Structural features of fetal membranes in preterm labor
Objective: to investigate structural alterations in the fetal membranes during preterm labor (PL) in the absence of inflammatory changes.Nizyaeva N.V., Karapetyan A.O., Gapaeva M.D., Sinitsyna V.A., Baev O.R.
Materials and methods. The fetal membranes of 25 placentas were analyzed: 14 from spontaneous PL at 28-36 weeks, 7 from term spontaneous labor during uncomplicated pregnancy; 4 from preterm operative delivery for fetal hypoxia, premature detachment of a normally situated placenta, and impaired fetal functional status at 27-32 weeks’ gestation. A histological (hematoxylin and eosin staining) and immunohistochemical studies on paraffin-embedded fetal membrane sections were performed using primary monoclonal antibodies to the mesenchymal cell marker vimentin (Spring Bioscience), to the marker of angiogenesis CD34 (Spring Bioscience), and to the macrophage marker CD68 (Spring Bioscience), as well as to the innate immunity receptor NOD-1 (Invitrogen). Immunohistochemical study was done using a closed Ventana immunostainer (Roche, UK).
Results. Staining with antibodies to CD34, CD68, vimentin, and NOD-1 revealed the staining of mesenchymal cells and their processes, as well as macrophages located in the center of above structures. Increased angiogenesis in the compact layer and decidual plate was ascertained to weaken membrane mechanical strength during PB, and there were multiple defects and microfractures in these structures.
Conclusion. Thus, the results of the investigation indicate that PL is due to a set of certain factors: decreased membrane mechanical strength, increased angiogenesis in the compact layer, and multiple microdefects and fractures, which lowers the mechanical strength of the fetal membranes.
Keywords
Fetal membranes have selective permeability to various molecules, they participate in exchange of amniotic fluid and have a complex histological structure. Amniotic epithelium contacts with amniotic fluid. The compact layer consists of connective tissue elements and is the most resistant. Below the compact layer there is cytotrophoblast (called «smooth chorion», as opposed to the chorion of placental villi), with underlying layer of decidual cells contacting with uterine cavity [1, 2].
The term “preterm labor” (PL) is used to refer to childbirth, which occurs at gestational age 22 to 37 weeks with newborns weight more than 500 g [3]. PL is a polyetiological complication of pregnancy. The most studied cause of PL is premature rupture of membranes associated with inflammatory changes (membranitis) caused by microbial agents [1, 2, 4, 5]. An intra-amniotic infection is believed to occur in every third case of PL, however in other preterm deliveries the main cause remains unknown. Moreover, no clear relationship between the degree of membranes inflammatory infiltration and onset of PL was found [6-8].
The aim of the research was to study structural changes of fetal membranes after PL in the absence of inflammation.
Materials and Methods
Fetal membranes of 25 placentas were analyzed. The studied group consisted of 14 women with spontaneous labor at 28-36 weeks of gestation. The comparison group included 7 women with uncomplicated pregnancy and spontaneous onset of labor at term (normal control – NC) and 4 women with emergency preterm cesarean section at 27-32 weeks due to fetal distress, placental abruption (early control – EC).
Exclusion criteria were signs of inflammatory changes in membranes detected by histological examination (the presence of neutrophils, plasmocytes, lympho-macrophage infiltration of membranes) [1, 9].
On macroscopic examination the fetal membranes were grabbed with forceps 3 cm away from the side of the rupture and twisted like a roll, then dissected across with a sharp knife or scalpel into parts 0.3-0.4 cm thick [2]. The dissected tissue fragments were fixed in 10% formalin.
Histological (hematoxylin and eosin staining) and immunohistochemical studies were carried out on serial paraffin sections 4 μm thick using primary monoclonal antibodies to the mesenchymal cell marker vimentin (clone SP20, ready for use; Spring Bioscience), angiogenesis marker CD34 (clone QBenD, ready for use; Spring Bioscience), macrophage marker CD68 (clone SP251, ready for use; Spring Bioscience) and to the innate immunity receptor NOD-1 (nucleotide-binding oligomerization domain-containing protein-1) (1: 100, Invitrogen). The immunostainer Ventana (Roche, UK) with closed detection kit was used for immunohistochemical study. The protocol for automated staining of samples included all stages of the standard immunohistochemical procedure: dewaxing of sections, their unmasking, endogenous peroxidase blocking, incubation with primary and secondary antibodies. View Universal DAB, Detection Kit were used as visualization system. The reaction products became detectable when brown staining of the cell membrane and cytoplasm appeared. For negative control, samples of the studied sections underwent a standard immunohistochemical procedure without incubation with primary antibodies. A positive control for each antibody was selected in accordance with specifications of the manufacturer.
Results
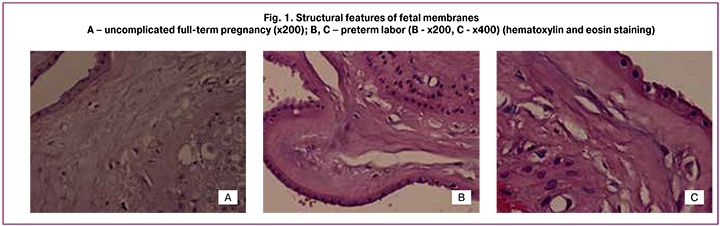
On histological examination of the fetal membranes samples in PL and full-term pregnancy groups, edema was observed; it appeared mainly in the form of rarefaction sites of compact layer, and single macrophages were also visualized (Fig.1А-C). In PL group morphological changes of fetal membranes were the most pronounced: the compact layer was with sections of a mesh or it was cellular due to multiple closely located capillary-like structures or stromal channels (by analogy with the villous placenta tree), often with multiple micro- and macro-ruptures from 1 to several tens of millimetres. Desquamation of amniotic epithelium and stratification of the membranes were observed frequently. Moreover, the dystrophic changes, areas of compact layer and decidual plate necrosis were present. In EC group the edema was not pronounced, macrophages were single or absent. Immunohistochemical study of the compact layer of fetal membranes revealed vimentin+ mesenchymal cells, mainly spindle-shaped, contacting each other with long processes this way forming a continuous chain; at the same time several cells were weakly CD34+. The decidual plates or compact layers capillaries were single or absent. Along with this, capillary-like structures consisting of stellate fibroblast-like cells with long thin processes, delimiting the space of the forming vessel were detected in NC and PL groups (Fig. 2A, 2B), in the center there was CD68+ macrophage (Fig. 2C, 2D) [2].
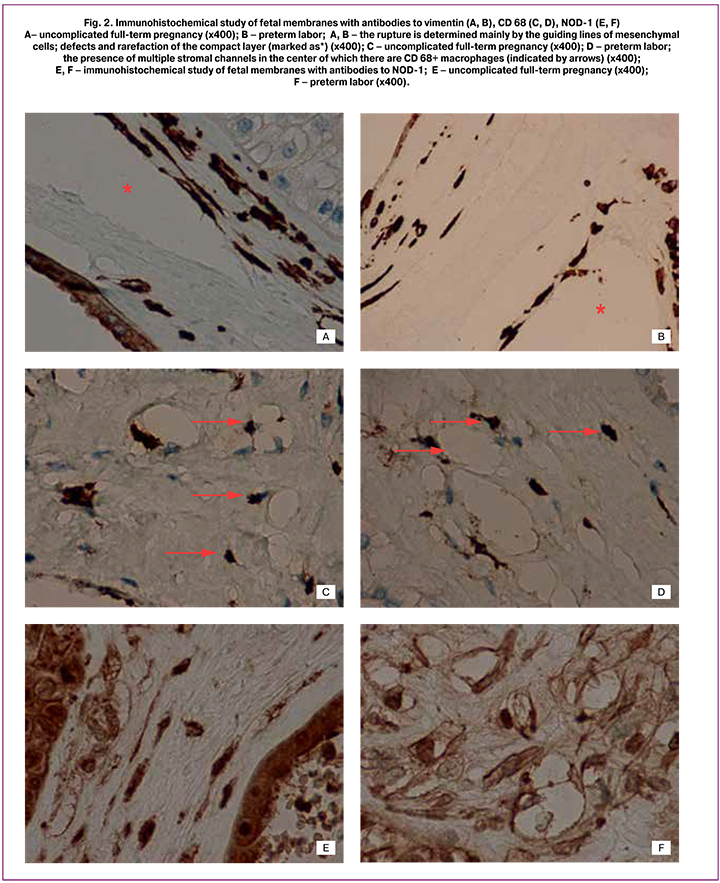
These structures are similar to stromal channels by its formation, which is a manifestation of early angiogenesis of the placental villous tree [2]. Like placental villi, mesenchymal cells forming the stromal channels of the membranes were intensely stained with vimentin and weakly CD34+. The endothelium of blood vessels and capillaries was intensely stained with CD34+ (Fig. 3A-E).
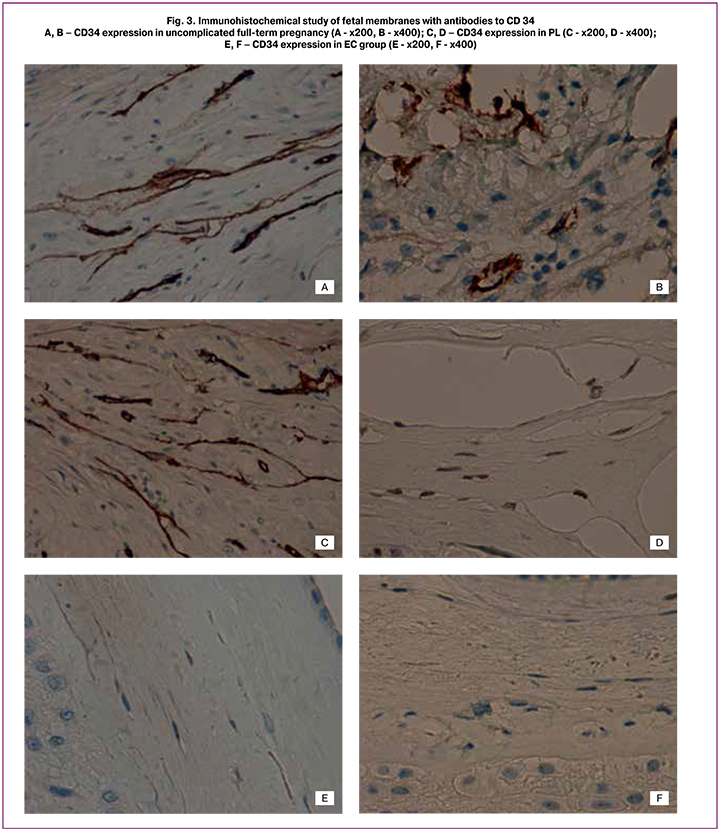
Along with the above mentioned changes, the number of blood vessels in decidual plate was increased, which also weakened the mechanical strength of the membranes. Maximum number of vessels was present in the decidual plate in PL group (more than 5 per high power field of view (x200)). In some observations of women with PL, the number of vessels was significantly increased up to the development of cystic transformation of the decidual plate compared to EC and NC [2]. In full-term pregnancy the number of vessels was 4-5 per high power field (x200), which was comparable with the PL group (p>0.05). In EC group, they were single and of smaller diameter (p<0.05).
It should be emphasized that in PL and by the end of full-term pregnancy microruptures in the fetal membranes compact layer were caused not by edema or necrosis, but they occurred mainly along capillary-like structures and stromal channels (Fig. 2A, 2B; 3A, 3B) [1, 2].
It is worth noting that the above-described capillary-like structures or stromal channels when stained with hematoxylin and eosin are masked as edema or artificial changes, which are often thought to be associated with disturbance of the material fixation technology or technical difficulties in the microtome in the manufacture of histological sections (Fig. 1).
In addition, brown staining of both macrophages and stromal cells was determined when using antibodies to the innate immunity receptor NOD-1 (Fig. 2E, 2F). This result clearly showed that macrophages were inside the lumen of stromal channels. At the same time, only macrophages were detected during staining with antibodies to CD68, stromal cells were mostly negative [10]. The analysis of micropreparations stained with antibodies to NOD-1 made it possible to visualize the mesenchymal cells and their processes that form the stromal channels, as well as the macrophages located in their lumen. We suppose that these changes are similar to changes in the placental villous tree and they are manifestations of early angiogenesis [1, 11].
Discussion
According to the existing data, one of the most studied cause of PL development is inflammation and associated changes of the membranes [1]. However, the histological signs of amnionitis and chorioamnionitis are present only in 50% of cases [12]. At the same time, premature rupture of the membranes and spontaneous labor onset before term can occur in the absence of inflammatory reaction in the placenta and membranes, for example, in patients with undifferentiated connective tissue dysplasia, when collagen synthesis is impaired, the “strength” of amniotic sac decreases and causes its rupture [6, 8, 13-16]. The received data indicate that at full-term pregnancy there is an increase in vascularization of the membranes, leading to their rupture and labor onset. The development of these changes in earlier pregnancy can be the reason of PL. Probably, similar process occurs when paper or postage stamp easily comes off in the presence of small microperforations, the membranes tend to rupture due to increased angiogenesis and stromal channels appearance.
Our results are important in understanding PL pathogenesis. Currently, one of the methods for PL predicting is the detection of placental alpha-microglobulin (PAMG). The concentration of this protein in amniotic fluid is high, however, it cannot be determined in vaginal mucus. The appearance of laboratory-determined levels of this protein in vaginal mucus in the absence of clinical manifestations of membranes rupture indicates the excretion of amniotic fluid through microperforation foramina (microruptures) of the membranes and possible premature rupture of the membranes and/or PL in a short time [17].
Conclusion
Thus, the results of our study show that PL occurs due to a combination of certain factors leading to a decrease in mechanical strength of membranes, caused by an increase in compact layer and decidual plate angiogenesis, as well as multiple microdefects and ruptures.
References
- Benirschke K., Burton G.J., Baergen R.N. Pathology of the human placenta. Sixth ed., Springer. 2012.
- Милованов А.П. Патология системы мать-плацента-плод: руководство для врачей. М.: Медицина; 1999. 448 с. [Milovanov A.P. Pathology of the mother-placenta-fetus system: a guide for doctors. М.: Medicine; 1999. 448 p. (InRuss.)]
- Сухих Г.Т., Серов В.Н., Адамян Л.В. Преждевременные роды. Клинические рекомендации (протокол). М. 2014. 35с.[Sukhikh G.T., Serov V.N., Adamyan L.V. Premature birth. Clinical recommendations (protocol). M. 2014.35s. (InRuss.)]
- Fahey J.O. Clinical management of intra-amniotic infection and chorioamnionitis: a review of the literature. J Midwifery Womens Health. 2008; 53(3): 227-235.
- Tita A.T., Andrews W.W. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010; 37(2): 339-354.
- Steel J.H., Malatos S., Kennea N., Edwards A.D., Miles L., Duggan P., Reynolds P.R., Feldman R.G., Sullivan M.H. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005; 57(3): 404-411.
- Farraj A.A. Randomized placental and cord blood sampling culture in women with preterm and term labour to detect infection. East Mediterr Health J. 2000; 6: 272-275.
- Queiros da Mota V., Prodhom G., Yan P., Hohlfheld P., Greub G., Rouleau C. Correlation between placental bacterial culture results and histological chorioamnionitis: a prospective study on 376 placentas. J Clin Pathol. 2013; 66: 243-248.
- Низяева Н.В. Гистологические критерии воспалительных изменений плодных оболочек плаценты и пуповины. Международный журнал прикладных и фундаментальных исследований. 2018; 2: 202-207. [Nizyaeva N.V. Histological criteria of the inflammatory diseases of the placental villus tree. International Journal of applied and fundamental research. 2018; 2: 202-207. (InRuss.)]
- Nizyaeva N., Karapetian A., Sinitsyna V., Pavlovich S., Kan N., Baev O. NOD-1 expression in placental villi in pregnancy-related complications. Virchows Archiv. 2018; 473: S1.
- Burton G.J., Sebire N.J., Myatt L., Tannetta D., Wang Y.L., Sadovsky Y. et al. Optimising sample collection for placental research. Placenta. 2014; 35: 9-22.
- Баев О.Р., Васильченко О.Н., Кан Н.Е., Клименченко Н.И., Митрохин С.Д., Тетруашвили Н.К., Ходжаева З.С., Шмаков Р.Г., Дегтярев Д.Н., Тютюнник В.Л., Адамян Л.В. Преждевременный разрыв плодных оболочек. Преждевременное излитие вод. Акушерство и гинекология. 2013; 9: 123-134. [Baev O.R., Vasilchenko O.N., Kan N.E., Klimenchenko N.I., Mitrokhin S.D., Tetruashvili N.K., Khodzhaeva Z.S., Shmakov R.G., Degtyarev D.N., Tyutyunnik V.L., Adamyan L.V. Clinical guidelines for preterm amniorrhea. Obstetrics and Gynecology. 2013; 9: 123-134. (InRuss.)].
- Кан Н.Е., Санникова М.В., Донников А.Е., Климанцев И.В., Амирасланов Э.Ю., Ломова Н.А., Кесова М.И., Костин П.А., Тютюнник В.Л., Сухих Г.Т. Клинические и молекулярно-генетические факторы риска преждевременного разрыва плодных оболочек. Акушерство и гинекология. 2013; 4: 14-18. [Kan N.E., Sannikova M.V., Donnikov A.E., Klimantsev I.V., Amiraslanov E Yu., Lomova N.A., Kesova M.I., Kostin P.A., Tyutyunnik V.L., Sukhikh G.T. The clinical and molecular genetic risk factors of premature rupture of membranes in pregnant women with undifferentiated connective tissue dysplasia. Obstetrics and Gynecology. 2013; 4:14-18. (In Russ.]
- Кан Н.Е., Санникова М.В., Амирасланов Э.Ю., Тютюнник В.Л. Клинические предикторы прогнозирования преждевременного разрыва плодных оболочек. Вопросы гинекологии, акушерства и перинатологии. 2013; 12(3):12-18. [Kan N.E., Sannikova M.V., Amiraslanov E.Yu., Tyutyunnik V.L. Clinical predictors in prognosis of premature rupture of membranes. Gynecology, obstetrics and perinatology. 2013; 12(3): 12-18. (in Russ.)].
- Kan N.E., Tuytyunnik V.L., Donnikov A.E., Sannikova M.V., Sukhikh G.T. Association of ESR1 gene polymorphism with preterm rupture of fetal membranes. Bulletin of Experimental Biology and Medicine. 2013; 156: 12: 811-814.
- Nizyaeva N.V., Sukhacheva T.V., Kulikova G.V., Nagovitsyna M.N., Poltavtseva R.A., Kan N.E., Tyutyunnik V.L., Pavlovich S.V., Serov R.A., Shchyogolev A.I. Ultrastructural Characteristics of Placental Telocytes. Bulletin of Experimental Biology and Medicine. 2017; 31.
- Lee S.M., Romero R., Park J.W., Kim S.M., Park C.W., Korzeniewski S.J., Chaiworapongsa T., Yoon B.H. The clinical significance of a positive Amnisure test in women with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2012; 25(9): 1690-98.
Received 21.01.2019
Accepted 22.02.2019
About the Authors
Nizyaeva Natalia V., Ph.D., senior researcher, Pathology Department; National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation; E-mail: niziaeva@gmail.com, mob.: +7-926-248-28-93 ORCID: orcid.org/0000-0001-5592-5690117997, Russia, Moscow, Ac. Oparina str. 4.
Каrapetian Аnnа О., physician of the first Maternity Department; National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation. 117997, Russia, Moscow, Ac. Oparina str. 4.
Gapaeva Маsаrа D., postgraduate student; first Maternity Department; National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation 117997, Russia, Moscow, Ac. Oparina str. 4.
Sinitsyna Veronica A., senior laboratory assistant, Pathology Department; National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, 117997, Russia, Moscow, Ac. Oparina str. 4.
Baev Oleg R., PhD, MD, Professor, Head of the first Maternity Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation 117997, Russia, Moscow, Ac. Oparina str. 4.
I.N. Sechenov First Moscow State Medical University of Ministry of Healthcare of Russian Federation. Department of Obstetrics, Gynecology, Perinatology and Reproductology; 119991, Russia, Moscow, Trubetskaya str. 8/2 ORCID: orcid.org/0000-0001-8572-1971, SPIN 5058-7295 8 (495) 438-11-88. E-mail: o_baev@oparina4.ru
For citation: Nizyaeva N.V., Karapetyan A.O., Gapaeva M.D., Sinitsyna V.A., Baev O.R. Structural features of fetal membranes in preterm labor.
Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019;(8):63-9 (in Russian).
https://dx.doi.org/10.18565/aig.2019.8.63-69



