Serum 25-hydroxycalciferol levels in cholecalciferol prophylaxis during breastfeeding
The high prevalence of vitamin D deficiency in pregnant women and their newborns causes a number of complications that can be prevented. Nowadays, the use of prophylactic doses of vitamin D during pregnancy and lactation remains debatable and requires further study.Novikova T.V., Zazerskaya I.E., Kuznetsova L.V., Vasilyeva M.Yu., K.A. Rudenko K.A.
Objective: To evaluate the saturation of the body of a woman and a newborn who was breastfed during vitamin D prophylaxis administered to mother.
Materials and methods: The study included 110 women who recently gave birth and 100 newborns. Biological samples were collected for subsequent identification and retrospective assessment of serum 25-hydroxycalciferol levels. During breastfeeding, women of group I (n=54) received 400 IU cholecalciferol, women of group II (n=56) received 1400 IU cholecalciferol. The children were exclusively breastfed and received 500 IU cholecalciferol additionally. The evaluation of 25(OH)D level was carried out using a chemiluminescent method after childbirth and after 6 months of breastfeeding.
Results: Vitamin D deficiency and insufficiency were detected in 98/110 (89%) women who recently gave birth: deficiency was identified in 43/110 (39%) women, insufficiency was detected in 56/110 (50.9) women. Vitamin D deficiency was detected in 78/100 (78%) newborns and insufficiency was revealed in 22/100 (22%) newborns. Umbilical blood cord concentration of 25(OH)D corresponded to 67% of the content of 25(OH)D in the mother’s blood serum. After the use of vitamin D at a dose of 400 IU, deficiency and insufficiency persisted in 43/46 (93.5%) women; when vitamin D was administered at a dose of 1400 IU, deficiency and insufficiency were detected in 28/48 (58.3%) women. Deficiency and insufficiency persisted in all children in the study regardless of the prophylactic dose of cholecalciferol taken by mother.
Conclusion: Umbilical blood cord concentration of 25(OH)D is 1.5 times lower than one in the maternal serum. Prophylactic doses of cholecalciferol are insufficient in common vitamin D deficiency. It is recommended to determine 25(OH)D level when planning pregnancy and during lactation.
Keywords
Vitamin D deficiency and insufficiency are a significant worldwide public health problem. Vitamin D deficiency and insufficiency in pregnant women and women who recently gave birth reach 70–82.2% in St. Petersburg [1, 2]. Among children, vitamin D deficiency occurs in 35% under the age of 6 months, in 20% under the age of one year, in 45% in the second year of life and in 62% in the third year [1]; among newborns, vitamin D deficiency and insufficiency range from 73% to 94%, however, due to the lack of resources and for the purpose of financial efficiency, the level of vitamin D is usually not identified in newborns. Severe vitamin D deficiency in the mother contributes to the development of deficiency in her newborn and can cause hypocalcemia which can subsequently lead to seizures and cardiomyopathy, cause the development of rickets, growth retardation, atopic manifestations and the appearance of eczema [2, 3]. Vitamin D deficiency in children of the first year of life is associated with an increased risk of developing respiratory diseases, asthma, diabetes mellitus and many other conditions associated with vitamin D deficiency in the future [3–5]. It is certainly necessary to study the relationship between the serum 25-hydroxycalciferol levels in mother and child; the correlation should be initially determined in the umbilical cord blood in order to prevent timely development of vitamin D deficiency in newborns. The issue of the use of prophylactic doses of cholecalciferol during breastfeeding remains relevant as well. There are a lot of studies in the Russian Federation devoted to the use of prophylactic doses of cholecalciferol during pregnancy, but there are practically no such studies on the use of cholecalciferol during breastfeeding which is of the greatest interest for further research. According to a number of foreign guidelines and some domestic ones, the recommended preventive dose remains 400–500 IU of cholecalciferol; however, there are no studies in St. Petersburg on the effectiveness of this dose during breastfeeding, and therefore our study seems to be relevant.
The aim of the study was to evaluate the saturation of the body of a woman and a newborn who was breastfed during vitamin D prophylaxis administered to mother.
Materials and methods
A total of 140 women who recently gave birth were examined on the 3rd–5th day after delivery; 110 out of 140 women as well as 100 of their newborns were included in the study. All women were the residents of St. Petersburg, Russia. The criteria for inclusion in the study were puerperas on the 3rd–5th day after birth, age from 20 to 35 years, signed informed consent, desire for prolonged breastfeeding, taking cholecalciferol in prophylactic doses. There were the following exclusion criteria from the study: diseases of the endocrine system, kidney diseases, diseases of the gastrointestinal tract, oncological diseases, skin diseases, rheumatic diseases, taking medications that affect the absorption of vitamin D and bone metabolism, bronchial asthma with the use of systemic glucocorticoid therapy, refusal of breastfeed. During pregnancy, all women received a multivitamin complex containing at least 200 IU of cholecalciferol.
Biological samples of maternal blood and umbilical cord blood were collected after childbirth in a 10 ml vacuum tube. The samples of venous blood were collected in Vacutainer tubes according to the standard procedure and delivered to the laboratory within 30 minutes after sampling. The serum was centrifuged for 10 minutes at 1000 rpm, and the samples were stored in a freezer at -86°C until the time of analysis. The evaluation of 25(OH)D level was carried out using a chemiluminescent method with kits and calibrators from Roche Diagnostics (Germany) for the Architect 2000 analyzer (USA). Serum 25-hydroxycalciferol levels were evaluated retrospectively. In order to assess the level of vitamin D, a classification was used. According to this classification, the patients were divided into three normative gradation classes on the basis of the concentration level of 25 (OH)D in serum: vitamin D deficiency corresponds to level 25(OH)D below 20 ng/ml (50 nmol/L), vitamin D insufficiency refers to 25 (OH)D within the range of 21–30 ng/ml (51–74 nmol/L), the normal level corresponds to 25 (OH)D ≥ 30 ng/ml [1, 6].
During breastfeeding, all women were prescribed prophylactic doses of cholecalciferol: group I (n=54) received 400 IU of vitamin D and 1000 mg of calcium (in tablets), group II (n=56) received 1400 IU of vitamin D and 1000 mg of calcium (in tablets and additionally 1000 IU of oil-based vitamin D); the biological samples of woman’s blood (n=94) and the child’s blood (n=60) were collected again after 6 months. The children were exclusively breastfed and received 500 IU of vitamin D additionally on the recommendation of a pediatrician. The study was conducted at the Almazov National Medical Research Center, at the maternity and obstetric departments. Laboratory studies were performed at the Central Clinical and Diagnostic Laboratory headed by Elena Vasilyeva.
Statistical analysis
The results of the study were statistically processed using STATISTICA 10 En software package (StatSoft Inc., USA) at Biostatistics Research Laboratory, Almazov National Medical Research Center. The normality of the distribution of sample concentration values 25(OH)D and their logarithms were evaluated using Kolmogorov–Smirnov and Lilliefors tests. Standard methods of descriptive statistics (mean value (M (SD)) were used to describe quantitative variables. Pearson correlation coefficient r was used to analyze correlations. The main characteristics of the related samples were compared using the Wilcoxon criterion. The significance level of 0.05 was accepted for all statistical tests.
Results
The average age of the women who recently gave birth was 32.8 (3.2) years. According to their obstetric and gynecologic history, the average age of menarche was 11.3 (2.1) years, menstrual cycle disorders, such as oligomenorrhea, occurred in 7/110 (6.4%) women, miscarriages were revealed in 5/110 (4.3%) women who recently gave birth. The current pregnancy occurred spontaneously in all the examined patients. There were 44/110 (40%) primiparas and 73/110 (66%) multiparas. BMI >25 kg/m2 was noted in 37/110 (34%) women. Low calcium intake from food sources was revealed in 75/110 (82%) women. Among pregnancy complications, there was gestational diabetes mellitus in 55/110 (50%) women, autoimmune thyroiditis in 37/110 (34%) women, preeclampsia in 33/110 (30%) women. Pregnancy ended in spontaneous vaginal delivery in 83/110 (75%) women, cesarean section was performed in 28/110 (25%) cases due to developing fetal hypoxia. All newborns were in satisfactory condition and stayed with their mothers.
Vitamin D deficiency and insufficiency were detected in 98/110 (89%) women who recently gave birth (n=110): 43/110 (39%) of them had deficiency, 56/110 (50.9%) had insufficiency. The average value of 25(OH)D after childbirth was 15.8 (2.8) ng/ml and corresponded to vitamin D deficiency.
The assessment of 25(OH)D level in the umbilical cord blood of a newborn (n=100) was carried out. Vitamin D deficiency was revealed in 78/100 (78%) newborns and vitamin D insufficiency was detected in 22/100 (22%) newborns. The average value of 25(OH)D was 12.2 (2.07) ng/ml and corresponded to vitamin D deficiency. Wilcoxon’s criterion for related samples confirmed the significance of the differences between 25(OH)D level in umbilical cord blood and maternal serum on the 3rd-5th day after birth (p<0.0001).
Since the distribution of the values of both these indicators was logarithmically normal (Kolmogorov–Smirnov test), their relationship becomes clinically significant. The diagram in Figure 1 shows that the concentration of 25(OH)D in umbilical cord blood was lower by more than 1.5 times (above the dotted line) than in maternal serum (less than 67% content of 25(OH)D in the woman’s blood serum) in almost all cases.
The dynamics of 25(OH)D level in women and newborns was analyzed during breastfeeding when the patients received the prophylactic doses of cholecalciferol (Fig. 2). After receiving vitamin D 400 IU, there was an increase in women to 4.98 (4.64) ng/ml and an average value of 25 (OH)D corresponded to vitamin D insufficiency, the limits of the normal indicators were detected in 3/46 (6.5%) patients, which suggests that the dose administered during lactation was not enough (Table). When vitamin D was prescribed at a dose of 1400 IU, an increase in 25 (OH)D in women was 12.81 (4.64) ng/ml (p<0.001, Wilcoxon test) and the normal level of 32.3 (1.2) ng/ml was revealed in 20/48 (41.7%) patients; vitamin D insufficiency was observed in 22/48 (45.8%) women (Table).
After estimating the concentration values of 25(OH)D level in blood, we calculated the number of women who reached the target concentration of 25(OH)D (≥30 ng/ml) after 6 months (Fig. 2; Table).
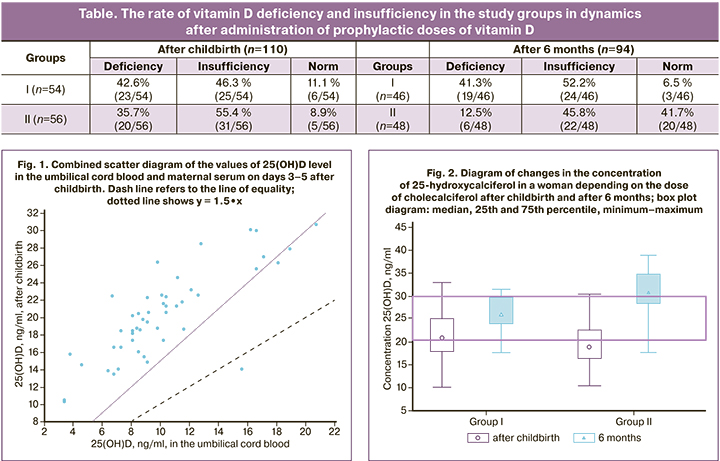
After evaluating the content of 25(OH)D level in the blood of newborns (n=60) after 6 months, there was no statistically significant increase in 25(OH)D level depending on the dose of vitamin D taken by the mother during breastfeeding (Fig. 3). The average values of 25(OH)D level in all newborns corresponded to vitamin D deficiency – 15.36 (4.67) ng/ml; after taking vitamin D at a dose of 1400 IU, the maximum concentrations in the blood serum of newborns also corresponded to vitamin D deficiency – 17.2 (1.6) ng/ml, vitamin D deficiency [25 (OH)D<10 ng/ml] was registered in 16/100 (16%) children. In addition to breastfeeding, the children received supplements of vitamin D 500 IU per day.
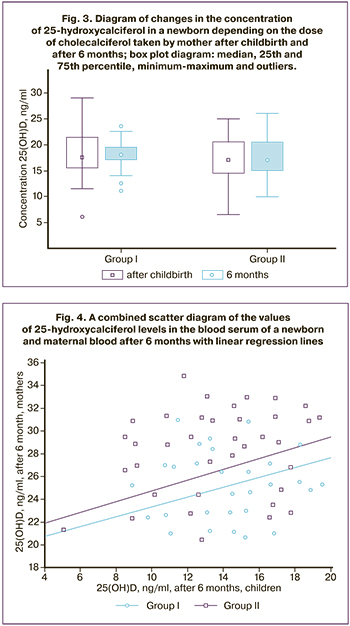
This was an analysis of the relationship between the level of 25-hydroxycalciferol in the blood serum of a newborn and maternal blood after 6 months. For group I: r=0.543, p=0.001, 95% confidence interval (CI) for r: CI=[0.256; 0.742]; for group II: r=0.400, p=0.030, 95% CI=[0.074; 0.645]; linear regression equations, respectively, y = 0.43x + 19.00 and y = 0.47x + 20.02 (Fig. 4).
Wilcoxon test for longitudinal comparison showed no differences in both cases (p=0.26; p=0.10).
This study showed that prophylactic doses (400 IU and 1400 IU) of vitamin D taken by mother during 6 months of breastfeeding did not lead to a change in the level of 25-hydroxycalciferol in the blood serum of children.
Therefore, a dose of 400 IU of vitamin D taken during breastfeeding is not effective for the prevention of vitamin D deficiency and insufficiency for a woman and a child. A dose of 1400 IU of vitamin D taken by mother during 6 months of breastfeeding makes it possible to compensate for the initial vitamin D deficiency in a woman, but does not affect the content of 25 (OH)D in a child.
Discussion
Normal pregnancy and breastfeeding are accompanied by changes in vitamin D metabolism and phosphorus-calcium metabolism. Transplacental calcium transfer during pregnancy has been proved to be 50 mg/day at the beginning of the first trimester and it reaches 330 mg/day in the third trimester. During breastfeeding, the average daily calcium secretion into breast milk is about 210-400 mg. Some women who breastfeed twins can experience daily calcium loss of up to 1000 mg per day which significantly exceeds the demand for calcium during pregnancy [1]. There are several adaptive mechanisms to the growing load on mineral metabolism with increasing gestational age. These mechanisms include, first of all, increased absorption of calcium in the intestine due to the synthesis of calbindin, decreased excretion of calcium by the kidneys, synthesis of active metabolites of vitamin D by placental tissue [6]. The maternal kidney, placenta and embryonic kidneys provide the necessary activity of 1α-hydroxylase which converts inactive metabolites of vitamin D into the active form. Thus, the concentration of 1.25(OH)2D3 increases already in the first trimester of pregnancy and the concentration continues to grow and becomes several times higher than one before pregnancy [5, 6]. During breastfeeding, there is no additional source of synthesis of the active metabolite of vitamin D. There is an increase in a number of biologically active and hormonal substances that promote bone resorption and thus maintain calcium homeostasis, which confirms the need for exogenous calcium and vitamin D supplementation during pregnancy and breastfeeding. Given nearly total prevalence of vitamin D deficiency and insufficiency in pregnant women and puerperas, newborns remain at risk for the development of vitamin D deficiency. The provision of the fetus and newborn with vitamin D has a linear correlation with the maternal vitamin D content. Concentration of 25(OH)D in the umbilical cord blood of a child composes 50-80% of the level of 25 (OH)D in the maternal blood, regardless of the gestation period [6]. The characteristics of vitamin D metabolism in fetuses, newborns and premature infants have not been sufficiently studied. Vitamin D passes through the placenta by passive or facilitated transport and mainly in the form of 25(OH)D; then it is metabolized into 1.25(OH)2D3 in the fetal kidneys [7]. This ability was noted already at 8 weeks gestation. The level of 1.25(OH)2D3 increases significantly in a woman during pregnancy. This is due to an increase in the synthesis of 1.25(OH)2D3 in the maternal kidneys, as well as the appearance of extrarenal synthesis of this metabolite in the trophoblast, decidual tissue and placenta. The content of 1.25(OH)2D3 in the fetus and in the umbilical cord blood is lower than in adults and significantly lower than in maternal blood. In newborns, the synthesis of 1.25(OH)2D3 is activated during the first day of life in response to a decrease in calcium concentration and an increase in parathyroid hormone in the blood.
According to foreign studies, the concentration of vitamin D in newborns ranges from 50% to 70% of the concentration in their mothers [8]. Infants born to mothers with vitamin D deficiency may have delayed growth and large fontanelles, craniotabes and wrist ossification centers [9, 10].
Despite the fact that vitamin D deficiency is completely preventable, multicenter studies demonstrate a high prevalence of vitamin D deficiency in many European and Asian countries [11–17]. Vitamin D deficiency and insufficiency occur in 80-97% of maternity centers in St. Petersburg, in spite of administration of a prophylactic 500 IU dose of vitamin D during pregnancy. At the same time, 100% of newborns have vitamin D deficiency or insufficiency [14, 15]. Vitamin D deficiency significantly leads to an increase in the number of premature births [18, 19], it accompanies the development of preeclampsia [20, 21], gestational diabetes [21–24] and bacterial infections [24, 25]. According to the results of a systematic review and meta-analysis [21–25], as well as a meta-analysis of 10 studies [25], randomized controlled trials and 31 observational studies [24], vitamin D deficiency (25(OH)D<20 ng/ml) is significantly associated with the risk of developing preeclampsia [20, 22].
Maternal vitamin D deficiency is an important risk factor for vitamin D deficiency in newborns and children. Congenital rickets which is quite rare in industrialized countries occurs in case of severe maternal vitamin D deficiency during pregnancy. Therefore, screening for vitamin D deficiency should be a routine part of the preconception examination of pregnant women and in case of detecting a deficiency, it should be compensated in a timely manner by additional vitamin D intake.
Vitamin D deficiency is a public health problem [18, 19]. It is necessary to pay special attention to vitamin D supplements during pregnancy and lactation in order to avoid congenital and nutritional rickets in early infancy [19, 20, 22, 26, 27]. The current recommendations for the use of prophylactic doses of cholecalciferol indicate prophylactic doses of 400 and 500 IU, the latest guidelines indicate a dose of cholecalciferol of 1200 IU which was studied in the research. The dose of cholecalciferol 400 IU was proved ineffective for a woman during lactation and for a child due to the lack of influence on the level of 25-hydroxycalciferol [26, 27]. It is possible to administer cholecalciferol at a dose of 1400 IU in case of initial vitamin D deficiency, however, this dose has no effect on the content of 25-hydroxycalciferol in a child either. Due to the widespread deficiency and insufficiency of vitamin D in St. Petersburg, it is recommended to determine the level of 25-hydroxycalciferol to all women from risk groups in order to compensate for its deficiency and insufficiency in a timely manner and prescribe therapeutic doses. If it is not possible to determine the level of 25-hydroxycalciferol, the minimum preventive dose should be at least 1400 IU of cholecalciferol and additional cholecalciferol supplements should be prescribed to children, according to modern recommendations of pediatricians [19].
Conclusion
Umbilical blood cord concentration of 25(OH)D is 1.5 times lower than one in the maternal serum.
Prophylactic doses of cholecalciferol are insufficient in common vitamin D deficiency in pregnant women and newborns.
It is recommended to determine 25-hydroxycalciferol level when planning pregnancy, during lactation and in the presence of risk factors for vitamin D deficiency.
References
1. Новикова Т.В., Хазова Е.Л., Кузнецова Л.В., Кустаров В.Н. Взаимосвязь между уровнем 25-гидроксикальциферола в пуповинной крови и крови матери. Лабораторная служба. 2021; 10(2): 18-21. [Novikova T.V., Khazova E.L., Kuznetsova L.V., Kustarov V.N. The relationship between the level of 25-hydroxycalciferol in umbilical cord blood and maternal blood. Laboratory service. 2021; 10(2): 18-21 (in Russian)].
2. Хазова Е.Л., Зазерская И.Е. Эпидемиология дефицита и недостаточности витамина D. Сезонные колебания насыщенности организма витамином D у беременных Санкт-Петербурга. Витамин D и репродуктивное здоровье женщины. 2017: 31-43. [Khazova E.L., Zazerskaya I.E. Epidemiology of vitamin D deficiency and insufficiency. Seasonal fluctuations in vitamin D saturation in pregnant women in St. Petersburg. Vitamin D and reproductive health of women. 2017: 31-43 (in Russian)].
3. Захарова И.Н., Боровик Т.Э., Вахлова И.В., Горелов А.В., Гуменюк О.И., Гусев Е.И. и др. Национальная программа «Недостаточность витамина D у детей и подростков Российской Федерации: современные подходы к коррекции. М.: Изд-во Педиатръ; 2018. 96 c. [Zakharova I.N., Borovik T.E., Vakhlova I.V., Gorelov A.V., Gumenyuk O.I., Gusev E.I. et al. National program «Vitamin D deficiency in children and adolescents of the Russian Federation: modern approaches to correction». Moscow; 2018. 96 p. (in Russian)].
4. Dawodu A., Salameh K.M., Al-Janahi N.S., Bener A., Elkum N. The Effect of High-Dose Postpartum Maternal Vitamin D Supplementation Alone Compared with Maternal Plus Infant Vitamin D Supplementation in Breastfeeding Infants in a High-Risk Population. A Randomized Controlled Trial. Nutrients. 2019; 11(7): 1632. https:/doi.org/10.3390/nu11071632.
5. Amegah A.K., Klevor M.K., Wagner C.L. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta-analysis of longitudinal studies. PLoS One. 2017; 12(3): e0173605. https:/doi.org/10.1371/journal.pone.0173605.
6. Palacios C., Kostiuk L.K., Pena-Rosas J. Cochrane Database of Systematic Reviews. Vitamin D supplementation for women during pregnancy. 26 July 2019. https:/doi.org/10.1002/14651858.CD008873.pub4.
7. Общественная организация «Российская ассоциация эндокринологов». Клинические рекомендации «Дефицит витамина D». 2021. [Public organization "Russian Association of Endocrinologists". Clinical Recommendations "Vitamin D Deficiency". 2021. (in Russian)].
8. Karlsson C., Obrant K.J., Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2002; 12(10): 828-34. https:/doi.org/10.1007/s001980170033.
9. Feldman A.G., Sokol R.J. Neonatal Cholestasis. Neoreviews. 2013; 14(2): 10.1542/ neo.14-2-e63. https:/doi.org/10.1542/neo.14-2-e63.
10. Van der Pligt P., Willcox J., Szymlek-Gay E.A., Murray E., Worsley A., Daly R.M. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients. 2018; 10(5): 640. https:/doi.org/10.3390/nu10050640.
11. Pludowski P., Holick M.F., Grant W.B., Konstantynowicz J., Mascarenhas M.R. et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018; 175: 125-35. https://doi.org/10.1016/j.jsbmb.2017.01.021.
12. Kuhn J., Trotz P., Stangl G.I. Prevalence of vitamin D insufficiency and evidence for disease prevention in the older population. Z Gerontol Geriatr. 2018; 51(5): 567-72. https:/doi.org/10.1007/s00391-018-1390-z.
13. Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017; 18(2): 153-65. https:/doi.org/10.1007/s11154-017-9424-1.
14. Institute of Medicine (US); Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross A.C., Taylor C.L., Yaktine A.L., Del Valle H.B., eds. Dietary Reference Intakes for Calcium and Vitamin D. 2011. https:/doi.org/10.17226/13050.
15. Хазова Е.Л., Новикова Т.В., Беляева Е.Н., Шелепова Е.С., Кустаров В.Н. Взаимосвязь уровней кальцидиола в крови матери в третьем триместре беременности и новорожденного. Эффективная фармакотерапия. 2020; 16 (22): 10-3. [Khazova E.L., Novikova T.V., Belyaeva E.N., Shelepova E.S., Kustarov V.N. Relationship of Calcium Levels in Series Materials in the Third Trimester of Pregnancy and the Newborn. Effective Pharmacotherapy. 2020; 16 (22): 10-3. (in Russian)]. https:/doi.org/10.33978/ 2307-3586-2020-16-22-10-13.
16. Sofi N.Y., Jain M., Kapil U., Seenu V., Lakshmy R. et al. Reproductive factors, nutritional status and serum 25(OH) D levels in women with breast cancer: A case control study. J Steroid Biochem Mol Biol. 2018; 175: 200-4. https:/doi.org/10.1016/j.jsbmb.2017.11.003.
17. Kovacs C.S. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011; 40(4): 795-826. https:/doi.org/10.1016/j.ecl.2011.08.002.
18. Новикова Т.В., Зазерская И.Е., Кузнецова Л.В., Шелепова Е.С., Хазова Е.Л. Витамин D и показатели минерального обмена после родов при применении профилактических доз холекальциферола. Журнал акушерства и женских болезней. 2019; 68(5): 45-53. [Novikova T.V., Zazerskaya I.E., Kuznetsova L.V., Shelepova E.S., Khazova E.L. Vitamin D and mineral metabolism after childbirth with the use of preventive doses of cholecalciferol. Journal of Obstetrics and Women's Diseases. 2019; 68(5): 45-53. (in Russian)].
19. Баранов И.И., Дорофейков В.В., Зазерская И.Е., Заплатников А.Л., Захарова И.Н. и др. Междисциплинарное руководство по профилактике и лечению дефицита витамина D в прегравидарном периоде, во время беременности и после родов. Санкт-Петербург; 2020. [Baranov I.I., Dorofeikov V.V., Zazerskaya I.E., Zaplatnikov A.L., Zakharova I.N. et al. Interdisciplinary guidelines for the prevention and treatment of vitamin D deficiency in the preconception period, during pregnancy and after childbirth. St. Petersburg; 2020 (in Russian)].
20. Малявская С.И., Карамян В.Г., Кострова Г.Н., Лебедев А.В. Обеспеченность витамином D рожениц и новорожденных в диаде «мать-дитя» в условиях приарктической зоны РФ в зимний период. Акушерство и гинекология. 2018; 3: 58-62. [Malyavskaya S.I., Karamyan V.G., Kostrova G.N., Lebedev A.V. The provision of vitamin D in parturients and newborn infants in the mother-child dyad under the conditions of the Subarctic Zone of the Russian federation in the winter season. Obstetrics and Gynecology. 2018; 3: 58-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.3.58-62.
21. Мальцева Л.И., Васильева Э.Н., Денисова Т.Г. Значение дефицита витамина D для развития тяжелых форм преэклампсии у женщин группы высокого риска. Акушерство и гинекология. 2018; 9: 120-5. [Maltseva L.I., Vasilyeva E.N., Denisova T.G. Implication of vitamin D deficiency in the development of severe forms of preeclampsia in women at high risk. Obstetrics and Gynecology. 2018; 9: 120-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.9.120-125.
22. Сергунина О.А., Балушкина А.А., Кан Н.Е., Тютюнник В.Л. Препараты кальция в профилактике осложнений беременности. Акушерство и гинекология. 2015; 1: 111-5. [Sergunina O.A., Balushkina A.A., Kan N.E., Tyutyunnik V.L. Calcium supplements in the prevention of pregnancy complications. Obstetrics and Gynecology. 2015; 1: 111-5. (in Russian)].
23. Kiely M.E, Wagner C.L, Roth D.E. Vitamin D in pregnancy: Where we are and where we should go. J Steroid Biochem Mol Biol. 2020; 201: 105669. https:/doi.org/10.1016/j.jsbmb.2020.105669.
24. Tous M., Villalobos M., Iglesias L., Fernandez-Barres S., Arija V. Vitamin D status during pregnancy and offspring outcomes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2020; 74(1): 36-53. https:/doi.org/10.1038/s41430-018-0373-x.
25. Tan M.L., Abrams S.A., Osborn D.A. Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health. Cochrane Database Syst Rev. 2020; 12(12): CD013046. https:/doi.org/10.1002/ 14651858.CD013046.pub2.
26. Miyamoto T., Miyakoshi K., Sato Y., Kasuga Y., Ikenoue S. et al. Changes in bone metabolic profile associated with pregnancy or lactation. Sci Rep. 2019; 9(1): 6787. https://doi.org/10.1038/s41598-019-43049-1.
27. Trivedi M., Faridi M.M.A., Aggarwal A., Madhu S.V., Malhotra R.K. Oral Vitamin D Supplementation to Mothers During Lactation-Effect of 25(OH)D Concentration on Exclusively Breastfed Infants at 6 Months ofAge: A Randomized Double-Blind Placebo-Controlled Trial. Breastfeed Med. 2020; 15(4): 237-45. https:/doi.org/10.1089/bfm.2019.0102.
Received 22.02.2022
Accepted 21.03.2022
About the Authors
Tatiana V. Novikova, PhD, Assistant of the Department of Obstetrics and Gynecology, Institute of Medical Education, V.A. Almazov Scientific Research Center,Ministry of Health of Russia, tanyanovikova.85@mail.ru, https://orcid.org/0000-0001-8758-6857, eLibrary SPIN: 7143-2088, 197341, Russia, St. Petersburg, Akkuratov str., 2.
Irina E. Zazerskaya, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology, V.A. Almazov Scientific Research Center, Ministry of Health of Russia, +7(921)948-83-40, zazera@mail.ru, 197341, Russia, St. Petersburg, Akkuratov str., 2.
Lyubov V. Kuznetsova, PhD, Head of Research Laboratory of Reproduction and Women's Health, Institute of Perinatology and Pediatrics, V.A. Almazov Scientific Research Center, Ministry of Health of Russia, https://orcid.org/0000-0002-8175-7886, eLibrary SPIN: 5873-2280, 197341, Russia, St. Petersburg, Akkuratov str., 2.
Margarita Yu. Vasilyeva, PhD student of the Department of Obstetrics and Gynecology, Institute of Medical Education, V.A. Almazov Scientific Research Center, Ministry of Health of Russia; obstetrician-gynecologist of the Obstetrical Department of the Perinatal Center, V.A. Almazov Scientific Research Center, eLibrary SPIN: 8495-1800, 197341, Russia, St. Petersburg, Akkuratov str., 2.
Ksenia A. Rudenko, Clinical Resident, Senior Laboratory Assistant, Department of Obstetrics and Gynecology, V.A. Almazov Scientific Research Center, Ministry of Health of Russia, xeniaruru@yandex.ru, ResearcherID: ABC-1438-2021, eLibrary SPIN: 3534-4785, 197341, Russia, St. Petersburg, Akkuratov str., 2.
Corresponding author: Tatiana V. Novikova, +7(921)849-68-79, tanyanovikova.85@mail.ru
Authors’ contributions: Novikova T.V - collection of clinical material after childbirth, statistical processing of the results; Vasilyeva M.Yu. - presentation of the material, summary; Kuznetsova L.V. - description of techniques; Rudenko K.A. - formation of a list of references.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study has been performed within the state scientific research No. 056-00109-21-02.
Ethical Approval: The Research Ethics Committee of the V. A. Almazov NMRC approved the clinical trial as part of the dissertation titled "Bone metabolic status after childbirth and during lactation” (Code 14.01.01 - Obstetrics and Gynecology, dated June 22, 2021).
Acknowledgement: The authors express their gratitude to Viktor A. Bart, PhD, Leading Researcher, Almazov National Medical Research Center, and Elena Yu. Vasilyeva, Head of the Central Clinical Diagnostic Laboratory, Almazov National Medical Research Center.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: T.V. Novikova, I.E. Zazerskaya, L.V. Kuznetsova, M.Yu. Vasilyeva, K.A. Rudenko. Serum 25-hydroxycalciferol levels in cholecalciferol prophylaxis during breastfeeding. Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2022; 3: 97-103 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.97-103



