Gam-COVID-Vac (Sputnik V) vaccine has no adverse effect on ovarian reserve in reproductive-age women
Relevance. There is limited evidence on the effect of various vaccines on the human reproductive system. Potential adverse effects of vaccines on fertility are associated with autoimmune disorders, which might cause gonadal damage. Currently, several studies are underway investigating the impact of COVID -19 vaccines on human fertility. To our best knowledge, there is only one study published which demonstrated no adverse effects of the COVID -19 vaccine on assisted reproductive technology outcomes.Dolgushina N.V., Drapkina YU.S., Krechetova L.V., Ivanets T.Yu., Menzhinskaya I.V., Gus A.I., Bayramova G.R., Sukhikh G.T.
Aim. To investigate the effect of the Gam-COVID-Vac (Sputnik V) vaccine on ovarian reserve and antiphospholipid antibody level in reproductive-age women.
Materials and methods. The prospective study included 51 women vaccinated against COVID-19 with Gam-COVID-Vac (Sputnik V) vaccine. The inclusion criteria were age from 18 to 45, preserved menstrual function, normal ovarian reserve, no history of COVID-19, negative PCR test result for SARS-CoV-2 and negative SARS-CoV-2 IgG antibody test before vaccination, no pregnancy, and no history of serious illnesses. Clinical evaluation was carried out twice - immediately before immunization and 90 days after the first vaccine component administration. The antral follicle count was measured by pelvic ultrasound. Serum levels of AMH, FSH, TSH, estradiol, antiphospholipid antibodies (aPL) M and G isotypes against cardiolipin (aCL), β2-glycoprotein-1 (aβ2-GP-1), annexin V (aAn V), phosphatidylserine (aPS), and IgG antibodies against SARS-CoV-2 were measured by enzyme immunoassay.
Results. There were no significant changes in hormones levels and antral follicle counts before and after vaccination, including in women of advanced reproductive age (≥37 years). After immunization, aPL antibody levels did not differ significantly from the baseline. There was no correlation between aPL antibody level dynamics and level of FSH and AMH, which indirectly demonstrates no possible autoimmune effect of vaccination on women's fertility.
Conclusion. This is the first study investigating the effect of the Gam-COVID-Vac vaccine on ovarian reserve parameters and aPL antibody levels. The preliminary results prove that the Gam-COVID-Vac vaccine in women of reproductive age does not adversely impact ovarian reserve.
Keywords
Currently, vaccination against COVID -19 is carried out globally. Vaccination rates reach high numbers in some countries, for example, it is 73.8% in Malta. The percentage of the vaccinated population in Russia was 11.98% as of July 2, 2021 ( https: // yandex.ru/covid19/stat ).
Despite the worldwide vaccination there is little data on the impact of different types of vaccines on human reproduction [1–4]. The possible adverse effect of vaccines on reproductive organs and tissues is associated with autoimmune disorders which might have gonadotoxic effect and lead to endocrine organs failure and antiphospholipid syndrome (APS). Also, rheumatic disorders can cause secondary infertility within ASIA syndrome. For example, systemic lupus erythematosus (SLE) in women leads to autoimmune oophoritis, and as a result, premature ovarian failure; in men it causes spermatogenesis disorders. APS is diagnosed in more than a third of women with SLE. The menstrual cycle is often irregular in women with autoimmune thyroiditis, and they are in high risk for infertility and miscarriage [5–6]. Theoretically vaccination can initiate APS. For example, IgM antibodies produced after tetanus toxoid vaccination interact with both the toxin and cardiolipin connected with β2-glycoprotein-1 (β2-GP-1). In addition, it was shown that tetanus toxoid vaccination together with adjuvants (aluminum hydroxide or glycerol) develops APS in mice without any autoimmune disorders before vaccination [7]. In another study it was demonstrated that mice immunization with a subunit cytomegalovirus peptide vaccine also leads to the similar syndrome [8]. A possible homology between tetanus toxoid or other antigens used for vaccination and human peptides, as well as the use of adjuvants, may be sufficient to induce APS and cause reproductive failure [9].
To date, there are a few incomplete studies on the effect of COVID-19 vaccination on reproductive function (www. clinicaltrials.gov). A recently published study has not shown any negative effect of vaccination on spermatogenesis in men [10]. Similar data was obtained by Russian researchers [11]. When it comes to vaccination effect on female fertility, there is only one research published, where no negative impact of vaccination on assisted reproduction results was found [12]. Due to the lack of knowledge, the study of COVID-19 vaccine effect on the female reproductive function is extremely relevant. Due to the fact that among all Russian vaccines, only Gam-COVID-Vac (Sputnik V) has passed all the stages of clinical trials, it was chosen for studying the effect of vaccination on ovarian reserve in women [13].
The aim of the study was to evaluate the effect of Gam-COVID-Vac (Sputnik V) vaccine on ovarian reserve and the level of antiphospholipid antibodies (aPL) in women of reproductive age.
Materials and methods
A prospective study included 51 women who were vaccinated against COVID-19 with Gam-COVID-Vac (Sputnik V). The criteria for inclusion in the study were age from 18 to 45 years, preserved menstrual function, normal ovarian reserve, the absence of a previous history of COVID-19, a negative RT-PCR result in SARS-CoV-2 testing, negative SARS-CoV-2 IgG results prior to vaccination. Non-inclusion criteria were contraindications to vaccination according to the instructions, pregnancy and breastfeeding, oncological and rheumatic disorders of any localization, autoimmune diseases, tuberculosis, chronic systemic infection, severe allergic reactions in history, hormone therapy and any vaccination carried out 30 days before inclusion in the study, treatment with immunoglobulins and immunomodulatory drugs 3 months before inclusion in the study. The exclusion criteria were COVID-19 during the vaccination period, a severe undesirable reaction resulting in the cancelled 2nd dose of the vaccine, and the patient’s refusal to continue vaccination.
The patients were examined twice: immediately before vaccination and 90 days after the first dose was injected. Gynecologic examination was performed, blood samples were taken on the 2nd –5th day of the menstrual cycle. The number of antral follicles was determined during the ultrasound examination of the pelvic organs on the 5th –7th day of the menstrual cycle.
The concentration of follicle-stimulating hormone (FSH), anti-Müllerian hormone (AMH) and estradiol level was determined in blood serum with an electrochemiluminescent method on an automatic immunochemical analyzer “Cobas e411” (Roche Diagnostics GmbH, Germany) using commercial kits from this manufacturer. The concentration of thyroid-stimulating hormone (TSH) was determined in blood serum using an immunochemiluminescent method on an automatic immunochemical analyzer “IMMULITE® 2000” (Siemens, USA). The analysis of blood for IgM and IgG antiphospholipid antibodies (aPL) to cardiolipin (aCL), β2-glycoprotein-1 (aß2-GP1), annexin V (aAnV), phosphatidylserine (aPS) was carried out with enzyme immunoassay using “ORGENTEC Diagnostika” kits (Germany). IgG antibodies to SARS-CoV-2 in blood serum were determined with enzyme immunoassay using the test system “A set of reagents for detecting IgG specific to SARS-CoV-2 spike using enzyme immunoassay” (RPC “Diagnostic Systems”, Russia). The results were assessed using spectrophotometer “Infinite F50” (TECAN, USA).
Ovarian reserve in women was assessed using AMH, FSH and antral follicle counts (AFC) parameters in blood serum. Ovarian reserve was considered normal when the level of AMH ≥ 1.2 ng/mL, the level of FSH <12 IU/L and AFC ≥ 5 follicles in both ovaries [14].
Statistical analysis
The statistical software package Statistica 10 (USA) was used for statistical analysis. The data were represented as absolute values and % for categorical data, medians (with an interquartile interval) for quantitative data. The data were compared using pairwise testing methods, namely, paired Wilcoxon test for related samples due to the abnormal distribution of data for all values. Normal distribution of the data was evaluated using the Shapiro–Wilk criterion. The correlation was analyzed using Spearman’s criterion. The differences between the statistical values were considered statistically significant at р˂0.05.
The study was approved by the Local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Results
The average age of the women was 31.0 (26.0–36.0) years, the body mass index (BMI) was 22.7 (19.4–25.5) kg/m2 . The assessment of clinical and anamnestic data revealed that the prevalence of gynecological disorders in the participants of the study was low: endometriosis was noted in 4 women (7.8%), uterine fibroids – in 4 patients (7.8%), endometrial polyps – in 2 women (3.9%), polycystic ovary syndrome (PCOS) – in 2 women (3.9%), precancerous condition of the cervix – in 7 women (12.9%), chronic tonsillitis – in 12 patients (23.5%), chronic gastritis – in 12 women (23.5%), chronic cystitis – in 3 women (5.9%), pollinosis – in 4 patients (7.8%).
The vaccine was generally well tolerated by the patients, there were no serious adverse events associated with the injection of the vaccine. The reaction to the vaccine was more frequently observed after injecting the 2nd dose and was short-term (1–2 days). Local reaction at the injection site was observed in 34 women (66.7%), subfebrile temperature was noted in 39 patients (76.5%), febrile temperature was observed in 6 patients (11.7%), intoxication symptoms such as headache and/or myalgia were observed in 23 cases (45.1%), and 3 women (5.9%) did not report any reactions to the vaccine.
SARS-CoV-2 IgG antibodies were detected in almost all vaccinated patients, only in one patient (1.9%) their level did not reach the threshold value for positivity.
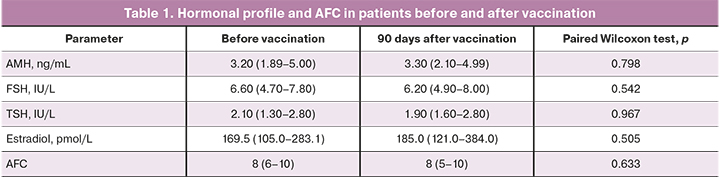
The comparison of the average parameters of hormonal profile and AFC before and after vaccination did not reveal significant differences in hormone level and AFC (Table 1).
The comparison of the dynamics of AMH, FSH level and AFC in patients of late reproductive age (LRA) (≥ 37 years, n=11) ones in patients of early reproductive age (ERA) (< 37 years, n=40) did not reveal any significant differences. The delta of AMH level in LRA group was 0.10 (-0.24–0.50) ng/ml, in ERA group it was 0.07 (-0.19–0.55) (p>0.05). The delta of FSH level in LRA patients was (-0.30) (-1.80–0.90) IU/L and in ERA group (-0.15) (-0.77–1.20) IU/ml (p>0.05).
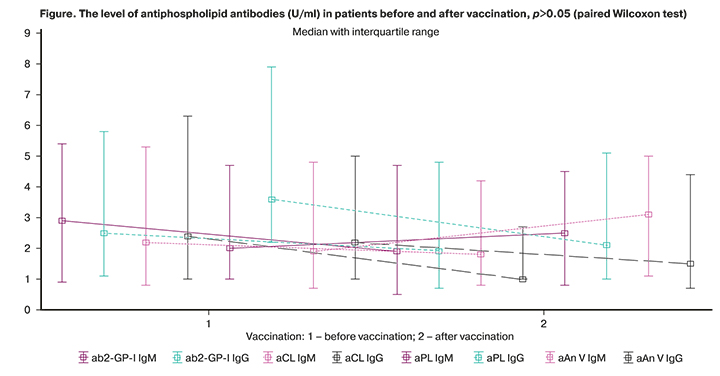
The level of aPL was subsequently analyzed before and after vaccination (Figure 1). There was a slight decrease in the level of aβ2-GP-1 IgM, aCL IgG, aPL IgG and aAnV IgG and an increase in aAnV IgM after vaccination compared to the initial values. The level of aPL did not exceed the reference values almost in all patients. The increase in the level of aPL antibodies above the reference values was observed only in 3 (5.9%) women.
There was a correlation analysis of the dependence of changes in the level of AMH, FSH and AFC. The results of this analysis did not reveal any significant correlations (p>0.05).
The vaccine has no effect on ovarian function in women, but there is evidence of a negative effect of COVID-19 on fertility. The analysis revealed a wide representation of receptors for SARS-CoV-2 (angiotensin-converting enzyme (ACE2), transmembrane serine protease 2 (TMPRSS2), CD147 (basigin) in human reproductive organs and tissues [15–19]. The study carried out by Chinese scientists with multivariate hierarchical linear modeling showed that COVID-19 was significantly associated with a decrease in AMH levels (β = -0.191; 95% CI: -1.177–0.327; p=0.001), which proves a negative effect of COVID-9 on fertility [20].
Conclusion
This is the first study devoted to the effect of the Gam-COVID-Vac vaccine on the ovarian reserve and aPL level in women. All patients included in the study initially had a normal ovarian reserve and did not experience any severe somatic diseases. Factors that could influence the excessive autoimmune response after vaccination were also excluded from the study.
According to the preliminary data of the study, Gam-COVID-Vac does not have a negative effect on the ovarian reserve in women, including patients of late reproductive age. In addition, there is no increase in the level of aPL antibodies; no connection was found between the level of aPL antibodies and level of FSH and AMH, which may indirectly indicate the absence of possible autoimmune effect of vaccination on fertility. The research will continue and further study will include 200 women.
References
- Wacholder S., Chen B.E., Wilcox A., Macones G., Gonzalez P., Befano B. et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ. 2010; 340: c712. https://dx.doi.org/10.1136/bmj.c712.
- Panagiotou O.A., Befano B.L., Gonzalez P., Rodríguez A.C., Herrero R., Schiller J.T. et al. Effect of bivalent human papillomavirus vaccination on pregnancy outcomes: long term observational follow-up in the Costa Rica HPV Vaccine Trial. BMJ. 2015; 351: h4358. https://dx.doi.org/10.1136/bmj.h4358.
- Wiesen A.R., Littell C.T. Relationship between prepregnancy anthrax vaccination and pregnancy and birth outcomes among US army women. JAMA. 2002; 287(12): 1556-60. https://dx.doi.org/10.1001/jama.287.12.1556.
- Catherino W.H., Levi A., Kao T.C., Leondires M.P., McKeeby J., Segars J.H. Anthrax vaccine does not affect semen parameters, embryo quality, or pregnancy outcome in couples with a vaccinated male military service member. Fertil. Steril. 2005; 83(2): 480-3. https://dx.doi.org/10.1016/j.fertnstert.2004.07.965.
- Carp H.J.A., Selmi C., Shoenfeld Y. The autoimmune bases of infertility and pregnancy loss. J. Autoimmun. 2012; 38(2-3): J266-74. https://dx.doi.org/10.1016/j.jaut.2011.11.016.
- Перминова С.Г. Бесплодие у женщин с аутоиммунной патологией щитовидной железы. Медицинский совет. 2012; 7: 40-4. [Perminova S.G. Infertility in women with autoimmune thyroid pathology. Medical Council. 2012; 7: 40-4. (in Russian)].
- Zivkovic I., Stojanovic M., Petrusic V., Inic-Kanada A., Dimitrijevic L. Induction of APS after TTd hyper-immunization has a different outcome in BALB/c and C57BL/6 mice. Am. J. Reprod. Immunol. 2011; 65(5): 492-502. https://dx.doi.org/10.1111/j.1600-0897.2010.00922.x.
- Gharavi A.E., Pierangeli S.S., Espinola R.G., Liu X., Colden-Stanfield M., Harris E.N. Antiphospholipid antibodies induced in mice by immunization with a cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002; 46(2): 545-52. https://dx.doi.org/10.1002/art.10130.
- Cruz-Tapias P., Blank M., Anaya J.M., Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr. Opin. Rheumatol. 2012; 24(4): 389-93. https://dx.doi.org/10.1097/BOR.0b013e32835448b8.
- Safrai M., Reubinoff B., Ben-Meir A. BNT162b2 mRNA Covid-19 vaccine does not impair sperm parameters. medRxiv preprint. May, 2021. https://dx.doi.org/10.1101/2021.04.30.21255690.
- Елагин В.В., Адамян Л.В., Вечорко В.И., Дорошенко Д.А., Дашко А.А., Филиппов О.С., Степанян А.А., Медведева И.В. Вакцинация против COVID-19 и репродуктивное здоровье мужчин (предварительные данные). Проблемы репродукции. 2021; 27(4). [Elagin V.V., Adamyan L.V., Vechorko V.I., Doroshenko D.A. et al. Vaccination against COVID-19 and reproductive health of men (preliminary data). Russian Journal of Human Reproduction. 2021; 27(4). (in Russin)]. DOI 10.17116/repro2021270410.
- Orvieto R., Noach-Hirsh M., Segev-Zahav A., Haas J., Nahum R., Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod. Biol. Endocrinol. 2021; 19(1): 69. https://dx.doi.org/10.1186/s12958-021-00757-6.
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397(10275): 671-81. https://dx.doi.org/10.1016/S0140-6736(21)00234-8.
- Клинические рекомендации. Женское бесплодие. М.: Российское общество акушеров-гинекологов, Российская ассоциация репродукции человека; 2021. 81с. [Clinical recommendations "Female infertility." 2021. 81 p. (in Russian)].
- Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020; 9(1): 45. https://dx.doi.org/10.1186/s40249-020-00662-x.
- Jing Y., Run-Qian L., Hao-Ran W., Hao-Ran C., Ya-Bin L., Yang G. et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020; 26(6): 367-73. https://dx.doi.org/10.1093/molehr/gaaa030.
- Smedts A.M., Lele S.M., Modesitt S.C., Curry T.E. Expression of an extracellular matrix metalloproteinase inducer (basigin) in the human ovary and ovarian endometriosis. Fertil. Steril. 2006; 86(3): 535-42. https://dx.doi.org/10.1016/j.fertnstert.2006.01.042.
- Li K., Nowak R.A. The role of basigin in reproduction. Reproduction. 2019; REP-19-0268.R1. https://dx.doi.org/10.1530/REP-19-0268.
- Долгушин Г.О., Романов А.Ю. Влияние SARS-COV-2 репродукцию человека. Акушерство и гинекология. 2020; 11: 6-12. [Dolgushin G.O., Romanov A.Yu. Effects of SARS-CoV-2 on human reproduction. Obstetrics and Gynecology. 2020; 11: 6-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.6-12.
- Ding T., Wang T., Zhang J., Cui P., Chen Z., Zhou S. et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: An Observational Study. Front. Med. 2021; 8: 635255. https://dx.doi.org/10.3389/fmed.2021.635255.
Received 16.07.2021
Accepted 20.07.2021
About the Authors
Nataliya V. Dolgushina, Dr. Med. Sci., Deputy Director – Head of the Department of Research Administration, V.I. Kulakov NMRC for OG&P, Ministry of Healthcareof the Russian Federation. Tel.: +7(495)438-49-77. E-mail: n_dolgushina@oparina4.ru 4 Oparin str., 117997, Moscow, Russia.
Yulia S. Drapkina, Ph.D., Obstetrition-Gynecologist at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation. E-mail: julia.drapkina@gmail.com. 4 Oparin str., 117997, Moscow, Russia.
Lyubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation.
Tel.: +7(495)438-11-83. E-mail: k_l_v_@mail.ru. 4 Oparin str., 117997, Moscow, Russia.
Tatiana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation.
Tel.: +7(495)438-25-66. E-mail: t_ivanets@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Irina V. Menzhinskaya, Dr. Med. Sci., Senior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation. Tel.: +7(915)345-06-59. E-mail: i_menzinskaya@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Alexander I. Gus, Dr. Med. Sci., Professor, Head of the Department Ultrasound Diagnostic Imaging, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation. Tel.: +7(985)231-97-44. E-mail: a_gus@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Gyuldana R. Bayramova, Dr. Med. Sci., Clinical Care Supervisor at the Polyclinic Department, V.I. Kulakov NMRC for OG&P, Ministry of Healthcare
of the Russian Federation. Tel.: +7(909)994-77-00. E-mail: g_bairamova@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of the RAS, Director of the V.I. Kulakov NMRC for OG&P, Ministry of Healthcare of the Russian Federation.
Tel.: +7(495)438-18-00. E-mail: secretariat@oparina4.ru. 4 Oparin str., 117997, Moscow, Russia.
For citation: Dolgushina N.V., Drapkina Yu.S., Krechetova L.V., Ivanets T.Yu., Menzhinskaya I.V., Gus A.I., Bairamova G.R., Sukhikh G.T. Gam-COVID-Vac (Sputnik V) vaccine has no adverse effect on the ovarian reserve in reproductive-age women.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2021; 7: 81-86 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.81-86



