC=0,832 (рис. 2В).
При сравнении групп СЗРП и ПЭ обнаружено, что при СЗРП количество мтДНК в крови было выше на 31% (р<0,05, рис. 1). Схожие данные, повышение мтДНК у беременных с СЗРП также получены в работах Colleoni с соавт. (2010) и Mandò с соавторами (2014) [20, 23]. Не выявлено статистически значимых различий между группами с ПР и СЗРП (р>0,05).
Заключение
Одновременно с повышением эффективности энергопродукции митохондриями, которое в норме происходит при беременности, повышается продукция свободных радикалов, в частности АФК и АФА. Избыточное образование свободных радикалов на фоне недостаточной работы антиоксидантных систем, наблюдаемое при патологическом течении беременности, оказывает повреждающее воздействие на клетки и ткани, в результате чего в кровь может попадать большее количество мтДНК. В нашей работе при сравнении группы с ФБ и групп с осложненной беременностью мы показали, что повышение уровня мтДНК в крови в I триместре положительно коррелирует с развитием осложнений гестации и позволяет выделить женщин с возможным риском развития ПР, ПЭ и СЗРП. Остается не выясненным вопрос, предшествует ли наблюдаемое повышение уровня мтДНК в крови развитию патологии, носит ли оно компенсаторный характер или является следствием протекающих патологических процессов. Однако такое изменение количества копий мтДНК доступно для определения и представляет интерес в качестве возможного маркера развития осложнений гестации, поскольку вопрос раннего выявления и своевременного проведения комплекса профилактических мероприятий остается актуальным в акушерской практике.



