С конца XX века во всем мире, в том числе в Российской Федерации, зарегистрирован неуклонный рост числа бесплодных супружеских пар. По данным эпидемиологических исследований в различных регионах России, бесплодие составляет от 8 до 17,8% и имеет тенденцию к дальнейшему росту [1]. За период с 1995 по 2013 г. количество бесплодных пар увеличилось в 1,9 раза в абсолютных числах. Методы вспомогательных репродуктивных технологий (ВРТ) становятся все более востребованными, поскольку позволяют преодолеть бесплодие в браке за относительно короткий временной промежуток [2]. Одним из наиболее эффективных методов ВРТ является экстракорпоральное оплодотворение (ЭКО). По данным регистра Российской ассоциации репродукции человека, число циклов ЭКО в России возросло за последние 15 лет более чем в 10 раз и составило 95 628 циклов в 2014 году [3]. В связи с демографическим спадом и наличием стабильного ежегодного увеличения количества циклов ЭКО в России было принято решение о включении этой процедуры в программу ОМС [4]. В 2017 г. на основании экспертных оценок определена ориентировочная потребность регионов в числе процедур ЭКО, которая составила 1000 циклов процедур ЭКО на 1 млн. чел. населения [5].
Однако эффективность ЭКО как метода лечения бесплодия, по данным мировой статистики, не превышает 40%, и число неудач не снижается [6]. В попытках повысить эффективность программ ВРТ разрабатываются новые препараты гонадотропинов, улучшается степень их очистки, повышается удобство дозирования и доставки препаратов. Продолжаются исследования по сравнению различных протоколов стимуляции овуляции мочевыми и рекомбинантными гонадотропинами, препаратами, содержащими чистый ФСГ и комбинированными препаратами с лютеинизирующим гормоном (ЛГ). В настоящее время убедительных доказательств по преимуществу одних протоколов или видов гонадотропинов над другими в неселективной популяции пациентов не получено [7–14], однако, в отдельных подгруппах пациенток (с «бедным ответом», с синдромом поликистозных яичников, с наружным генитальным эндометриозом, моложе и старше 35 лет) получены данные о возможных преимуществах одних протоколов и видах гонадотропинов над другими [15–21]. Тем не менее, при проведении исследований выбирается определенная группа больных, исключаются или минимизируются дополнительные факторы, способные оказать влияние на анализ результатов, тогда как в рутинной клинической практике пациенты более гетерогенны. Поэтому изучение клинико-экономической эффективности в использовании различных препаратов гонадотропинов в тех или иных протоколах в рутинной клинической практике представляет особый интерес.
В связи с вышеизложенным было проведено наблюдательное исследование с последующим сравнительным фармакоэкономическим анализом эффективности применения рекомбинантного фолликулостимулирующего гормона (р-ФСГ) пролонгированного действия (Элонва) в протоколах ЭКО с антагонистами ГнРГ в сравнении с р-ФСГ (Гонал-Ф, Пурегон) ежедневного введения.
Цель исследования: Оценка целесообразности применения р-ФСГ пролонгированного действия (Элонва) в протоколах ЭКО с антагонистами гонадотропин – рилизинг гормона (ГнРГ) в сравнении с р-ФСГ (Гонал-Ф, Пурегон) ежедневного введения с точки зрения фармакоэкономических преимуществ.
Материалы и методы
Данное исследование представляет из себя комплексное многоэтапное фармакоэкономическое исследование, состоящее из следующих блоков: наблюдательного проспективного исследования с ретроспективным сбором данных из первичной медицинской документации пациенток и фармакоэкономического исследования с применением математического моделирования. Все математические расчеты были произведены в программе Microsoft Excel 2016.
Дизайн наблюдательного проспективного исследования
Проведено многоцентровое наблюдательное исследование с ретроспективным сбором данных пациенток из первичной медицинской документации, прошедших ЭКО в региональных центрах ВРТ как коммерческих, так и государственных на территории РФ с целью оценки клинической эффективности и качества жизни пациенток. В программе приняли участие 15 центров ВРТ из 7 городов РФ: Москва, Санкт-Петербург, Новосибирск, Уфа, Ростов-на-Дону, Тюмень, Екатеринбург. В общей сложности были оценены 613 циклов ЭКО с антагонистами ГнРГ и применением препаратов Элонва, Гонал-Ф и Пурегон. Решение о назначении того или иного препарата принималось непосредственно врачом в зависимости от клинических показаний (возраста, показателей овариального резерва или ожидаемого ответа на стимуляцию, факторов бесплодия). Исследование не предусматривало стороннего вмешательства в лечебный процесс. Сбор данных осуществлялся по индивидуальным регистрационным картам пациентки и стандартизированной валидной форме анкеты опроса для оценки качества жизни пациентки EQ-5D-3L [22].
В качестве основного оценочного критерия клинической эффективности терапии была взята доля пациенток, которым в протоколе ЭКО был произведен перенос эмбриона/бластоцисты. Дополнительно были проанализированы клинические показатели эффективности, такие как число полученных ооцитов и число перенесенных эмбрионов /бластоцист.
Оценка качества жизни производилась 2 методами:
- опросник EQ-5D-3L
- визуально-аналоговая шкала (EQ-VAS)
Данные методы оценки качества жизни носят гипотетический характер. Полученные значения результатов полезности (Utility – полезность или прогностический показатель состояния здоровья) любого из этих методов лежат в интервале от 0,00 до 1,00. Расчет значения качества жизни QALY (Quality-adjusted life years – количество приобретенных в результате медицинского вмешательства лет качественной жизни) рассчитывалось на основе формулы: QALY = Utility × временной интервал, для которого производится расчет (количество лет).
Во время исследования проводилась два замера с разницей в ≈7 дней. Исходя из этого были посчитаны QALY для одной недели. Для пересчета состояний здоровья по опроснику качества жизни использовался ключ перевода в индексы полезности (Utility) на примере Польши, так как для России такого ключа пока не существует.
Дизайн фармакоэкономического исследования
Для сравнительной оценки экономической целесообразности применения препаратов р-ФСГ проводилось кабинетное фармакоэкономическое исследование, состоящее из 3 последовательных этапов: мета-анализа данных, математического моделирования и статистической обработки данных и фармакоэкономический анализ: анализ затрат, анализ «затраты-эффективность», анализ влияния на бюджет и анализ «затраты-полезность» [23,24].
При анализе затрат учитывались только прямые медицинские затраты, связанные с процедурой ЭКО: тариф ОМС на ЭКО, стоимость препаратов р-ФСГ. Для расчетов стоимости прямых медицинских затрат были использованы актуальные тарифы Фонда ОМС РФ за 2018 год, а именно, тарифы на стационарную медицинскую помощь в каждом регионе РФ. Стоимость препаратов была рассчитана на основании данных государственных закупок (http://www.zakupki.gov.ru).
Анализ «затраты-эффективность» в исследовании использовался для сравнения соотношения совокупных затрат на проведение процедуры ЭКО и эффективности для каждого анализируемого препарата.
Анализ влияния на бюджет учитывался только в рамках тарифа ОМС на ЭКО и демонстрировал долю стоимости препарата в составе тарифа ОМС.
Анализ «затраты-полезность» применялся для определения уровня затрат на 1 единицу полезности, который позволяет в большей степени отразить восприятие пациентом важности и ценности для него медицинского вмешательства (в литературе встречается как «точка зрения» пациента).
Результаты исследования
Описание выборки
В анализ включены данные 613 пациенток, которым была проведена стимуляция овуляции разными препаратами р-ФСГ в рамках протоколов ЭКО с антагонистами ГнРГ в 15 региональных центрах ВРТ России (613 циклов ЭКО) с использованием опросников качества жизни (EQ-5D-3L и визуально-аналоговая шкала (VAS)) с мая по июль 2018 года. Средний возраст по всей выборке пациенток составил 33 года (мин. – 19; макс. – 46). Значение индекса массы тела (ИМТ) пациенток в среднем составило 22,7 (±4,4) кг/м². Полученное среднее значение ИМТ согласно интерпретации рекомендаций ВОЗ является нормой.
Пациентки были разбиты на 4 группы в зависимости от применяемого препарата р-ФСГ для стимуляции овуляции: 296 пациенткам в рамках стимуляции овуляции применяли препарат Элонва (средняя дозировка 150 мкг, однократное применение); в дальнейшем в анализе участвовали только 289 пациенток, так как у 7 пациенток не было данных; 224 пациенткам – Гонал-Ф (средняя ежедневная дозировка 200 МЕ, среднее количество дней приема 7,7 и средняя суммарная доза 1540 МЕ); 82 пациенткам – Пурегон (средняя ежедневная дозировка 200 МЕ, среднее количество дней приема 5,6 и средняя суммарная доза 1120 МЕ) и 11 пациенткам – препарат Элонва с добавлением других р-ФСГ. Группа из 11 пациенток (препарат Элонва с добавлением других р-ФСГ) была исключена из дальнейших расчетов в связи с малочисленностью выборки и большой погрешностью данных в сравнении с другими группами. На момент проведения анкетирования среднее количество попыток ЭКО в расчете на 1 пациентку в каждой группе препарата было равнозначным и не превышало 2-х.
 Наиболее частой причиной бесплодия были мужской фактор и бесплодие трубного происхождения. При сравнении групп пациенток по уровню АМГ, который свидетельствует, в том числе об уровне овариального резерва, оказалось, что доля пациенток с низким овариальным резервом (уровень АМГ за последние 6 месяцев <1,2 нг/мл) больше в 2 раза в группе женщин, использующих препарат Элонва (29%), чем в группах, использующих Гонал-Ф (15%) и Пурегон (11%). Также среднее количество антральных фолликулов в расчете на пациентку в группе Элонва (9,4) меньше чем в группах сравнения (Гонал-Ф – 12,9; Пурегон – 11,6). Такие входные данные способствуют более «бедному ответу» на стимуляцию овуляции в группе препарата Элонва.
Наиболее частой причиной бесплодия были мужской фактор и бесплодие трубного происхождения. При сравнении групп пациенток по уровню АМГ, который свидетельствует, в том числе об уровне овариального резерва, оказалось, что доля пациенток с низким овариальным резервом (уровень АМГ за последние 6 месяцев <1,2 нг/мл) больше в 2 раза в группе женщин, использующих препарат Элонва (29%), чем в группах, использующих Гонал-Ф (15%) и Пурегон (11%). Также среднее количество антральных фолликулов в расчете на пациентку в группе Элонва (9,4) меньше чем в группах сравнения (Гонал-Ф – 12,9; Пурегон – 11,6). Такие входные данные способствуют более «бедному ответу» на стимуляцию овуляции в группе препарата Элонва.
Анализ эффективности
Анализ эффективности проводился в группе пациенток, не имевших отмены переноса эмбрионов и осложнений ЭКО. Показатели количества полученных ооцитов, перенесенных эмбрионов у пациенток в группе Элонва уступают таковым в группах сравнения (таблица 1), что является закономерным, с учетом того, что в группу Элонва попало большее число пациенток с низким овариальным резервом, что послужило причиной и более «бедного ответа» на стимуляцию овуляции. Анализ клинических показателей ЭКО из таблицы 1 был произведен только по пациенткам, которым был произведен перенос эмбриона.
Таким образом, перенос эмбрионов проведен у 75% (216 из 289) пациенток, использующих монотерапию препаратом Элонва, тогда как при назначении Гонал-Ф – в 67% случаев (150 из 224 пациенток), Пурегон – в 70% случаев (57 из 82 пациенток).
Основной причиной отмены переноса эмбрионов стал риск развития синдрома гиперстимуляции яичников (СГЯ), он составил 57% (94 пациентки из 165) от всех случаев отмены. Другими причинами стали: отсутствие эмбрионов, пригодных для переноса (19%), результаты преимплантационной генетической диагностики (6%). Риск развития СГЯ чаще всего отмечался в группе на препарате Пурегон – 23% случаев (19 из 82 пациенток); в группе на препарате Гонал-Ф риск развития СГЯ был отмечен в 19% случаев (43 из 224 пациенток), в группе на препарате Элонва – всего в 11% (32 из 296) пациенток. Подтвержденный диагноз СГЯ был отмечен у 23% (3 из 19) пациенток в группе на препарате Пурегон; 58% (25 из 43) пациенток – в группе на препарате Гонал-Ф; 44% (14 из 32) пациенток – в группе на препарате Элонва.
В общей сложности у 7% (45 из 602) пациенток были зафиксированы случаи осложнения ЭКО, где в подавляющем большинстве были диагностированы СГЯ (93% от всех случаев) и кровотечение после пункции фолликулов (7% от всех случаев). В группе на препарате Элонва осложнения у пациенток возникали в 5% случаев, что сравнимо с группой на препарате Пурегон (4%) и в 2,4 раза реже, чем в группе Гонал-Ф (12%). (рис. 1). Из всех диагностированных случаев СГЯ, только одной пациентке, использующей терапию препаратом Гонал-Ф, понадобилось стационарное лечение, в остальных случаях для коррекции осложнения назначалось амбулаторное и консервативное лечение.
По результатам проведенного анализа, была выдвинута гипотеза о том, что применение препарата Элонва в пересчете на одну пациентку более эффективно с точки зрения затрат, с учетом показателя эффективности – числа пациенток без осложнений, прошедших процедуру ЭКО с переносом эмбриона/бластоцисты, что необходимо было подтвердить фармакоэкономическим анализом.
 Оценка качества жизни
Оценка качества жизни
Во время стимуляции овуляции ухудшение качества жизни было отмечено у 24–31% пациенток по методу опросника EQ-5D-3L (обработка результатов опросника проводилась по валидированной методике по совокупности данных) или у 21–28% пациенток по методу визуально-аналоговой шкалы (EQ-VAS обработка результатов опросника проводилась по валидированной методике по совокупности данных) во всех группах сравнения. У большинства же пациенток (60–70%) не было отмечено изменения качества жизни. Улучшение качества жизни было отмечено у 5–9% пациенток во всех группах. При усреднении показателей изменения качества жизни по методу опросника EQ-5D-3L по группам препаратов, в группе Элонва было отмечено снижение качества жизни на 1,7 процентных пункта от изначального, в группах Гонал-Ф и Пурегон – снижение на 2,7 и 1,5 процентных пункта соответственно. По методу EQ-VAS в группе Элонва было отмечено снижение качества жизни всего на 1,3 процентных пункта, тогда как в группах с р-ФСГ ежедневного введения снижение составило 2,3 процентных пункта (2,2 и 2,5 в группах Гонал-Ф и Пурегон соответственно) (рис. 2).
Оба используемых метода оценки качества жизни являются валидными. Однако, оценка изменения качества жизни по методике EQ-VAS является наиболее предпочтительной при дальнейших фармакоэкономических расчетах с точки зрения наличия наиболее значимых различий по показателю изменения качества жизни между сравниваемыми препаратами, а также, по причине того, что метод EQ-VAS, несмотря на то, что является более субъективным методом оценки, является более чувствительным методом изменения, что особенно важно при ультракоротком временном интервале замера изменения качества жизни.
Анализ затрат
В соответствии с постановлением Правительства РФ (приказ Министерства здравоохранения РФ от 30 августа 2012 г. № 107н) в рамках базовой программы ОМС застрахованным лицам осуществляются мероприятия по применению ВРТ (ЭКО). На основании данных наблюдательного исследования, основным источником финансирования ЭКО для пациенток является ФОМС (66% пациенток). В государственном секторе прямые медицинские затраты находятся на уровне тарифа ОМС на ЭКО. Согласно классификации, принятой при составлении тарифов ОМС, процедуру ЭКО можно разделить на следующие условные этапы:
I этап ЭКО: стимуляция суперовуляции;
I–III этапы ЭКО: стимуляция суперовуляции, получение ооцитов, экстракорпоральное оплодотворение и культивирование эмбрионов с последующей криоконсервацией эмбрионов;
I–IV этап ЭКО: полный цикл экстракорпорального оплодотворения с переносом эмбрионов в полость матки и последующей криоконсервацией эмбрионов.
В таблице 2 представлены тарифы ОМС на ЭКО в зависимости от региона.
Стоимость препаратов была рассчитана на основании базы данных государственных закупок за период январь 2017 – август 2018. Стоимость рассчитывалась с учетом дозировки на каждую пациентку в группе и длительности приема препарата (таблица 3). В случае применения препарата Элонва для получения клинического эффекта было достаточно однократного введения, без последующего применения других р-ФСГ. Анализ затрат показывает, что итоговая стоимость препарата Элонва ниже препарата Гонал-Ф на 20% и препарата Пурегон – на 5%.
Анализ «затраты-эффективность»
Так как в тариф ОМС на ЭКО включена лекарственная терапия, в том числе стоимость препаратов р-ФСГ, то анализ «затраты-эффективность» проводился на основании прямых медицинских затрат: по стоимости тарифа ОМС на законченный случай процедуры ЭКО и отдельно – по стоимости курса лечения препаратами р-ФСГ. Расчет показателей «затраты-эффективность» – CER (cost-effectiveness ratio) был произведен по каждому региону, где проводилось наблюдательное исследование (таблица 4).
Для расчетов был взят полный тариф ОМС (I–IV этапы). В среднем разброс стоимости региональных тарифов от усредненного значения находится в пределах 20%, как в большую, так и в меньшую сторону.
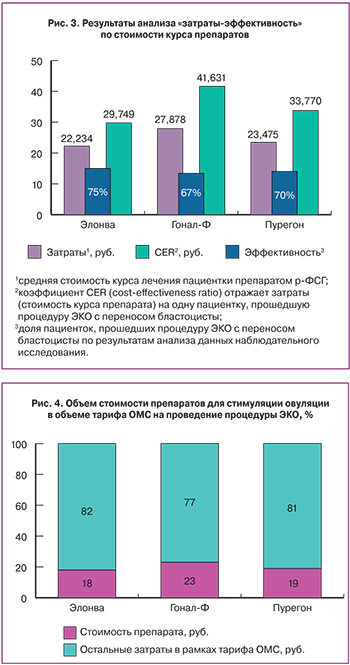
Анализ «затраты-эффективность» показывает, что препарат Элонва способствует наиболее эффективному использованию тарифов ОМС на ЭКО среди других препаратов р-ФСГ. На один случай переноса бластоцисты при использовании препарата Элонва, тариф ОМС будет затратоэффективней на 10% в сравнении с препаратом Гонал-Ф и на 7% – в сравнении с препаратом Пурегон.
Отдельно проведенный анализ «затраты-эффективность» по стоимости препаратов р-ФСГ, показывает, что препарат Элонва является наиболее экономичным и эффективным среди сравниваемых препаратов р-ФСГ. На один случай переноса бластоцисты стоимость препарата Элонва на 29% дешевле в сравнении с препаратом Гонал-Ф и на 12% – в сравнении с препаратом Пурегон (рис. 3).
Анализ влияния на бюджет
Анализ влияния на бюджет проводился только в рамках тарифа ОМС на проведение процедуры ЭКО и демонстрирует какую долю занимает каждый из исследуемых препаратов р-ФСГ в объеме тарифа (рис. 4). Средний тариф ОМС по включенным в анализ регионам составляет – 122 993 руб.
В тарифе ОМС по ЭКО препарат Элонва имеет наименьшую долю (18%), в то время как Гонал-Ф занимает наибольшую (23%).
Анализ «затраты-полезность»
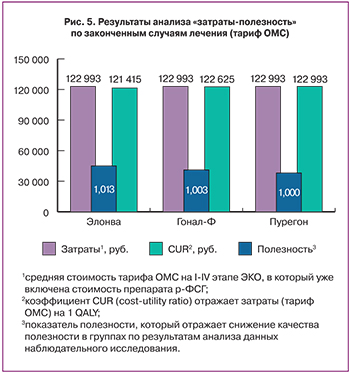
Анализ «затраты-полезность» показывает, что препарат Элонва экономичнее Гонала-Ф и Пурегона, поскольку расходы на один QALY при его применении на 2% и на 1,2% соответственно ниже таковых при использовании препаратов сравнения, что способствует наиболее эффективному использованию тарифа ОМС на ЭКО среди других препаратов р-ФСГ (рис. 5).
Выводы
Все препараты р-ФСГ в процедуре ЭКО в 98% случаев использовались в качестве монотерапии, без дополнительных инъекций других р-ФСГ. При этом доля пациенток, прошедших процедуру ЭКО с переносом эмбриона/ бластоцисты в группе на препарате Элонва (75%) оказалась выше, чем в остальных группах, даже при условии, что пациентки этой группы отличались более низкими показателями овариального резерва и более «бедным ответом» на стимуляцию овуляции.
Применение препарата Элонва в меньшей степени ухудшает качество жизни пациенток по сравнению с препаратом Гонал-Ф (по опроснику EQ-5D-3L и шкале EQ-VAS) и Пурегон (по шкале шкалы EQ-VAS).
Итоговая стоимость применения препарата Элонва ниже стоимости курса препарата Гонал-Ф на 20% и курса препарата Пурегон – на 5%.
Препарат Элонва является наиболее эффективным с точки зрения вложения затрат среди анализируемых препаратов р-ФСГ, и при этом достаточно однократного введения препарата для получения клинического эффекта. В расчете на один случай переноса бластоцисты стоимость препарата Элонва на 29% экономичнее в сравнении со стоимостью препарата Гонал-Ф и на 12%– в сравнении со стоимостью препарата Пурегон.
Препарат Элонва в сравнении с другими препаратами р-ФСГ способствует более эффективному расходованию средств ОМС: на один случай переноса бластоцисты при применении препарата Элонва без необходимости дополнительного приема других р-ФСГ, тарифы ОМС будут использованы эффективнее на 10%, в сравнении с применением препарата Гонал-Ф и на 7% – в сравнении с применением препарата Пурегон.
В анализе влияния на бюджет в рамках тарифа ОМС на проведение процедуры ЭКО препарат Элонва имеет наименьшую долю в стоимости тарифа (18%).
Анализ «затраты-полезность» показывает, что затраты на один QALY при применении препарата Элонва ниже на 1%, чем при применении препарата Гонал-Ф и ниже на 1,3%, чем при применении препарата Пурегон, что позволит эффективнее использовать средства ОМС (в рамках оплаты тарифа по ЭКО).



