Pregnancy course and outcomes in women with familial Mediterranean fever
Aim. To study pregnancy course and outcomes in patients with familial Mediterranean fever (FMF). Materials and methods. An observational cohort study compared pregnancy outcomes in 251 women with FMF and in 312 women without FMF. A group of women (4577) of reproductive age (18–49 years) with confirmed diagnosis of FMF was sequentially selected among 32,000 persons of Armenian nationality, who were examined for the presence of mutations in MEFV gene and their clinical, laboratory and genetic test results were included in the information register for the period 1998–2018 in the Center for Medical Genetics and Primary Health Care, Yerevan, Armenia. The main group included 211 patients with FMF. Inclusion criteria were: termination of pregnancy or abortion, obstetric complications, perinatal outcomes. The results were processed using the SPSS Statistics 16.0 software package. To study a possible relationship between a certain outcome and risk factor in the compared groups, the data were processed using the GraphPad Prism 4 and GraphPad Prism 5 software packages. Relative risk (RR) with 95% confidence interval (CI) was calculated. Results. Pregnancy complications associated with FMF were found as follows: preterm delivery (PTD<37 weeks) (RR=1.9; 95% CI 1.2–4.4, р=0.036); ectopic pregnancy (EP) (RR=1.5; 95% CI 1.1–2.7, р=0.018). In patients with FMF 149 pregnancies (59.3%) without complications resulted in 184 live births (73.3%) (RR=1.0; 95% CI 0.9–1.0, р=0.811). In patients without FMF 190 pregnancies (61.0%) resulted in 231 live births (74.0%). There were no significant differences among the groups with early (15.9% vs. 16.3%, p=0.326) and late miscarriages (1.6% vs. 3.2%, p=0.457), сesarean delivery (CD) (12.6% vs. 13.2%, p=0.794), premature detachment of a normally positioned placenta (PDNPP)(0.4% vs. 1.0%, p=0.431), intrauterine growth restriction (IUGR) (1.2% vs. 1.3%, p=0.930), preeclampsia (PE) (3.2% vs. 3.2%, p=0.996), intrauterine fetal hypoxia (2.4% vs.1.9%, p=0.699), antenatal fetal death (AFD) (2.4% vs. 3.5%, p=0.438), fertility treatment (24.7% vs. 23.7%, p=0.823), respectively. Perinatal outcomes in the groups were comparable: low Apgar scores at the 1st minute (<7) (1.6% vs. 2.5%, p=0.497) and and the 5th minute (0.5% vs. 0.4%, p=0.875), congenital malformations (CM) (1.6% vs. 2.6%, p=0.431) and perinatal mortality (PM) (3.2% vs. 4.5%, p=0.438), respectively. In 75.3% of patients with FMF, the average maternal age at delivery was 19–35 years, compared to 47.1% in the control group. Women without FMF were older (>36 years – 51.2%, p<0.0001). The average birth weight in babies (2500–4000 g) was lower in the main group (64.2% vs. 80.2%, p<0.0001). Assessment of pregnancy outcomes in 131 (52.2%) patients who were taking colchicine, and 120 (47,8%) who were not taking colchicine or not taking regularly did not show differences regarding CM, PE, PDNPP, IUGR, AFD, PM, late miscarriage, CD. Controlling FMF attacks in 88,5% of cases and more favourable pregnancy outcomes were due to intake of colchicine: early miscarriages (13.7% vs. 18.3%, p=0.326; respectively), PTD (5,3% vs. 8.3%; p=0.121),<37 weeks (5.3% vs. 8.3%, p=0.121; respectively), EP(4.6% vs. 9.2%, p=0.225), respectively, but the results did not reach statistical significance. Conclusion. Familial Mediterranean fever is associated with an increased risk of preterm delivery, lower birth weight and ectopic pregnancy. Perinatal outcomes in patients with and without FMF are comparable. Colchicine treatment of pregnant women with FMF has a beneficial effect on the clinical course of the disease and does not affect pregnancy outcomes.Sotskiy P.O.
Keywords
Familial Medeterranean fever (FMF) is a hereditary, genetically determined autoinflammatory disorder. The clinical picture is characterized by periodic attacks of fever and serositis, which may lead to an increase in uterine contractility and adverse pregnancy outcomes [1]. MEditerranean FeVer (MEFV) gene is located in the short arm of chromosome 166 (16р13.3) and comprises 10 exons. Most of missense mutations associated with FMF are in exons 2, 3, 5 and 10. Colchicine is the main drug in treatment of FMF. The effect of FMF on reproductive system and lifelong colchicine therapy raises concerns about their impact on pregnancy outcomes. At the end of the last century, the first works devoted to the study of reproductive function in patients with FMF were published [2–4]. Potential complications of FMF (amyloidosis), exposure to the disease (subclinical inflammation), and treatment with colchicine can seriously affect fertility and pregnancy [1, 5, 6]. Pregnancy outcomes in women with renal amyloidosis are of particular concern due to increased risk of abortion, stillbirth, or impaired renal function [7–9]. The studies in recent years showed a moderate trend towards better pregnancy outcomes in patients receiving colchicine [5]. According to the presented data, intake of colchicine should be continued during pregnancy, since refusal of colchicine treatment can worsen the symptoms and worsen fertility prognosis [5, 6, 10, 11]. There are few studies on the effects of FMF and colchicine on pregnancy cause and outcomes. Most publications are the reviews summarizing the previous results [12–15]. Therefore, we decided to study this problem among our female patients in Armenia.
Materials and methods
Observational cohort study was carried out, which compared pregnancy outcomes in 251 women with FM in 312 women without FMF. The study was approved by the local Ethics Committee. In the first step, a study group of women (cohort) was formed. It included 4577 women of reproductive age (18–49 years) with confirmed diagnosis of FMF. These women were selected among 32,000 persons of Armenian nationality, who were examined for the presence of mutations in MEFV gene and their clinical, laboratory and genetic test results were included in the information register for the period 1998–2018 in the Center for Medical Genetics and Primary Health Care, Yerevan, Armenia. In the second step, a study group with reproductive disorders was formed. It included 211 patients selected among women with confirmed diagnosis of FMF, and 162 patients among women without confirmed diagnosis of FMF. Pregnancy course and outcomes were studied in both groups. Inclusion criteria were: termination of pregnancy or abortion, obstetric complications, perinatal outcomes. Exclusion criteria were: age beyond the edge of reproductive age (less than 18 and more than 49 years), malignant neoplasms, cardiovascular and systemic diseases. The demographic data of all patients, their clinical and laboratory test results, as well as the results of obstetric and gynecological examination, antenatal care, childbirth delivery methods and perinatal outcomes were registered in the unified computerized perinatal database.
The diagnosis of FMF was confirmed using Tel Hashomer criteria [16] and molecular genetic test for 12 most common MEFV gene mutations in the Armenian population. The disease severity was estimated for each patient, taking into account the age of onset, the frequency of seizures, the presence of arthropathy, erysipelas, proteinuria and renal complications or inadequate response to colchicine. Clinical manifestations of FMF showed 3 levels of severity: mild (2–5), moderate (6–9), severe (>10) [16, 17].
Molecular genetic diagnostic testing for FMF
Molecular genetic testing was carried out under the leadership of Professor A.S. Airapetyan, Doctor of Biological Sciences, Head of the Laboratory of Genetics of Autoinflammatory Diseases of the Center of Medical Genetics and Primary Health Care. Whole peripheral blood was used to identify MEFV gene mutations. Blood was drawn from cubital vein and collected in test tubes containing EDTA to prevent blood clotting and DNA degradation. For DNA isolation, Ultra Clean Blood DNA Isolation Kits (MOBIO laboratories, Inc., USA) were used. Mutations were detected by multiplex amplification of the selected region of DNA containing the studied gene, with reverse hybridization of the obtained amplicons, control in the parallel study and visualization of mutations with enzymatic color reaction (Vienna Lab FMF&SAA1 Assay).
Amplification products were hybridized to the allele-specific oligonucleotide probes immobilized on test strips as parallel lines. The test covered 12 mutations of MEFV gene: E148Q, P369S, F479L, M680I (G/C), M680I (G/A), I692del, M694V, M694I, K695R, V726A, A744S, R761H.
The obstetric and gynecological examination included the standard antenatal protocol for monitoring high-risk pregnancy.
Statistical analysis
The results of the study were processed using SPSS Statistics 16.0 software package. The arithmetic mean (M) and standard deviation (CD) was used to describe quantitative data with normal distribution. Qualitative indicators were presented in absolute and relative values (%). Parametric methods of statistical analysis were used for normal distribution of characteristics and equality of variances in the compared groups. Significance in difference between the indicators (p) was tested by Student’s t-test. The Pearson χ2 test with Yates's correction for continuity was used to compare qualitative binary characteristics in the groups. For quantitative limitations, two-sided Fisher’s exact test was used. To study a possible relationship between a certain outcome and risk factor in the compared groups, the data were processed using GraphPad Prism 4 and GraphPad Prism 5 software packages. Relative risk (RR) with 95% confidence interval (CI) was calculated. Statistical significance of RR was assessed by calculating upper and lower bounds of 95% CI. The identified relationship between the risk factor and the outcome was considered statistically significant at p<0.05, if CI did not include the value 1, i.e. both bound values were either above or below 1. Thus, the results were considered statistically significant at RR > 1; р<0.05).
Results
The course and outcomes of 251 pregnancies was studied in 211 patients with FMF aged 18–45 years – average age 21.3 (6.4) years – in group 1 (the main group) and 312 pregnancies in 162 women without FMF – average age 31.4 (7.0) years – in group 2 (the control group). In group 1, 140 (59.3%) pregnancies developed without complications, and in group 2 – 190 (61.0%). Major complications of pregnancy are presented in Table 1. In patients with FMF most often complications were: early miscarriages – in 40 women in group 1 and in 51 women in group 2 (15.9% versus 16.3%, р=0.326) (RR=0.9; 95% CI 0.7–1.3); ectopic pregnancies – in 17 women in group 1 and in 9 women in group 2 (6.8% versus 2.9%) (RR=1.5; 95% CI 1.1–2.7; р=0,018); threatened abortion (TA) – in 13 women in group 1 and in 10 women in group 2 (5.2% versus 3.2%) (RR=1.3; 95% CI 0.7–2.5; р=0.236). The ratio was statistically significant only in cases of EP.
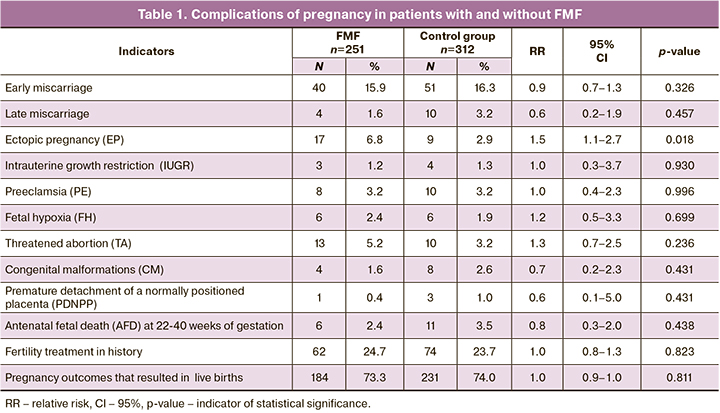
There were no significant differences between the groups, when comparing the following indicators: late miscarriages – 4 and 10, respectively (1.6% vs. 3.2%, р=0.457) (RR=0.6; 95% CI 0.2–1.9); PDNPP – 1 in group 1 and 3 in group 2 (0.4% vs. 1.0%, р=0.431) (RR=0.6; 95% CI 0.1–5.0); IUGR – 3 and 4, respectively (1.2% vs. 1.3%, р=0.930) (RR=1.0; 95% CI 0.3–3.7); PE – 8 and 10, respectively (3.2% vs. 3.2%, р=0.996) (RR=1.0; 95% CI 0.4–2.3); fetal hypoxia – 6 and 6, respectively (2.4% vs. 1.9%, р=0.699) (RR=1.2; 95% CI 0.5–3.3); AFD – 6 and 11, respectively (2.4% vs. 3.5%, р=0.438) (RR=0.8; 95% CI 0.3–2.0); CM – 4 and 8, respectively (1.6% vs. 2.6%, р=0.431) (RR=0.7; 95% CI 0.2–2.3). The number of pregnancies after infertility treatment was comparable in both groups: 62 and 74, respectively (24.7% vs. 23.7%, р=0.823) (RR=1.0; 95% CI 0.8–1.3).
The effect of colchicine on the clinical course of the disease and pregnancy outcomes in women with FMF was compared between 131 pregnant women (52.2%) treated with colchicine (group 1) and 120 pregnant women (47.8%) – not treated with colchicine or with irregular intake of colchicine (group 2). In group 1, there was a regular intake of colchicine at a dose of 0.5–2.0 mg/day. Treatment was started immediately after clinical diagnosis of FMF and continued in the periconceptional period and during pregnancy at the same dosage as before pregnancy. This allowed controlling FMF attacks and minimizing subclinical inflammation between the attacks. The frequency and intensity of the attacks during pregnancy was assessed. There were no cases of clinical worsening of FMF. In 116 (88.5%) women clinical course of FMF remained unchanged, and in 15 (11.5%) pregnant women the number of attacks decreased. In group 1, the incidence of TA and ectopic implantation was the same – 4.6% and 4.6% (6 and 6, respectively). The complications of pregnancies observed among the patients included early miscarriages – 18 (13.7%); late miscarriages – 2 (1.5%); IUGR – 1 (0.8%); fetal hypoxia and congenital malformations – 2 and 2 observations, respectively (1.5% and 1.5%); AFD – 3 (2.3%); PE – 4 (3.1%); PDNPP – 0 (0.0%). Seven pregnancies resulted in preterm delivery (PD) (5.3%), 6 – in induced labor (4.6%). Thirteen (9.9%) women had cesarean deliveries.
In group 2, one hundred and twenty pregnant women did not take colchicine or took it irregularly and at insufficient dosages (92 and 28 pregnancies, respectively) due to fears of teratogenic effects of the drug, as well as untimely diagnosis of FMF. Clinical worsening of FMF was in 102 pregnant women (85.0%), the number of attacks remained unchanged in 18 women (15.0%); in none of the cases clinical improvement was observed. The rate of TA in group 2 was 7.5% (9 observations); EP – 9.2% (11 observations), early miscarriages – 18.3% (22 observations); late miscarriages – 1.7% (2 observations); IUGR – 1.7% (2 observations); fetal hypoxia – 3.3% (4 observations); CM – 1.7% (2 observations); AFD – 2.5% (3 observations); PDNPP – 0.83% (1 observation); PE – 3.3% (4 observations). Ten pregnancies resulted in PD (8.3%); induced labor was in 6 women (5.0%). Eleven women (9.2%) had cesarean deliveries.
Thus, in patients with regular intake of colchicine at adequate dosages, the clinical course of the disease was controlled: the number of attacks remained unchanged, an increase in TA incidence (4.6% versus 7.5%; p=0.236) and fetal hypoxia (1.5% versus 3.3%; p=0.141) was detected. A more favorable trend was noted in relation to pregnancy outcomes. Early miscarriage (13.4% versus 18.3%; p=0.326); PD (5.3% versus 8.3%; p=0.121) and EP (4.6% versus 9.2%; p=0.225) was less common, although the results did not reach statistical significance. There were no significant differences in congenital malformations (1.5% versus 2.5%; p=0.365); late miscarriage (1.5% versus 1.7%; p=0.457); PE (3.1% versus 3.3%; p=0.996); PDNPP (0.0% versus 0.83%; p=0.631); IUGR (0.8% versus 1.7%; p=0.930); AFD (2.3% versus 2.5%; p=0.738); CD (9.9% versus 9.2%; p=0.811).
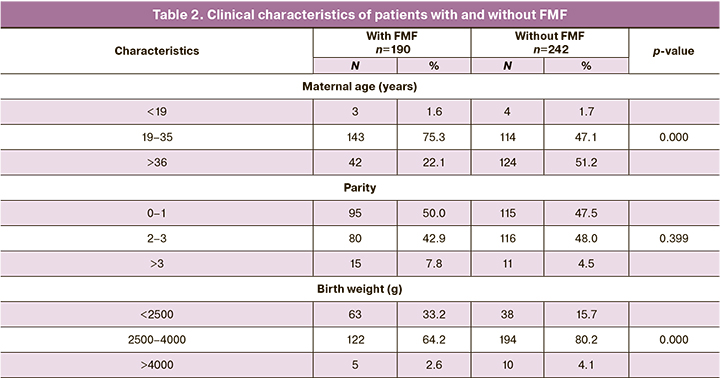
Clinical characteristics in women with and without FMF, whose pregnancies resulted in childbirths are shown in Table 2. In group 1, maternal age 19–35 years was prevalent – in 143 (75.3%) patients. In group 2, the women were older – 124 patients were aged >36 years (51.2%) (р<0.0001). In group 1, the parity number was: 1 – 50.0%; 2–3 – 42.9%; >3 – 7.8%; in group 2: 1 – 47.5%; 2–3 – 48.0%; >3 – 4.5% (р=0.399), respectively. Average birth weight of babies (2500.0-4000.0 g) born to 194 patients (80.2%) in group 2 was higher compared to low birth weight of babies born to 122 women (64,2%) (р< 0,0001) among the group of women with FMF.
The major method of delivery in women with FMF was spontaneous term labor – 130 (68.4%) (RR =1.1; 95% CI 0.7–1.8), which did not differ significantly from the control group – 153 (63.2%; р=0.212) (Table 3). In group of women with FMF the rate of spontaneous PD at <37 weeks was higher – 17 (8.9%) (RR=1.9; 95% CI 1.2–4.4) compared to the control group – 10 (4.1%; р=0.036). Cesarean deliveries were in 24 (12.6%) women (RR=1.0; 95% CI 0.5–2.0), in the control group – in 32 (13.2%; р=0.794). Spontaneous PDs at < 37 weeks by CS were in 7 women in group 1 (3.7%) (RR =0.5; 95% CI 0.2–0.9) and in 32 women in group 2 (13.2%; р<0.001). Statistically significant predominance of CSs at <37 weeks in group 2 can be explained by the difference in the age of patients in both groups. Older maternal age and higher number of multiple pregnancies was in the control group. Induced PDs were equally common in both groups. Comparing such complications as abnormal fetal presentation and placenta previa, it was found that in group 1 placenta previa was more common – in 9 cases (4.7%) (RR=1.3; 95% CI 0.5–3.2) compared to 5 observations in group 2 (2.1%; р=0.131). However, these indicators did not reach statistical significance.
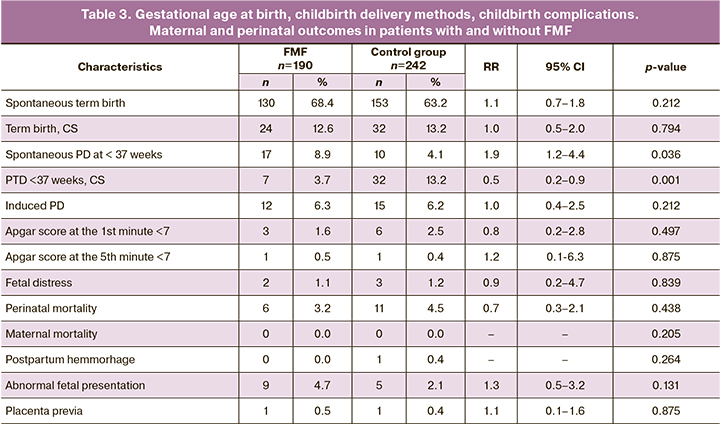
Perinatal outcomes, such as low Apgar scores (<7) at the 1st minute (1.6% vs. 2.5%; р=0.497) and at the 5th minute (0.5% vs. 0.4%; р=0.875); perinatal mortality (3.2% vs. 4.5%; р=0.438); CM (1.6% vs. 2.6%; р=0,431); pregnancy outcomes that resulted in live births (73.3% vs. 74.0%; р=0,811) were comparable between the groups. There was no maternal mortality in both groups.
Discussion
This study compared pregnancy outcomes in two groups: in patients with FMF (group 1) and without FMF (group 2). The women in the group with FMF were younger. The rates of successful pregnancy were surprisingly similar in both groups (59.3% and 61.0%). Most of the previous studies focused on the effect of colchicine on pregnancy outcomes in patients with FMF versus healthy women in the control groups. [2–5, 10, 18–25]. The most significant prospective and retrospective cohort study, which was conducted in Israel in 2004–2008 (Ben-Chetrit E. et al.), compared 179 pregnancies in women with FMF, who took colchicine, and 197 pregnancies in women who did not take colchicine, as well as 312 pregnancies in healthy women in the control group [5]. The authors reported that there were no differences between early (8.9%, 9.0% and 14.0%) and late miscarriages (1.1%, 3.0% and 2.0%), CM (0.5%, 2.0% and 0.6%). There was a moderate trend towards better outcomes in the group of patients, who received colchicine. This suggested that colchicine could be a useful drug to be taken during pregnancy [5]. The results obtained by us are consistent with these data. In the 70ies of XX century, when colchicine was not used, the frequency of pregnancy loss was higher in women with FMF, than in the general population [4]. Later studies showed high rates of early abortions due to peritonitis attacks and premature contraction of the uterus in patients, who did not take colchicine (20.2% versus 10.2%) [3], as well as high abortion rate among the patients with FMF compared to women in the control group [2].
In 2010, a large prospective observational cohort study was conducted in 2010 by Diav-Citrin O. et al. in cooperation with two Teratology Information Services in Israel [21]. 238 pregnant women who underwent therapy with colchicine (97.0% in the first trimester) and 964 pregnant women who did not receive colichicine therapy were studied. The authors reported that there were high rates of PDs. The rates of congenital anomalies were comparable between the groups: 10/221 (4.5%) and 35/908 (3.9%). The average duration of gestational age at birth was shorter, and the average weight at birth was lower. It was concluded that the use of colchicine did not imply increased cytogenetic risk, and increased rate of preterm births and low fetal weight was associated with FMF. Nabil Н. et al. presented the data on favourable pregnancy outcomes in 26 patients with FMF, who took colchicine before and after pregnancy [20]. Yasar O. et al. found higher rates of premature rupture of membranes, CS and low birth weight of babies in 47 patients with FMF compared to 138 women in the control group. The rate of PDs in patients with FMF was higher, than in women in the control group, but it was not statistically significant. The rates of PE, IUGR, gestational diabetes and stillbirths were comparable [18]. In 25 patients, colchicine intake and attacks were not associated with adverse pregnancy outcomes. Among 10 patients, who did not take colchicine, high rate of recurrent miscarriages was detected – 44.4% versus 8.1%; p<0.01.
This study compared pregnancies in patients with and without FMF with purpose of studying a possible relationship between the risk factors for FMF and pregnancy outcomes. Another important finding in the study referred to the intake of colchicine during pregnancy and its effect on the clinical course of FMF and pregnancy outcomes. 52.2% of studied women took colchicine since the first-time diagnosis of FMF including during pregnancy. The course and outcomes of pregnancy were comparable to those in the cohort of healthy women. The rates of some complications of pregnancy – early miscarriages, PD, EP was lower, although the results did not reach statistical significance. Fetal chromosomal abnormalities were not found. The frequency of non-chromosomal abnormalities was similar to that in the control group. Increased rates of EP and early miscarriages in the group of patients, who did not took colchicine or took it irregularly, can be explained by uncontrolled attacks of FMF, leading to strong uterine contractility, ectopic implantation, the threat of miscarriage, and termination of pregnancy. This finding supports an earlier hypothesis, that FMF attack with high levels of body temperature in early pregnancy may lead to early pregnancy loss [18].
In the largest published population-based study on pregnancy outcomes in patients with and without FMF, Ofir D. et al. were the first to describe independent risk factors associated with FMF [19]. Pregnancy course and outcomes in 239 patients with FMF, who took or did not take colchicine and 175 572 pregnant women without FMF. The authors stated that FMF was an independent risk factor for PD and suggested that recurrent peritoneal attacks of FMF could cause PD and mimic pregnancy complications: placental abruption or chorioamnionitis. Differential diagnosis was only possible, if FMF attacks were preceded with a prodromal period. Perinatal outcomes in patients with FMF were comparable with those in the group of women without FMF. There were no significant differences between the groups in the following indicators: low Apgar scores at the 1st minute (<7) and at the 5th minute (2.5% versus 4.0%, р=0.253 and 0.4% versus 0.6%, р=0.783; respectively); perinatal mortality (0.8% versus 1.3%, р=0.544); CM (4.6% versus 4.8%, р=0.888), whether or not colchicine was used [19]. They found a higher rate of infertility treatment in patients with FMF (5.4%) compared to women without FMF (1.9%), but in their opinion, the reason for this association remained unclear. [19]. In contrast to the previous study, the rate of infertility and IVF was comparable in our study, since the control group was composed of women with reproductive disorders. Perhaps this circumstance and older age of patients in the control group can explain the fact that we did not find a significant difference in the frequency of CSs (12.7% versus 13.1%), as other authors stated. [18, 19]. Rather frequent cesarean deliveries (18.0% versus 12.8%) could be explained by concerns about adverse pregnancy outcomes among women who received infertility treatment [19]. Using multiple logistic regression methods, the authors found that FMF was not an independent risk factor for CS, since CS was associated with other obstetric risk factors.
Thus, the results obtained in this study were consistent with most of the above studies. However, the remaining differences can be explained by the different number of women in the studied groups, the design of the study and regimen of colchicine therapy.
Conclusion
FMF is associated with an increased risk of PD, low birth weight and EP. Perinatal outcomes in patients with and without FMF are comparable. Colchicine treatment of pregnant women with FMF has a beneficial effect on the clinical course of the disease and does not affect pregnancy outcomes.
References
- Ben-Chetrit E., Levy M. Reproductive system in familial Mediterranean fever: an overview. Ann. Rheum. Dis. 2003; 62(10): 916-9. https://dx.doi.org/10.1136/ard.62.10.916.
- Mijatovic V., Hompes P.G., Wouters M.G. Familial Mediterranean fever and its implications for fertility and pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003; 108(2): 171-6. https://dx.doi.org/10.1016/s0301-2115(02)00417-7.
- Rabinovitch O., Zemer D., Kukia E., Sohar E., Mashiach S. Colchicine treatment in conception and pregnancy: two hundred thirty one pregnancies in patients with familial Mediterranean fever. Am. J. Reprod. Immunol. 1992; 28(3-4): 245-6. https://dx.doi.org/10.1111/j.1600-0897.1992.tb00805.x.
- Ehrenfeld M., Brzezinski A., Levy M., Eliakim M. Fertility and obstetric history in patients with familial Mediterranean fever on long-term colchicine therapy. Br. J. Obstet. Gynaecol. 1987; 94(12): 1186-91.https://dx.doi.org/10.1111/j.1471-0528.1987.tb02320.x.
- Ben-Chetrit E., Ben-Chetrit A., Berkun Y., Ben-Chetrit E. Pregnancy outcomes in women with familial Mediterranean fever receiving colchicine: is amniocentesis justified? Arthritis Care Res. (Hoboken). 2010; 62(2): 143-8. https://dx.doi.org/10.1002/acr. 20061.
- Michael O., Goldman R.D., Koren G., Team M. Safety of colchicine therapy during pregnancy. Can. Fam. Physician. 2003; 49: 967-9.
- Livneh A., Cabili S., Zemer D., Rabinovitch O., Pras M. Effect of pregnancy on renal function in amyloidosis of familial Mediterranean fever. J. Rheumatol. 1993; 20(9): 1519-23.
- Mordel N., Birkenfeld A., Rubinger D., Schenker J.G., Sadovsky E. Successful full-term pregnancy in familial Mediterranean fever complicated with amyloidosis: case report and review of the literature. Fetal Diagn. Ther. 1993; 8(2): 129-34. https://dx.doi.org/10.1159/000263761.
- Tutuncu L., Atasoyu E.M., Evrenkaya R., Mungen E. Familial Mediterranean fever-related nephrotic syndrome and successful full-term pregnancy. Arch. Med. Res. 2006; 37(1): 178-80. https://dx.doi.org/10.1016/j.arcmed.2005.04.013.
- Indraratna P., Virk S., Gurram D., Day R.O. Use of colchicine in pregnancy: a systematic review and meta-analysis. Rheumatology (Oxford). 2018; 57(2): 382-7. https://dx.doi.org/10.1093/rheumatology/kex353.
- Ozen S., Demirkaya E., Erer B., Livneh A., Ben-Chetrit E., Giancane G. et al. EULAR recommendations for the management of familial Mediterranean fever. Ann. Rheum. Dis. 2016; 75(4): 644-51. https://dx.doi.org/10.1136/annrheumdis-2015-208690.
- Dotters-Katz S., Kuller J., Price T. The impact of familial Mediterranean fever on women’s health. Obstet. Gynecol. Surv. 2012; 67(6): 357-64. https://dx.doi.org/10.1097/OGX.0b013e318259ed3a.
- Yanmaz M.N., Özcan A.J., Savan K. The impact of familial Mediterranean fever on reproductive system. Clin. Rheumatol. 2014; 33(10): 1385-8. https://dx.doi.org/10.1007/s10067-014-2709-9.
- Sarı İ., Birlik M., Kasifoğlu T. Familial Mediterranean fever: an updated review. Eur. J. Rheumatol. 2014; 1(1): 21-33. https://dx.doi.org/10.5152/eurjrheum.2014.006.
- Ozdogan H., Ugurlu S. Familial Mediterranean fever. Presse Med. 2019; 48(1, Pt. 2): e61-76. https://dx.doi.org/10.1016/j.Lpm.2018.08.014.
- Livneh A., Langevitz P., Zemer D., Zaks N., Kees S., Lidar T. et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997; 40(10): 1879-85. https://dx.doi.org/10.1002/art.1780401023.
- Mor A., Shinar Y., Zaks N., Langevitz P., Chetrit A., Shtrasburg S. et al. Evaluation of diseases severity in familial Mediterranean fever. Semin. Arthritis Rheum. 2005; 35(1): 57-64. https://dx.doi.org/10.1016/j.Semarthrit.2005.02.002.
- Yasar O., Iskender C., Kaymak O., Taflan Yaman S., Uygur D., Danisman N. Retrospective evaluation of pregnancy outcomes in women with familial Mediterranean fever. J. Matern. Fetal Neonatal Med. 2014; 27(7): 733-6. https://dx.doi.org/10.3109/14767058.2013.837446.
- Ofir D., Levy A., Wiznitzer A., Mazor M., Sheiner E. Familial Mediterranean fever during pregnancy: an independent risk factor for preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008; 141(2): 115-8. https://dx.doi.org/10.1016/j.ejogrb.2008.07.025.
- Nabil H., Zayed A., State O., Badawy A. Pregnancy outcome in women with familial Mediterranean fever. J. Obstet. Gynaecol. 2012; 32(8): 756-9. https://dx.doi.org/10.3109/01443615.2012.698667.
- Diav-Citrin O., Shechtman S., Schwartz V., Avgil-Tsadok M., Finkel-Pekarsky V., Wajnberg R. et al. Pregnancy outcome after in utero exposure to colchicine. Am. J. Obstet. Gynecol. 2010; 203(2): 144. e1-6. https://dx.doi.org/10.1016/j.ajog.2010.02.063.
- Yazicioğlu A., Turgal M., Senem O., Özyuncu Ö., Bekac M.S. Pregnancy outcome in women with familial Mediterranean fever: a retrospective analysis of 50 cases with a 10-year experience. Arch. Rheumatol. 2014; 29(2): 94-8. https://dx.doi.org/10.5606/ArchRheumatol.2014.3707.
- Ben-Chetrit E., Berkun Y., Ben-Chetrit E., Ben-Chetrit A. The outcome of pregnancy in the wives of men with familial Mediterranean fever treated with colchicine. Semin. Arthritis Rheum. 2004; 34(2): 549-52. https://dx.doi.org/10.1016/j.semarthrit.2004.07.004.
- Wang X., Chen C., Wang L., Chen D., Guang W., French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil. Steril. 2003; 79(3): 577-84. https://dx.doi.org/10.1016/s0015-0282(02)04694-0.
- Shimoni Y., Shalev E. Pregnancy and complicated familial Mediterranean fever. Int. J. Gynaecol. Obstet. 1990; 33(2): 165-9. https://dx.doi.org/10.1016/0020-7292(90)90591-8.
Received 03.09.2020
Accepted 23.12.2020
About the Authors
Pavel O. Sotskiy, Ph.D., obstetrician-gynecologist, Center of Medical Genetics and Primary Health Care, Yerevan, Armenia. Tel.: +374 41 188888. E-mail: pavel.sotskiy@ gmail.com.34/3 Abovyan str., 0001, Yerevan, Armenia.
For citation: Sotskiy P.O. Pregnancy course and outcomes in women with familial Mediterranean fever.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 61-68 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.61-68



