Structure of defects in sperm quality in men in the infertile couples and the algorithm for their management in level 3 healthcare facilities
Aim. To identify the structure of defects in sperm quality in men in infertile couples and to develop the algorythm for management of such patients. Materials and methods. Clinical laboratory testing of 4088 infertile couples (I) without established female factor; the control group – 365 fertile men (F), whose spouses became pregnant in the natural cycle for the last 12 weeks. Research type – analytical one-stage multicenter study. Results. The defects in sperm quality in group I were observed more often than in group F: cases of azoospermia – 4,2% and 0% (р<0,001), cryptozoospermia – in 1,6% and 0% (p<0,01), oligozoospermia – 17,4% and 2,2% (р<0,001), asthenozoospermia – 45,5% and 22,1% (р<0,001), teratozoospermia – 27,5% and 11,3% (р<0,001), oligospermia – 5,3% and 1,7% (p<0,05), antisperm antibodies (MAR-IgG>50%) – 12,3% and 4,8% (р<0,001), pyospermia – 17,1% and 8,1% (р<0,001), normal zoospermia – 47,0% and 70,0% (р<0,001) respectively. The medians and ranges of all the parameters of spermogram in group I were significantly worse than in group F (p <0.001), except for the volume and percentage of abnormal forms (p>0,05). In group I, the men with normal zoospermia had defects in sperm function: increased DNA fragmentation (51%), oxidative stress (50%), abnormal acrosomal reaction (46%) and protamination (30%). Conclusion. In 2/3 of cases, a decrease in male fertility is a consequence of functional disorders of spermatozoa. According to WHO-2010, in fertile men normozoospermia was observed in 70% of cases; this indicates that the ranges reference values vary among the regions. An algorithm for management of patients with various sperm disorders in level 3 healthcare facilities was proposed.Bozhedomov V.A., Nikolaeva M.A., Ushakova I.V., Bozhedomova G.E., Lipatova N.A., Kamarina R.N., Okhobotov D.A., Kamalov A.A.
Keywords
In half of the cases of infertility in married couples, male alone factor is present in (30%), or in combination with female infertility (20%) [1–3]. Impaired male fertility is often accompanied by the changes in the parameters of the ejaculate – a decrease in concentration, motility and proportion of sperm with normal forms – oligozoospermia, asthenozoospermia and teratozoospermia, respectively. However, the men who have lowest sperm concentration within reference values are not necessarily infertile. [4]. Conversely, male infertility may occur with formal «normozoospermia» in case of various functional sperm disorders: receptors interactions, including acrosome reactions, chromatin damage and other epigenetic dysfunctions, autoimmune response against sperm [3, 5–7]. In addition, the WHO experts note, that there may be regional variability in the volume of ejaculate in fertile men, and recommend that the laboratories should introduce their own reference values to be be calculated using the methods described in the guidelines “World Health Organization reference values for human semen” [4]. The unresolved issues of differential diagnosis of male infertility make treatment more difficult, since effective treatment based on only formal spermiological diagnosis is impossible. Special studies are necessary to clarify the etiopathogenetic mechanisms of subfertility. But there are still no clear algorithms linking the diagnosis and treatment for management of men in infertile couples [1–3, 7–10].
Purpose of the study: to identify the structure of defects in sperm quality in men in infertile couples and to develop the algorythm for management of patients in the specialized medical facilities.
Materials and methods
Type of the study – analytical one-stage multicenter study. Electronic medical records of 4088 men aged 18–50 years old, who in the period 2011–2020 were treated in third-level clinics where the authors are working, because of lacking pregnancy in marriage lasting more than 1 year, were reviewed. The time interval for inclusion in the study is based on the fact that a new edition of the WHO Guidelines for Sperm Research was introduced into clinical practice in 2010 [4], to some extent changing the work of laboratory diagnostics specialists. There were following conditions for inclusion in the study: the absence of known female causes of infertility (anomalies of the genital organs, obstruction of the fallopian tubes, anovulation, severe endometriosis, age over 35 years), as well as regular sex at least once a week. Other clinical and anamnestic factors were not considered. The examined patients from infertile couples were considered presumably subfertile, regardless of the results of sperm investigation and formed an “infertile” group (group B). The comparison group included 365 fertile men who applied for various reasons (medical examination, sexual problems, symptoms of the lower urinary tract diseases, etc.), with a spontaneous pregnancy in their female partners within 3 months preceding treatment (group F); the outcome of pregnancy was not taken into account.
The study of the ejaculate included the assessment of standard parameters (sperm volume, concentration, motility, morphology, etc.), as well as special tests for antisperm antibodies (ASA), oxidative stress (OS), DNA fragmentation, acrosome reaction (AR), chromatin condensation. While assessing the morphology of sperm, «strict criteria» were applied. ASA was determined by the MAR (mixed agglutination reaction) method (Ferti Pro N.V., Belgium) [4]: MAR-IgG = 10–49% was considered “moderate disorders”, MAR-IgG> 50% was considered “significant disorders”. In azoospermia, cryptozoospermia, severe oligozoospermia and asthenozoospermia, ASA was determined by an indirect method. The OS was assessed by measuring the production of reactive oxygen species (ROS) by the luminol-dependent chemiluminescence method [4, 11] using a «LKB-Wallac 1256 luminometer» (Finland) and a chemiluminometer-003 «UGATU» (Russia); chemiluminescence not more than 0.44 mV/s was considered to be normal [12]. To assess spontaneous and ionophore-induced A23187 AR, the method of double fluorescent staining of spermatozoa using fluoruscin-isothiocyanate-labeled lectin P. sativum (Sigma, USA) and tetramethylrhodamine-isothiocyanate-labeled lectin A. hypogaea (Sigma, USA) was used [13]; spontaneous AR was considered normal when there were not less than 15% of spermatozoa, and induced AR was considered normal when there were not less than 15% of spermatozoa [4]. Sperm apoptosis was characterized by DNA fragmentation, assessed by chromatin dispersion (Halosperm® Halotech DNA, SL, Spain) in an inert agarose gel with visual assessment under a microscope of halo formation after acid denaturation of DNA and lysis of nuclear proteins [14]; a fragmentation index of no more than 15% was considered the norm, fragmentation more than 30% was considered to have “significant violations”. Chromatin condensation was characterized by the number of spermatozoa with a high histone content [15]. We used aniline blue and eosin staining, which helped to identify the main nuclear proteins with a high lysine content, typical to immature spermatozoa. In the preparation with a magnification of x1000, the percentage of unstained cells (G0), spermatozoa with weak (G1) and intense (G2) staining was assessed; the index of protamination disorder (G0 x 0 + G1 x 1+ G2 x 2) was calculated; More than 30% of histone-positive spermatozoa were considered to be a violation, and the index of protamination violation was more than 40 [15].
Statistical analysis
Statistical analysis of the data was carried out using the «Statistica» package (StatSoft, USA). The analysis of the correspondence between the type of distribution of the trait and the law of normal distribution was carried out using the Shapiro–Wilk test (W). Group averages were presented as the median, 25%–75% and 5%–95% percentiles, a range of “captive values”. Outliers – were values that are far from the centeral distribution and don’t characterize it (possibly, the results of observation errors, or outliers). Outliers were determined in this way: via «Statistica» program, a box plot was built for each group of variables, in which the range of out-of-range values is indicated in the «legend» Charts. In our case, the «outliers» were the values calculated in accordance with the algorithm configured in the statistical package «Statistica» by default: «contours» – values greater than the interquartile range with a coefficient x 1.5; “Limits” – with a coefficient x 2. The use of 5–95% percentiles and a range of “captive values” is necessary not only to characterize the average trends in the distribution of values, but also to determine the reference limits for the estimated parameters. The significance of differences between the groups was checked using nonparametric Mann–Whitney (M–W) tests, signs, χ2; differences were considered significant when p <0.05.
Results
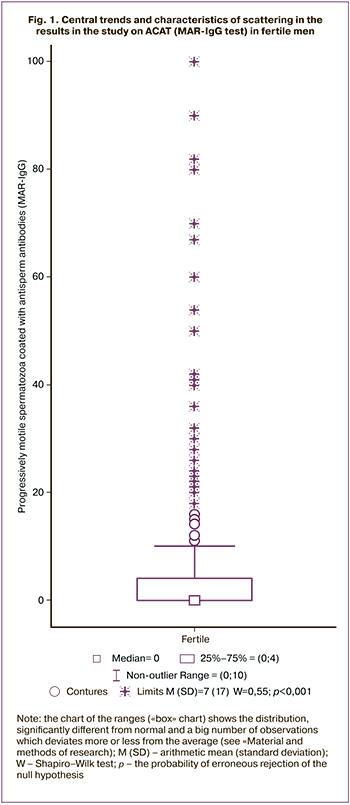
The analysis of the distribution of the values of all the estimated indicators of the ejaculate showed that it differed from normal : W for the volume of the ejaculate is 0.96 (p<0.001), the concentration of sperm is 0.79 (p<0.001), the proportion of progressively motile spermatozoa is 0.99 (p<0.001); The most pronounced deviations from the normal distribution were in the concentration of sperm leukocytes (W = 0.23; p <0.001) and the proportion of ASA-positive spermatozoa (W = 0.55; p <0.001; Fig. 1). Therefore, to characterize the central tendencies and variability of each parameter, we relied on the values of the median and percentiles.
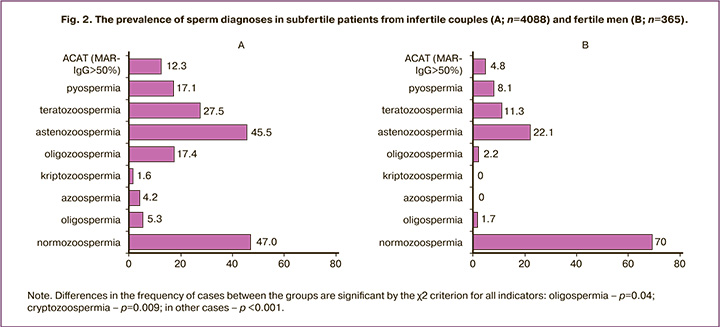
Various disturbances of sperm quality in men from infertile couples (group B) occurred significantly more often than in fertile men (group F) (Fig. 2): azoospermia – 4.2% and 0% of cases (p <0.001), cryptozoospermia – 1.6% and 0% (p=0.009), oligozoospermia – 17.4% and 2.2% (p <0.001), asthenozoospermia – 45.5% and 22.1% (p <0.001), teratozoospermia – 27.5% and 11.3% (p<0.001), oligospermia – 5.3% and 1.7% (p=0.04), АSA (MAR-IgG> 50%) – 12.3% and 4,8% (p<0.001), pyospermia – 17.1% and 8.1% (p<0.001).
Almost half (47.0%) of patients in group B had the concentration, motility and morphology of spermatozoa within the reference values, i.e. corresponded to the diagnosis «normozoospermia».
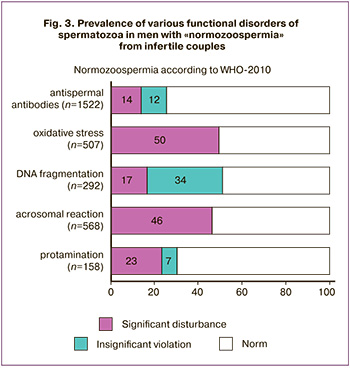 At the same time, every apparently normal man had one or another functional impairment of spermatozoa (Fig. 3): increased DNA fragmentation (51%), OS (50% of cases), impaired AR (46%) and protamination (30%); often such violations were combined. In 26% of men, ASA covered more than 10% of motile spermatozoa, incl. 14% of the ASAT-positive were at least 50% of the sperm. The average values and differences in the ranks of all spermogram indicators (table) in group B are significantly worse than in group F (p <0.001), except for the volume and percentage of abnormal forms (p> 0.05).
At the same time, every apparently normal man had one or another functional impairment of spermatozoa (Fig. 3): increased DNA fragmentation (51%), OS (50% of cases), impaired AR (46%) and protamination (30%); often such violations were combined. In 26% of men, ASA covered more than 10% of motile spermatozoa, incl. 14% of the ASAT-positive were at least 50% of the sperm. The average values and differences in the ranks of all spermogram indicators (table) in group B are significantly worse than in group F (p <0.001), except for the volume and percentage of abnormal forms (p> 0.05).
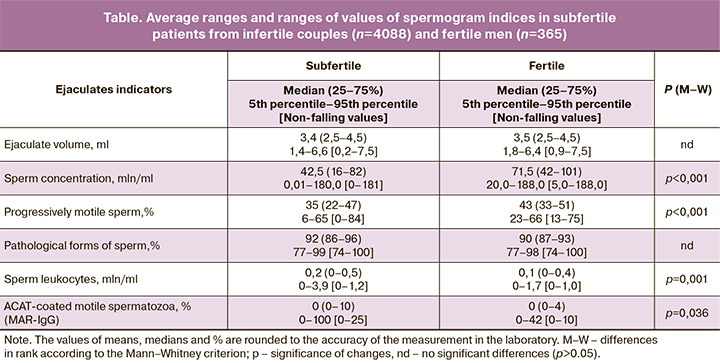
In fertile men, whose sexual partners became pregnant within the last three months (group F), the lower 5th percentile for volume was 1.8 ml, concentration was 20 million/ml, and the proportion of progressively mobile spermatozoa was 23%. For other important parameters of the ejaculate, where, on the contrary, the excess over the standard matters, the 95th percentile for sperm leukocytes was 1.7 million/ml, for ASAT (MAR-IgG) – 42%, pathological forms of spermatozoa – 98%. The use of the “non-falling out values” criterion showed slightly different results: for the concentration of sperm leukocytes, the “falling out” values were more than 1 million/ml, for MAR-IgG – more than 10%, for morphology – 100%.
Discussion
The established fact that the average values of concentration, mobility, morphology and other parameters of ejaculate in men from infertile couples are worse than in fertile ones, and the prevalence of azoospermia, cryptozoospermia, oligozoospermia, asthenozoospermia, teratozoospermia, pyospermia is higher than in fertile ones, is quite predictable. However from the data presented by us it can be seen that the decrease in the number of spermatozoa (azoospermia, cryptozoospermia, oligozoospermia) in the analyzed sample of subfertile men (n=4088) is in total about 25%; in another 5% of cases, oligospermia occurs. More often, there is a decrease in the quality of spermatozoa: a decrease in the proportion of progressively motile (46%) and an increase in the percentage of pathological forms (according to «strict criteria» 28%). In 47% of cases, normozoospermia occurs – i.e. men were presumed to be fertile. But at the same time, various functional disorders of spermatozoa were diagnosed in this subgroup: increased production of ROS by spermatozoa, which is a sign of sperm OS (50% of cases), increased DNA fragmentation (51% of cases), including those cases because of protamination violation (30%), their AR is violated (46%). Such violations can lead not only to the absence of pregnancy, but also to missed pregnancies and developmental anomalies, including when using the assisted reproductive technologies [1, 16–18]. Most likely, these disorders are interrelated, since OS is today considered as the leading pathogenetic mechanism of sperm damage in many clinical conditions: varicocele, infectious inflammatory processes, toxic effects, autoimmune reactions against spermatozoa, and others [1, 19–21]. In idiopathic forms of pathozoospermia, OS took place in up to 80% of cases; in infertility of unknown origin, i.e. functional disorders not diagnosed by standard sperm examination – in 30-40% [22, 23]. According to our data, with infertility of unknown genesis, OS occurred even more often – in 50% of cases. Establishing the presence of such functional disorders allows not only better understand the nature of childless marriages (infertility ceases to seem idiopathic), but also to make the treatment of such patients pathogenetically justified. Recently, a large group of authors published an article in which it was proposed to introduce a new diagnosis: «idiopathic infertility caused by OS» (Male Oxidative Stress Infertility / MOSI) [24]. The validity of this approach is confirmed by the results of our recent study, in which it was shown that the therapy of oligozoo-, asthenozo- and / or teratozoospermia using a complex of nutrient-antioxidants, widely used for the treatment of idiopathic male infertility [25], is effective at moderately elevated levels of ROS, and is less effective at significantly increased levels and has no effect at normal ROS levels [26]. This explains why the evidence for the efficacy of antioxidant nutrients in the empiric treatment of idiopathic infertility is highly controversial and has been ruled out as a recommended option in the latest EAU guidelines [1].
Given that we cannot unequivocally exclude the female factor of infertility (tubal factor, oligomenorrhea, endometriosis, and age over 35 are not exclusive causes of female infertility), we cannot claim that all men in group B were infertile. But at the same time, the presented data give sufficient reason to believe that the functional disorders of spermatozoa described above are a very common pathogenetic mechanism of male infertility, as in oligo-, astheno- and teratozoospermia (data in this article are not presented) and with normozoospermia. Therefore, laboratory tests to detect such functional disorders should be used as widely as possible in the examination of infertile couples and couples with miscarriage. It is necessary to reconsider the attitude to these methods as to «scientific», as argued in some manuals [1, 4], and more widely to introduce them into practical health care. We have already discussed the question of what methods of examination of men from infertile couples should be used in a medical and preventive institution of the first, second and third levels [7]. Since the implementation of studies of OS, chromatin structure, AR implies the use of rather complex special methods, equipment and qualifications of specialists, their use today, in our opinion, should be carried out in specialized medical institutions of the third level.
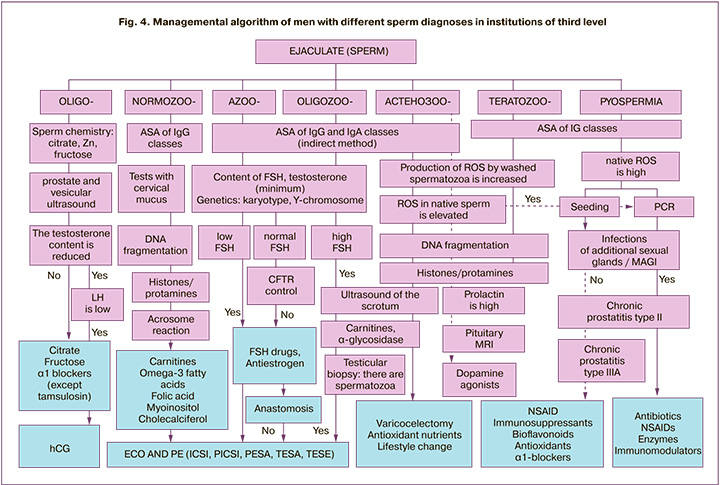
Figure 4 shows our algorithm for the management of men from childless couples, taking into account both traditional sperm conditions (oligozoospermia, asthenozoospermia and teratozoospermia, pyospermia) and various functional disorders. Following this algorithm, in our opinion, will allow the use of various possible treatments – gonadotropins, antiestrogens, antioxidant nutrients, antibiotics, etc. [1, 8, 25, 27] – not empirically, but pathogenetically justified.
In fertile men in our sample, ejaculate corresponds to the diagnosis «normozoospermia» according to WHO-2010 [4] only in 70% of cases. Since the surveyed group of fertile men is quite large – 365 people, and the fact that we took into account pregnancies that have occurred in the last 3 months, the data obtained, in our opinion, allow us to raise the question of introducing regional reference values for characterizing the sperm of fertile men in the Russian population. Regional differences in ejaculate rates in fertile men have been reported by the compilers of the WHO Semen Research Guidelines and recommended that laboratories implement their own reference ranges calculated using the methods described in this guide [4]. In particular, from the data presented in the article, it follows that the range of 20–188 mln / ml should be considered the normal concentration for the Russian population, and values less than 5 mln / ml are “outliers”, ie. not typical for fertile men. For progressively motile spermatozoa, the range of 23–66% should be considered normal, and less than 13% as “falling out”. For the result of the MAR-IgG test, the picture looks a little different: the upper 95%-percentile is 42%, and all values above 10% are “outliers”. There are certain differences in comparison with the reference values of the WHO Guidelines [4] and for other indicators of ejaculate.
It should be noted that the assessment of sperm morphology is really controversial today, depending not only on the research methods (color, number of counted visual fields, personnel qualifications), but also on the use of one or another classification, in particular, work on «strict» or «liberal”criteria [28, 29]. Our data confirm the point of view of those authors [29] who believe that the assessment of the morphology of spermatozoa with standard staining does not allow differentiating samples into fertile and sterile. According to our data, there were no significant differences in the percentage of pathological forms between the groups of fertile and subfertile men, and the range of “captive values” in the fertile group, like in subfertile men, reaches 100%. Obviously, the decrease in fertility is associated not with external defects in the structure of spermatozoa, but with their functional impairments, which can occur both in teratozoospermia and in normal morphology.
Perhaps, for clinical practice, it is rational to divide the results not only into “normal” and “pathological”, but give a space to a “gray zone” concept that characterizes subfertile patients [28]. We propose that going beyond the 5%–95% percentile range should be considered a significant risk factor for reduced fertility and is defined by the term “subfertility”, beyond the “outliers” by the term “male infertility”. Refinement of such regional criteria requires the continuation of large-scale multicenter studies in this direction with internal and external quality control of laboratory tests.
It should also be accepted that the concept of «fertile man», i.e. a man who gets his female partner pregnant, is not identical to the concept of a “healthy man” in the reproductive sense, since up to 40% of pregnancies terminate early due to poor sperm quality and associated fetal developmental disorders [16–18].
Conclusion
In more than 2/3 cases, the decrease in male fertility is due not to a small number of spermatozoa, but to their functional disorders: OS, chromatin damage, AR, ASA disorders. The data on regional features of the range of reference values of fertile men were obtained. Laboratory criteria for the use of the terms «subfertility» and «male infertility» were proposed. An algorithm for managing patients with various sperm conditions in specialized institutions of the third level is proposed (Fig. 4).
References
- Salonia A. (Chair), Bettocchi C., Carvalho J., Corona G., Jones T.H., Kadi-oglu A. et al. EAU Guidelines on sexual and reproductive health. European Association of Urology; 2020. 232p.
- Barratt C.L.R., Björndahl L., De Jonge C.J., Lamb D.J., Martini F.O., McLachlan R. et al. The diagnosis of male infertility: an analysis of the evi-dence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update. 2017; 23(6): 660-80. https://dx.doi.org/10.1093/humupd/dmx021.
- Agarwal А., Majzoub А., Parekh N., Henkel R. A schematic overview of the current status of male infertility practice. World J.Mens Health. 2020; 38(3): 308-22. https://dx.doi.org/10.5534/wjmh.190068.
- WHO laboratory manual for the examination and processing of human se-men. 5th ed. WHO; 2010. 271p.
- Belloc S., Benkhalifa M., Cohen-Bacrie M., Dalleac A., Amar E., Zini A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil. Steril. 2014; 101(6): 1588-93. https://dx.doi.org/10.1016/j.fertnstert.2014.02.006.
- Oud M.S., Volozonoka L., Smits R.M., Vissers L.E.L.M., Ramos L., Veltman J.A.A systematic review and standardized clinical validity assessment of male infertility genes. Hum. Reprod. 2019; 34(5): 932-41. https://dx.doi.org/ 10.1093/humrep/dez022.
- Божедомов В.А. Мужской фактор бездетного брака – пути решения проблемы. Урология. 2016; 1 (Приложение 1): 28-34. [Bozhedomov V.A. The male factor in childless marriage – problem-solving strategies. Urology. 2016; 1(Suppl. 1): 28-34. (in Russian)]. PMID: 28247744
- Cavallini G., Beretta G., eds. Clinical management of male infertility. Hei-delberg: Springer Cham; 2015. 187p. https://dx.doi.org/10.1007/978-3-319-08503-6.
- Чалый М.Е., Ахвледиани Н.Д., Харчилава Р.Р. Мужское бесплодие. Урология. 2016; 1 (Приложение 1): 2-17. [Chaly M.E., Akhvlediany N.D.,Kharchilava R.R. Male infertility. Urology, 2016, 1 (Suppl. 1): 2-17(in Russian)].
- Корнеев И.А., Зассеев Р.Д., Исакова Э.В., Кинунен А.А., Бичевая Н.К. Оказание медицинской помощи с применением вспомогательных репродуктивных технологий у мужчин: обзор клинических рекомендаций и алгоритм маршрутизации пациентов. Проблемы репродукции. 2018; 24(4): 59-65. [Korneev I.A., Zasseev R.D., Isakova E.V., Kinunen A.A.,Bichevaya N.K. Provision of medical care using assisted reproductive technologies in men: a review of clinical guidelines and a patient routing algorithm. Reproduction problems. 2018; 24 (4): 59-65.(in Russian)].
- Agarwal A., Deepinder F. Determination of seminal oxidants (reactive oxygen species). In: Lipshults L.I., Howards S.S., Niederberger C.S., eds. Infertility in the male. 4th ed. 2009: 618-32.
- Божедомов В.А., Громенко Д.С., Ушакова И.В., Торопцева М.В., Галимов Ш.Н., Александрова Л.А., Теодорович О.В., Сухих Г.Т. Оксидативный стресс сперматозоидов в патогенезе мужского бесплодия. Урология. 2009; 2: 51-6. [Bozhedomov V.A., Gromenko D.S., Ushakova I.V., Toroptseva M.V., Galimov Sh.N., Alexandrova L.A., Teodorovich O.V., Sukhikh G.Т. Sperm oxidative stress in the pathogenesis of male infertility. Urology. 2009; 2: 51-6. (in Russian)]. PMID: 19526875
- Nikolaeva M.A., Golubeva E.L., Kulakov V.I., Sukhikh G.T. Evaluation of stimulus-induced acrosome reaction by two-color flow cytometric analysis. Mol. Hum. Reprod. 1998; 4(3): 243-50. https://dx.doi.org/10.1093/molehr/4.3.243.
- Gosalvez J., Lopez-Fernandez C., Fernandez J.L. Sperm chromatin dispersion test: technical aspects and clinical applications. In: Zini A., Agarwal A., eds. Sperm chromatin: biological and clinical applications in male infertility and assisted reproduction. Springer; 2011: 151-70. https://dx.doi.org/10.1007/978-1-4419-6857-9.
- Jenkins T.G., Benjamin R.E., Carrell D. Assays used in the study of sperm nuclear proteins. In: Zini A., Agarwal A., eds. Sperm chromatin: biological and clinical application in male infertility and assisted reproduction. Springer; 2011: 233-41. https://dx.doi.org/10.1007/978-1-4419-6857-9.
- ESHRE Early Pregnancy Guidline Development Group. Guideline of the European Society of Human Reproduction and Embryology. Recurrent pregnancy loss. 2019: 81-4. https://dx.doi.org/10.1093/hropen/hoy004.
- McQueen D.B., Zhang J., Robins J.C. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil. Steril. 2019; 112(1): 54-60. https://dx.doi.org/10.1016/j.fertnstert.2019.03.003.
- Pereira N., Neri Q.V., Cozzubbo T., Cheung S., Rosenwaks Z., Palermo G. Male infertility and assisted reproduction. In: De Jonge C.J., Barratt C.L.R., eds. The sperm cell: production, maturation, fertilization, regeneration. 2nd ed. Cambridge University Press; 2017: 193-207. https://dx.doi.org/10.1017/9781316411124.
- Божедомов В.А., Семенов А.В., Божедомова Г.Е., Липатова Н.А., Пацановская Г.М. Репродуктивная функция мужчин при хроническом простатите: клинико-анамнестические и микробиологические аспекты. Урология. 2015; 1: 70-8. [Bozhedomov V.A., Semenov A.V., Bozhedomova G.E., Lipatova N.A., Patsanovskaya G.M. Reproductive function of men in chronic prostatitis: clinical, anamnestic and microbiological aspects. Urology. 2015; 1: 70-8. (in Russian)].
- Галимов Ш.Н., Божедомов В.А., Галимова Э.Ф., Павлов В.Н., Сухих Г.Т.Мужское бесплодие: молекулярные и иммунологические аспекты. М.: ГЭОТАР-Медиа; 2020. 208 с. [Galimov Sh.N., Bozhedomov V.A., Galimova E.F., Pavlov V.N., Sukhikh G.T. Male infertility: molecular and immunological aspects. M.: GEOTAR-Media; 2020; 208p. (in Russian)].IBSN 978-5-9704-5334-6.
- Aitken R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017; 84(10): 1039-52. https://dx.doi.org/10.1002/mrd.22871.
- Ko E.Y., Sabanegh E.S. Jr., Agarwal A. Male infertility testing: reactive ox-ygen species and antioxidant capacity. Fertil. Steril. 2014; 102(6): 1518-27. https://dx.doi.org/10.1016/j.fertnstert.2014.10.020.
- Wagner H., Cheng J.W., Ko E.Y. Role of reactive oxygen species in male infertility: an updated review of literature. Arab. J. Urol. 2017; 16(1): 35-43. https://dx.doi.org/10.1016/j.aju.2017.11.001.
- Agarwal A., Parekh N., Kumar M.P.S., Henkel R., Shah R., Homa S.T. et al.Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Mens Health. 2019; 37(3): 296-312. https://dx.doi.org/10.5534/wjmh.190055.
- Smits R.M., Mackenzie-Proctor R., Yazdani A., Stankiewicz M.T., Jordan V., Showell M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2019, (3): CD007411. https://dx.doi.org/10.1002/14651858.CD007411.pub4.
- Божедомов В.А., Камалов А.А., Божедомова Г.Е., Козлова В.И., Кама-рина Р.А., Епанчинцева М.А. Применение комплекса нутриентов при идиопатическом мужском бесплодии в форме астено- и/или тератозоос-пермии: поиск предикторов эффективности лечения (предварительные результаты). Урология. 2018; 5: 53-9. [Bozhedomov V.A., Kamalov A.A., Bozhedomova G.E., Kozlova V.I., Kamarina R.A., Epanchintseva M.A. Application of a complex of nutrients for idiopathic male infertility in the form of astheno- and/or teratozoospermia: search for predictors of treatment efficacy (preliminary results). Urology, 2018; 5: 53-9. (in Russian)]PMID: 30575350
- Omar M.I., Pal R.P., Kelly B.D., Bruins H.M., Yuan Y., Diemer T. et al. Benefits of empiric nutritional and medical therapy for semen parameters and pregnancy and live birth in couples with idiopathic infertility: a sys-tematic rewiew and meta-analysis. Eur. Urol. 2019, 75(4): 615-25. https://dx.doi.org/10.1016/j.eururo.2018.12.022.
- Björndahl L. What is normal semen quality? On the use and abuse of refer-ence limits for the interpretation of semen analysis results. Hum. Fertil. (Camb.). 2011; 14(3): 3179-86. https://dx.doi.org/10.3109/14647273.2011.580823.
- Gatimel N., Moreau J., Parinaud J., Leandri R.D. Sperm morphology: as-sessment, pathophysiology, clinical relevance and state of the art in 2017. Andrology. 2017; 5(5): 845-62. https://dx.doi.org/10.1111/andr.12389.
Received 24.08.2020
Accepted 07.10.2020
About the Authors
Vladimir A. Bozhedomov, Doctor of Medical Sciences, Professor, Leading Researcher, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Professor of the Department of Urology and Andrology of the Faculty of Fundamental Medicine and Head of “Men’s Health” Clinic, Medical Scientific and Educational Center, M.V. Lomonosov Moscow State University.Tel.: +7(495)782-65-89. E-mail: vbojedomov@mail.ru. 4, Oparina str., Moscow, 117997, Russia.
Marina A. Nikolaeva, Candidate of Biological Sciences, Leading Researcher, Laboratory of Clinical Imunology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(916)481-19-50. E-mail: nikolaeva_ma@mail.ru. 4, Oparina str., Moscow, 117997, Russia.
Irina V. Ushakova, Candidate of Biological Sciences, Embryologist, the Assisted Reproductive Technologies Department, V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Tel.: +7(985) 928-92-87. E-mail: irisun77@mail.ru. 4, Oparina str., Moscow, 117997, Russia.
Galina E. Bozhedomova, Medical Technologist,”Men’s Health” Clinic, Medical Scientific and Educational Center, M.V. Lomonosov Moscow State University, Polyclinic №3, Administration of the President of the Russian Federation. Tel.: +7(495)782-65-89. E-mail: bozhiedomova@bk.ru.
27/10, Lomonosovsky prospect, Moscow, 119991, Russia; 31, Groholsky per., Moscow, 129090, Russia.
Natalia A. Lipatova, Candidate of Medical Sciences, Clinical Laboratory Diagnosis Physician, Polyclinic №3, Administration of the President of the Russian Federation.
Tel.: +7(985)929-19-65. E-mail: li_na1328@mail.ru. 31, Groholsky per., Moscow, 129090, Russia.
Rimma A. Kamarina, Clinical Laboratory Diagnosis Physician, Polyclinic №3, Administration of the President of the Russian Federation.
Tel.: +7(926)241-91-89. E-mail: Kamarina.rim@yandex.ru. 31, Groholsky per., Moscow, 129090, Russia.
Dmitry A. Okhobotov, Candidate of Medical Sciences, Associate Professor of the Department of Urology and Andrology, Faculty of Fundamental Medicine; Urologist,
”Men’s Health” Clinic, Medical Scientific and Educational Center, M.V. Lomonosov Moscow State University. Tel.: +7 (926) 172-72-90. E-mail: 14072003m@gmail.com.
27/10, Lomonosovsky prospect, Moscow, 119991, Russia.
Armais A. Kamalov, Academician of the Russian Academy of Sciences, Doctor of Medical Sciences, Professor, Director of the Medical Research and Education Center and Head of the Department of Urology and Andrology, M.V. Lomonosov Moscow State University. Tel.: +7(495)531-27-37. E-mail: priemnaya@mc.msu.ru.
27/10, Lomonosovsky prospect, Moscow, 119991, Russia.
For citation: Bozhedomov V.A., Nikolaeva M.A., Ushakova I.V., Bozhedomova G.E., Lipatova N.A., Kamarina R.N., Okhobotov D.A., Kamalov A.A. The structure of defects in sperm quality in men in the infertile couples and the algorithm for their management in level 3 healthcare facilities.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 11: 159-167 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.159-167