Comparative analysis of techniques for diagnosing retrocervical endometriosis
Objective. To estimate the diagnostic informative value of instrumental examinations in patients with retrocervical endometriosis (RCE).Almova I.K., Khilkevich E.G., Chuprynin V.D., Balashov I.S., Gus A.S., Bychenko V.A., Matronitsky R.B., Serov V.N.
Subjects and methods. According to the extent of RCE and a concurrence of pelvic organ diseases, 120 patients were divided into 4 subgroups: IA) 30 patients with RCE; IB) 30 patients with RCE and with colon involvement; IC) 30 patients with RCE and endometrioid ovarian cyst; ID) 30 patients with RCE concurrent with uterine myoma. The extent of RCE was determined according to the data of gynecological examination, transvaginal ultrasound (TVUS), MRI, magnetic resonance imaging (MRT), colonoscopy, laparoscopy, and histological examination of surgical material.
Results. TVUS was more informative in endometrioid ovarian cysts (95%) and intestinal lesions (87.3%) than in the isolated form of RCE (68.75%). MRI had the highest diagnostic value in identifying endometrioid infiltrates spreading to the intestinal wall (98.6%), or ovarian lesions (97.4%). The sensitivity of TVUS and MRI in uterine fibroids concurrent with RCE was 36.6% and 89.6%, respectively. Both TVUS and MRI in unobvious pelvic adhesions were uninformative (29.13% and 59.23%, respectively).
Conclusion. Only laparoscopy enables full visualization of a pathological focus and access to organs. One should not deemphasize the role of preoperative examination including TVUS, MRI, and colonoscopy. Each of the techniques has its own diagnostic value and does not replace, but provides additional information.
Keywords
Endometriosis is a disease characterized by the presence of endometrium-like epithelium and stroma outside the endometrium and myometrium [1–5]. The term “extensive endometriosis” implies a massive and deep location of the ectopic endometrial implants, usually in the Douglas pouch, anterior rectal wall, posterior uterine wall, uterus, and uterosacral ligaments. This leads to obliteration of the recto-uterine pouch with a change in its anatomy [1, 6]. It should be noted that the endometriotic infiltration of retrocervical adipose tissue (infiltrative form) is extremely rare as an independent localization, and usually combined with endometriotic lesions of the pelvic peritoneum, ovaries and/or adenomyosis, involving the bowel and urinary tract [7].
Gynecological examination remains the first-line diagnostic modality for retrocervical endometriosis (RCE) [8]. The early diagnosis of RCE is complex because the clinical presentation is highly variable, and available imaging techniques, including transvaginal ultrasound (TVUS) and magnetic resonance imaging (MRI), have low sensitivity for detection of RCE at the early stages [9–12]. At present, specific immunohistochemical, molecular, and morphological screening tests for endometriosis do not exist [9, 13, 14].
This study aimed to investigate the diagnostic performance of instrumental diagnostic techniques in patients with RCE.
Material and methods
From 2016 to 2018, a total of 120 reproductive-age patients received surgical interventions for RCE at the Department of Surgery (Head - V.D. Chuprynin, Ph.D.) of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Based on the extent of RCE and its combinations with endometriotic lesions in pelvic organs, 120 patients were divided into 4 subgroups: subgroup IA of patients with RCE (n = 30); subgroup IB of patients with RCE and colon endometriosis (n = 30); subgroup IC of patients with RCE and endometriotic ovarian cysts (n = 30); subgroup ID of patients with RCE and concomitant uterine fibroids (n = 30).
All patients were enrolled in the study after providing informed consent that was recorded according to the standards of the Ethics Committee of the Ministry of Health of the Russian Federation. The study was approved by the Expert Commission on Medical Ethics of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Inclusion criteria were as follows: patients aged 18–45 years, informed consent to participate in the study, the presence of RCE.
Exclusion criteria: infectious diseases, malignant neoplasms, acute inflammatory diseases of the pelvic organs, severe extragenital pathology, previous six-month hormonal therapy for endometriosis.
The extent of RCE was estimated by gynecological examination, TVUS, MRI, colonoscopy, laparoscopy, and histological examination of biopsy specimens.
TVUS of pelvic organs was performed at the Department of Functional Diagnostics of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia (Head - Professor Gus A.I., Dr.Med.Sci.) using Aloka ProSound Alpha 10 (Japan) and Toshiba Xario (Japan) with transabdominal and transvaginal 3.5 and 5.0 MHz probes.
MRI was performed in the Department of Radiology (Head - Bychenko V.G., Ph.D.) of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia according to the standard technique on Magnetom Verio MRI 3 T scanner (Siemens Medical Systems, Germany) and Signa HDxT 1.5T MRI scanner (General Electric Medical Systems, USA).
Surgical interventions were performed under general endotracheal anesthesia using a standard technique with a patient in the Trendelenburg position.
All patients underwent hysteroscopy for diagnostic and treatment purposes using a Karl Storz rigid hysteroscope Hamou I (30°) and Hopkins II (30°) (Germany) with a 5 mm outer diameter.
A pathomorphological examination was carried out at the Department of Anatomic Pathology of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia (Head - Professor A.I. Shchegolev).
Data were entered in a specially designed spreadsheet-based form. Statistical analysis was performed using the Statistica V10, SPSS Statistics 22, and R v.3.5. statistical packages.
Quantitative variables were expressed as the median (Me) and interquartile range (IQR). The statistical significance of between-group differences for continuous variables was tested with the Mann–Whitney test; Kruskal-Wallis was used to test quantitative parameters between more than two groups. Categorical variables were reported as counts and proportions (%) and compared using the χ² test; the Fisher exact test was used if the expected count for a cell table was less than 5. The critical level of significance when testing statistical hypotheses was considered at p <0.05. Performance characteristics of diagnostic tests were calculated using 2x2 contingency tables.
Results
The mean age of RCE patients in 4 subgroups was 33.5 (30.0, 37.0) years ranging from 21 to 45 years; there were no significant age differences between the subgroups (p = 0.06). Findings of TVUS, MRI, colonoscopy, and laparoscopy were compared depending on the extent of RCE, the degree of endometriotic infiltration of pelvic organs, and the severity of adhesion.
Ultrasonography
Early stages of RCE are not easy to diagnose by ultrasound scanning because retrocervical lesions are dense and have indistinct margins.
 On TVUS, RCE appears as semilunar or oval hypoechoic nodular formations measuring 1.0 to 4.5 cm. The most characteristic sonographic features of endometriotic lesions were as follows: dense masses in rectovaginal adipose tissue, located both retrocervically and eccentrically to the cervix with local tenderness; the masses had heterogeneous echotexture, blurred margins, irregular outer contours and sizes ranging from 0.8 to 4.5 cm. Hypoechoic, mildly hyperechoic, and hyperechoic lesions were detected in 78/115 (67.8%), 31/115 (26.9%), and 9/115 (7.8%) patients, respectively.
On TVUS, RCE appears as semilunar or oval hypoechoic nodular formations measuring 1.0 to 4.5 cm. The most characteristic sonographic features of endometriotic lesions were as follows: dense masses in rectovaginal adipose tissue, located both retrocervically and eccentrically to the cervix with local tenderness; the masses had heterogeneous echotexture, blurred margins, irregular outer contours and sizes ranging from 0.8 to 4.5 cm. Hypoechoic, mildly hyperechoic, and hyperechoic lesions were detected in 78/115 (67.8%), 31/115 (26.9%), and 9/115 (7.8%) patients, respectively.
In patients of IA subgroup, the mean diameter of retrocervical endometriotic lesions as measured by TVUS and laparoscopy was 2.2 cm and 2.1 cm, respectively; the results are presented in Fig. 1 (p = 0.001).
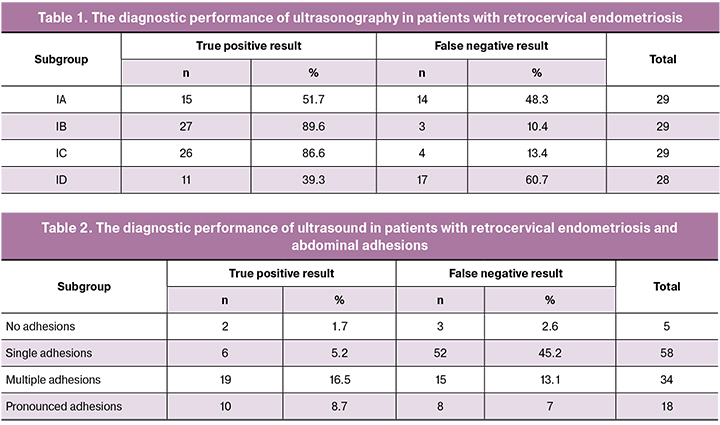
In patients with an isolated form of RCE (subgroup IA), TVUS had a sensitivity of 68.75%, specificity of 91.12%, positive predictive value of 76.77%, and negative predictive value of 87.11%.
The typical sonographic appearance of endometriotic bowel lesions is an anechoic oval nodule measuring 0.5 to 3.0 cm in diameter and 0.6 cm to 3.0 cm in length (Fig. 2, a, b).

During TVUS in the area of retrocervical lesions, 21/29 (72.4%) patients in IB subgroup indicated that tenderness was evoked by the pressure of the transvaginal probe.
TVUS in patients of IB subgroup had a sensitivity of 87.3%, specificity of 74.6%, positive predictive value of 77.8%, and negative predictive value of 56.8%.
A discrepancy between TVUS and surgical diagnosis occurred in 3 (10.4%) patients. A false-negative result was detected in cases of adhesive obliteration of the Douglas pouch and fixation of the anterior rectal wall.
In patients of IB subgroup, the mean diameter of retrocervical endometriotic lesions as measured by TVUS and laparoscopy was 3.0 cm and 5.1 cm, respectively; the results are shown in Fig. 3 (p = 0.001).
Sonographic features of endometriotic cysts in patients of subgroup IC were presented as tumor-like formations located posteriorly or along the uterine side, a thick-walled capsule and a fine intra-cystic suspension.
In 26/29 (86.6%) patients, the diagnosis of endometriotic cysts made by TVUS was confirmed by laparoscopy. However, there was a discrepancy between preoperative ultrasonography findings and surgical diagnosis in 4/29 (13.4%) patients who had a false-negative diagnosis. In these patients, the endometriotic ovarian cysts were interpreted as functional cysts, and the size of the lesions was less than 2.0 cm.
 As shown in fig. 4 comparing the findings of TVUS and laparoscopy, the mean diameter of endometriotic cysts as measured by TVUS and laparoscopy was 4.1cm and 6.8 cm, respectively(p = 0.001).
As shown in fig. 4 comparing the findings of TVUS and laparoscopy, the mean diameter of endometriotic cysts as measured by TVUS and laparoscopy was 4.1cm and 6.8 cm, respectively(p = 0.001).
The assessment of the diagnostic accuracy of TVUS for detecting RCE in combination with endometriotic ovarian cysts (subgroup IC) showed a sensitivity of 95%, specificity of 93.18%, positive predictive value of 89.13%, and negative predictive value of 78, 57%.
In patients with RCE and concomitant uterine fibroids (ID subgroup), the discrepancy between TVUS findings and surgical diagnosis occurred in 17/28 (60.7%) patients. A false-negative diagnosis was made in cases of multiple fibroids, which probably made it difficult to visualize RCE lesions.
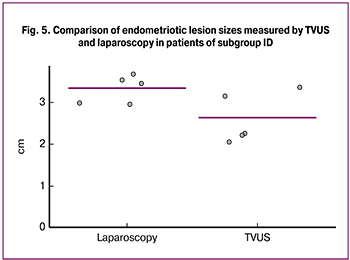
In patients of ID subgroup, the mean diameter of retrocervical endometriotic lesions as measured by TVUS and laparoscopy was 2.6 cm and 3.3 cm, respectively (p = 0.001); the results are shown in Fig. 5.
The sensitivity of TVUS for the detection of RCE in patients of the ID subgroup was 36.6%, the specificity, positive predictive value, and negative predictive value were 67.82%, 77.16%, and 66.72%, respectively.
The results of TVUS for all (IA-ID) subgroups are presented in Table. 1.
The diagnostic performance of ultrasonography in patients varied depending on the location of endometriotic lesions. So, the diagnostic performance of TVUS for the detection of endometriotic ovarian cysts and bowel lesions was higher than for isolated forms of RCE.
As it is known, endometriosis causes inflammation of the surrounding tissues, which leads to the formation of scar tissue, both thin, dense, and multiple. In our study, the abdominal adhesions were classified according to the system developed by D.N. Blacenko in 1956.
The analysis of the diagnostic performance of ultrasonography for detecting abdominal adhesions in patients with RCT is presented in Table. 2.
The sensitivity of TVUS for detecting adhesions in patients with RCE was 29.13%; specificity, positive predictive value, and negative predictive value were 60.0%, 91.67%, and 3.12%, respectively.
In our study, TVUS was found to have sufficient diagnostic performance for the detection of RCE involving the colon (IB subgroup) and in combination with endometriotic ovarian cysts (IC subgroup). However, detecting isolated RCE (IA subgroup) and RCE in combination with fibroids (ID subgroup) proved to be the most difficult. The diagnostic performance of TVUS for detecting pelvic adhesive process was low.
These observations confirm the need for using additional diagnostic modalities to guide pre-surgical planning in patients with RCE.
Magnetic resonance imaging
Patients with RCE had endometriotic infiltrates in the recto-uterine pouch with the involvement of the cervix (Figure 6a and b).
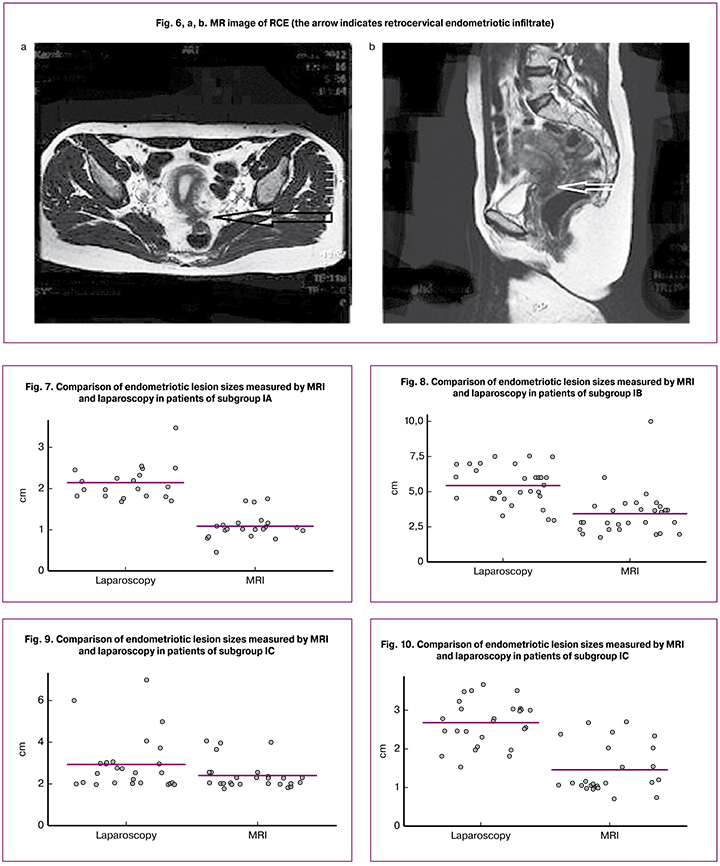
In our study, 110 (91.7%) patients with RCE underwent MRI. RCE lesions typically have various MRI appearances, including infiltration of the posterior uterine wall, broad uterine ligament, uterosacral ligament, and peritoneum of the Douglas pouch spreading to the bowel wall with or without signs of infiltration.
Findings of MRI and laparoscopy were compared depending on the extent of RCE, the degree of endometriotic infiltration of pelvic organs, and the severity of adhesion.
In patients of subgroup IA, the mean diameter of endometriotic lesions as measured by MRI and laparoscopy was 1.1cm and 2.1 cm, respectively (p = 0.001); the results are presented in Fig. 7 (p = 0.001).
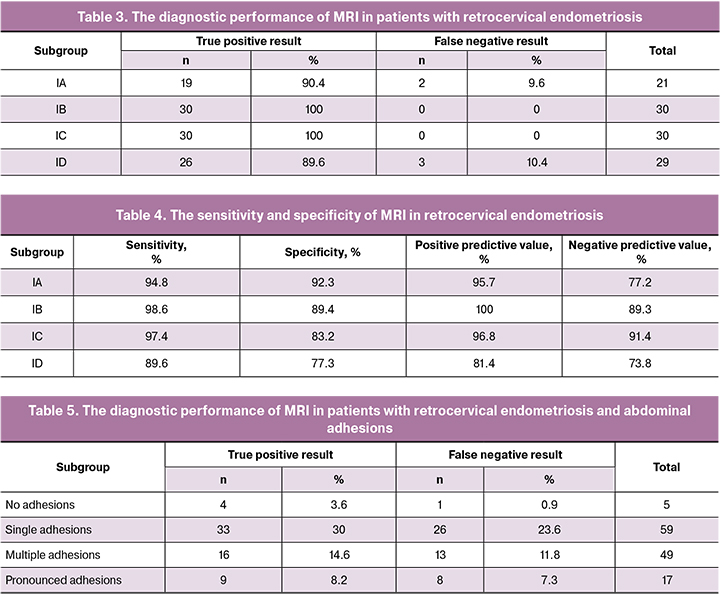
For the isolated form of RCE (IA subgroup), MRI had a sensitivity of 94.8%, a specificity of 92.3%, positive predictive value of 85.7%, and a negative predictive value of 77.2%.
When endometriotic infiltrate spreads to the bowel wall (IB subgroup), endometriosis was visualized as an irregular mass measuring from 1.0 to 4.8 cm in diameter. Evaluation of the endometriotic invasion of the bowel showed that submucosal, mucosal and muscle layers were affected in 6, 8, and 11 patients, respectively. The mean size of endometriotic infiltrates as measured by MRI and laparoscopy was 3.4 cm and 5.4 cm, respectively; the data are presented in Fig. 8 (p = 0.001).
In this subgroup, MRI had a sensitivity of 98.6%, a specificity of 89.4%, positive predictive value of 100.0%, and a negative predictive value of 89.3%.
In patients with endometriotic ovarian cysts (subgroup IC), the MRI scan shows complex cystic masses, often thick-walled, and an MR signal from the contents characteristic of hemoglobin biodegradation products.
As shown in fig. 9, the mean size of the endometriotic lesion in patients (subgroup IC) as measured by MRI and laparoscopy was 3.4 cm and 5.4 cm, respectively. These findings indicate that MRI is an accurate method for identifying pathological lesions, but the extent of endometriotic lesions and their exact sizes can only be determined intraoperatively.
It should be noted that in all patients with endometriotic cysts, MRI data were confirmed by laparoscopy.
The evaluation of the diagnostic accuracy of MRI for detecting RCE in combination with endometriotic ovarian cysts (subgroup IC) showed a sensitivity of 97.4%, specificity of 83.8%, the positive predictive value of 98.8%, and negative predictive value of 91.4%.
In patients with RCE in combination with uterine fibroids (subgroup ID), a discrepancy between MRI and surgical diagnosis occurred in 3/29 (10.4%) patients. The mean diameter of endometriotic lesions, according to MRI and laparoscopy was 1.4 cm and 2.6 cm, respectively (p = 0.001); the results are shown in Fig. 10.
The sensitivity of MRI for detecting RCE in patients of subgroup ID was 89.6%; specificity, positive predictive value, and negative predictive value were 77.3%, 81.4%, and 73.8%, respectively.
However, according to our results, 10.4% (3/29) patients with RCE in combination with uterine fibroids and 9.6% (2/21) patients with an isolated form of REC had false-negative results on MRI, respectively. The analysis of the results is presented in Tables 3 and 4.
As shown in fig. 11, the mean size of retrocervical lesions in patients of the entire study cohort as measured by MRI and laparoscopy was 2.1 cm and 3.4 cm, respectively (p = 0.001).
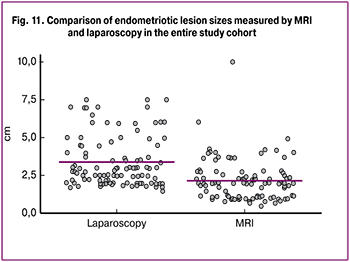 We performed an analysis of the diagnostic performance of MRI for detecting abdominal adhesions in patients with RCE; the data are presented in Table. 5.
We performed an analysis of the diagnostic performance of MRI for detecting abdominal adhesions in patients with RCE; the data are presented in Table. 5.
The sensitivity of MRI for detecting abdominal adhesions in patients with RCE was 59.23%; the specificity, positive predictive value, and negative predictive value were 20%, 94.44%, and 2.08%.
As a result of the study, we revealed a coincidence of the intraoperative diagnosis with the results of MRI in patients of subgroups IB and IC. The greatest diagnostic value of MRI is in the detection of endometriotic infiltrates spreading to the bowel wall with or without ovarian involvement, in which the accuracy of the diagnostic test was 100%. The indisputable advantage of MRI is accuracy and high resolution, which allows the determination of the nature of the pathological formation, localization, and the extent of the pathological process. Pelvic MRI allows the infiltration of the muscular/serosal layers of the bowel wall to be detected. This enables timely and less traumatic surgery while avoiding complications such as bowel stenosis.
Thus, the most informative non-invasive diagnostic modality is MRI, which, due to its high resolution, provides excellent visualization of the pelvic organs and their structure. However, in patients with pelvic adhesions, both TVUS and MRI are uninformative. Of great diagnostic value is the method with multiple and pronounced adhesions of the small pelvis. This is important because it is well known that pelvic adhesions are an indirect sign of pelvic endometriosis.
Colonoscopy
All patients in subgroup IB (n = 30) underwent diagnostic colonoscopy taking into account clinical manifestations, findings of TVUS, and pelvic MRI. In all cases, a colonoscopy was performed for the first time and was accompanied by a biopsy of suspicious lesions.
When characterizing the endoscopic picture, attention was paid to the presence of an infiltrate protruding into the bowel lumen, narrowing of the lumen or colon rigidity in the affected area.
Analysis of colonoscopy findings showed that in 7 (23.3%), 9 (30%), and 8 (26.7%) patients endometriotic lesions were located in the upper rectal ampulla, the recto-sigmoid part of the rectum, and the sigmoid colon, respectively. In 6 (20%) patients, colonoscopy was not informative in the diagnosis of endometriosis.
According to colonoscopy, endometriotic lesions infiltrated the muscular-serosal, submucosal and mucosal layers of the colon in 6 (20%), 6 (20.0%), and 11 (36.7%) patients, respectively.
The discrepancy of intraoperative data with the results of colonoscopy was noted mainly in patients with lesions infiltrating muscular-serosal and submucosal layers of the colon; the results are presented in Table 6.

We also analyzed direct and indirect colonoscopic signs depending on the location and depth of the colon endometriosis. Direct signs included polyp-like growths in the area of the endometriotic lesion, focal retraction of the colon mucosa with a cyanotic bottom. Direct signs were confirmed by morphological examination of biopsy specimens. Indirect signs included submucosal lesions in the bowel wall with or without the lumen stenosis, a change in the mobility of the mucosa over the lesion, and a decrease in vascularization.
As presented in the table. 7, the colonoscopic assessment was equally accurate for detecting both direct and indirect signs of colon endometriosis (up to 100%) in case of mucosal involvement of the upper rectal ampulla and the recto-sigmoid part of the rectum. However, 14.29% of patients with endometriosis affecting the mucosa of the sigmoid colon had false-negative results.
Colonoscopy was highly effective in detecting direct signs of submucosal involvement (100%), but not effective in detecting endometriosis affecting the muscular-serosal layer of the colon.
For detecting bowel endometriosis, colonoscopy had a sensitivity of 80%, specificity of 95%, positive predictive value of 45.76%, and negative predictive value of 16.37%.
Despite the invasive nature of colonoscopy, it provided accurate information regarding the extent and depth of endometriotic invasion enabling decision-making and choice of optimal surgical intervention.
Laparoscopic assessment does not present problems in visualizing RCE and uterine fibroids. RCE was seen as1.0 to 3.0 cm lesions surrounded by dense sclerotic tissue located within the rectovaginal septum.
There is no doubt in the importance and accuracy of the laparoscopic diagnosis of RCE. However, the final diagnosis should be confirmed by histological examination of surgical specimens.
Discussion
In this study, we used well-known diagnostic tests for endometriosis, including TVUS, pelvic MRI, and colonoscopy. A more detailed examination of the patients was undertaken to clarify the depth and extent of RCE invasion, and topographic-anatomical relationships between pelvic organs. We analyzed the diagnostic performance of each diagnostic modality comparing them with each other and with intraoperative findings. In all cases, the diagnosis was confirmed by histological examination of the surgical specimens.
The obtained acoustic characteristics of endometriotic cysts and retrocervical endometriotic infiltrates are well known and described in the literature [15–17]. However, TVUS was found to have some limitations in assessing the severity of endometriosis and pelvic adhesions. Our data confirmed that diagnostic performance of ultrasound in patients was different, depending on the size and location of the endometriotic lesions. Thus, TVUS had higher sensitivity for detecting endometriotic ovarian cysts (95%) and bowel lesions (87.3%) than isolated forms of RCE (68.75%). The sensitivity of TVUS for detecting adhesions in patients with RCE was 29.13%; specificity, positive predictive value, and negative predictive value were 60.0%, 91.67%, and 3.12%, respectively.
In our study, TVUS had sufficient prognostic significance for detecting RCE involving the colon (IB subgroup) and in combination with endometriotic ovarian cysts (IB subgroup). However, detecting isolated RCE (IA subgroup) and RCE in combination with fibroids (ID subgroup) using TVUS proved to be the most difficult. The diagnostic performance of TVUS for detecting pelvic adhesions was low.
Currently, it is known that pelvic MRI allows the detection of not only endometriotic infiltrates, but also single lesions on the pelvic peritoneum or serosa, the extent of the colon wall involvement and pelvic adhesions of varying severity [18– 21].
As a result of the study, the intraoperative diagnosis with MRI findings coincided in patients of subgroups IB and IC. MRI had the greatest sensitivity for detecting endometriotic infiltrates involving the bowel wall (98.6%) or endometriotic ovarian cysts (97.4%). Pelvic MRI allows the detection of endometriotic lesions infiltrating the serosal-muscular layer of the bowel wall, which are difficult to identify by colonoscopy. However, TVUS and MRI had the lower sensitivity of 36.6% and 89.6%, respectively, for detecting RCE with uterine fibroids.
Therefore, MRI is the most informative non-invasive diagnostic modality for endometriosis. The most significant advantages are the high tissue contrast, the absence of invisible zones, and independence from the operator.
However, the sensitivity of TVUS and MRI for detecting insignificant pelvic adhesions in patients with RCE was only 29.13% and 59.23%, respectively. The method is of great diagnostic value in patients with multiple and significant pelvic adhesions [22].
Patients in subgroup IB who had RCE with colonic involvement underwent diagnostic colonoscopy accompanied by a biopsy of suspicious lesions.
It should be noted that bowel mucosa involvement was confirmed in 17 women, but only 8/30 (26.6%) patients reported rectal bleeding during menstruation. Bloating before and during menstruation and diarrhea was reported by 18/30 (60%) and 12/30 (40%) patients, respectively. These observations are consistent with the findings of other researchers [23–25].
Currently, the most accurate method for diagnosing external genital endometriosis is a laparoscopy. Modern endoscopic equipment allows visualization, including isolated endometriotic heterotopies, due to multiple image enlargements on the monitor, which is confirmed other researchers [26–27].
Conclusion
A distinctive feature of RCE is its ability to infiltrate underlying organs, the posterior vaginal fornix, and uterosacral ligaments. At more advanced stages, ectopic endometrial implants are located in the bowel and lateral pelvic walls. However, only a laparoscopy provides a comprehensive visualization and access to endometriotic tissue, since the RCE has no clear boundary, capsule, and is capable of infiltrating growth. However, the role of preoperative examination, including TVUS, MRI, and colonoscopy, should not be underestimated. Each of these modalities has its diagnostic value and does not replace others, but provides additional information. Accurate diagnosis of endometriosis to guide pre-surgical planning requires a comprehensive use of pelvic ultrasound, MRI, colonoscopy, and direct visualization of endometrial lesions through laparoscopic procedures with histological verification.
References
- Адамян Л.В., Андреева Е.Н., Аполихина И.А., Беженарь В.Ф. и др. Эндометриоз: диагностика, лечение и реабилитация. Федеральные клинические рекомендации по ведению больных. М., 2013. 65 c. [Adamjan L.V., Andreeva E.N., Apolihina I.A., Bezhenar’ V.F. i dr. Jendometrioz: diagnostika, lechenie i reabilitacija. Federal’nye klinicheskie rekomendacii po vedeniju bol’nyh. M., 2013. 65 c. (In Russian)].
- Challenges in the development of novel therapeutic strategies for treatment of endometriosis / Vanhie A. [et al.]. Expert opinion on therapeutic targets. 2016; 20(5): 593–600. DOI: 10,1517 / 14728222.2016.1118461
- Борисова А.В. Ранняя диагностика наружного генитального эндометриоза и его рецидивов путем определения липидного профиля методом масс-спектрометрии: дис. ... канд. мед. наук: 14.01.01. М., 2017. 192 с. [Borisova, A.V. Rannjaja diagnostika naruzhnogo genital’nogo jendometrioza i ego recidivov putem opredelenija lipidnogo profilja metodom mass-spektrometrii: dis. kand. med. nauk: 14.01.01. M., 2017. 192 s. (In Russian)].
- Чернуха Г.Е., Ильина Л.М., Адамян Л.В., Павлович С.В. Глубокий инфильтративный эндометриоз: послеоперационные рецидивы и возможные пути их профилактики. Акушерство и гинекология. 2015; 8: 39-46.[Chernukha G.E., Ilyina L.M., Adamyan L.V., Pavlovich S.V. Deep infiltrating endometriosis: Postoperative recurrences and possible ways of their prevention. Akusherstvo i Ginekologiya/Obstetrics and gynecology. 2015; 8: 39-46.(In Russian)].
- Ata B., Mumusoglu S., Aslan K., Seyhan A., Kasapoglu I., Avci B., Urman B., Bozdag G., Uncu G. Which is worse? Comparison of ART outcome between women with primary or recurrent endometriomas. Hum Reprod. 2017; 32(7): 1427-31. DOI: 10,1093 / humrep / dex099
- Сонова М.М., Адамян Л.В., Жорданиа К.И., Паяниди Ю.Г. Эндометриоз и рак яичников. Есть ли взаимосвязь? Общие патогенетические черты рака яичников и эндометриоза. Онкогинекология. 2013; 4: 30-40. [Sonova M.M., Adamyan L.V., Zhordania K.I., Payanidi Yu.G. Endometriosis and ovarian cancer. Is there a relationship? the common pathogenetic features of ovarian cancer and endometriosis. Onkoginekologija. 2013; 4: 30-40. (in Russian)].
- Kennedy S., Bergqvist A., Chapron C., et al. ESHRE guideline for the diagnosis and treatment ofendometriosis. Hum Reprod. 2005; 20: 2698–2704. PMID: 15980014 DOI: 10.1093/humrep/dei135
- Evans M.B., Decherney A.H. Fertility and Endometriosis. Clin Obstet Gynecol. 2017; 60 (3): 497-502. doi: 10.3892/etm.2018.6307
- Signorile P.G., Baldi A. Looking for an effective and non-invasive diagnostic test for endometriosis: where are we? Ann Transl Med. 2018; 6(Suppl 2): 106.doi: 10.21037/atm.2018.11.46. PubMed PMID: 30740427; PubMed Central PMCID:PMC6330624.
- Bulun S.E. Endometriosis. N Engl J Med. 2009; 360: 268-79. doi: 10.1056/NEJMra0804690
- Ballard K., Lowton K., Wright J.T. What›s the delay? A qualitative study of women›s experience of reaching a diagnosis of endometriosis. Fertil Steril. 2006; 86: 1296-301. doi:10.1016/j.fertnstert.2006.04.054
- D’Hooghe T.M., Mihalyi A.M., Sisma P., et al. Why we need a noninvasive diagnostic test for minimal to mild endometriosis with high sensitivity. Gynecol Obstet Invest 2006; 62:136-8. doi: 10.1159/000093120
- О D.F., Flores I., Waelkens E., et al. Noninvasive diagnosis of endometriosis: Review of current peripheral blood and endometrial biomarkers. Best Pract Res Clin Obstet Gynaecol 2018; 50: 72-83. doi: 10.1016/j.bpobgyn.2018.04.001
- Gupta D., Hull M.L., Fraser I., et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016; 4: CD012165. doi: 10.1002/14651858.CD012165.
- Exacoustos C., Zupi E., Piccione E. Ultrasound Imaging for Ovarian and Deep Infiltrating Endometriosis. Semin Reprod Med. 2017; 35(1): 5-24. doi: 10.1055/s-0036-1597127. Epub 2017 Jan 11. Review. PubMed PMID: 28076877.
- Reid S., Condous G. Update on the ultrasound diagnosis of deep pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017; 209: 50-54. doi: 10.1016/j.ejogrb.2016.02.040. Epub 2016 Mar 8. Review. PubMed PMID: 27080442.
- van den Bosch T.,, van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract Res Clin Obstet Gynaecol. 2018 Aug; 51: 16-24. doi: 10.1016/j.bpobgyn.2018.01.013. Epub 2018 Feb 14. Review. PubMed PMID: 29506961.
- Guerriero S., Saba L., Pascual M.A., Ajossa S., Rodriguez I., Mais V., Alcazar J.L.Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018 May; 51(5): 586-595. doi: 10.1002/uog.18961. Review. PubMed PMID: 29154402.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017; 108(6): 886-894. doi: 10.1016/j.fertnstert.2017.10.026. Review. PubMed PMID: 29202963.
- Guerriero S., Ajossa S., Minguez J.A., Jurado M., Mais V., Melis G.B, Alcazar J.L. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015; 46(5): 534-45. doi: 10.1002/uog.15667. Review. PubMed PMID: 26250349.
- Guerriero S., Alcázar J.L., Pascual M.A., Ajossa S., Perniciano M., Piras A., Mais V., Piras B., Schirru F., Benedetto M.G., Saba L. Deep Infiltrating Endometriosis:Comparison Between 2-Dimensional Ultrasonography (US), 3-Dimensional US, and Magnetic Resonance Imaging. J Ultrasound Med. 2018; 37(6): 1511-1521. doi: 10.1002/jum.14496. Epub 2017 Nov 30. PubMed PMID: 29193230.
- Abd El-Kader A.I., Gonied A.S., Lotfy Mohamed M., Lotfy Mohamed S. Impact of Endometriosis-Related Adhesions on Quality of Life among Infertile Women. Int J Fertil Steril. 2019; 13(1): 72-76. doi: 10.22074/ijfs.2019.5572. Epub 2019 Jan PubMed PMID: 30644248; PubMed Central PMCID: PMC6334013.
- Tomiguchi J., Miyamoto H., Ozono K., Gushima R., Shono T., Naoe H., Tanaka M., Baba H., Katabuchi H., Sasaki Y. Preoperative Diagnosis of Intestinal Endometriosis by Magnifying Colonoscopy and Target Biopsy. Case Rep Gastroenterol. 2017; 11(2): 494-499. doi: 10.1159/000475751.
- van der Wat J., Kaplan M.D. Modified Virtual Colonoscopy in the Diagnosis and Quantification of Bowel and Disseminated Endometriosis. Surg Technol Int. 2015; 26:19-24. PubMed PMID: 26054986.
- Milone M., Mollo A., Musella M., Maietta P., Sosa Fernandez L.M., Shatalova O, Conforti A., Barone G., De Placido G., Milone F. Role of colonoscopy in the diagnostic work-up of bowel endometriosis. World J Gastroenterol. 2015; 21(16): 4997-5001. doi: 10.3748/wjg.v21.i16.4997. PubMed PMID: 25945014; PubMed Central PMCID:PMC4408473.
- Kavoussi S.K., Lim C.S., Skinner B.D., Lebovic D.I., As-Sanie S. New paradigms in the diagnosis and management of endometriosis. Curr Opin Obstet Gynecol. 2016; 28(4): 267-76. doi: 1.1097/GCO.0000000000000288. Review. PubMed PMID:27306924.
- Liu X., Long Q., Guo S.W. Surgical History and the Risk of Endometriosis: A Hospital-Based Case-Control Study. Reprod Sci. 2016 Sep;23(9):1217-24. doi: 10.1177/1933719116632921. Epub 2016 Feb 25. PubMed PMID: 26919976.
Received 16.09.2109
Accepted 04.10.2019
About the Authors
Almova Indira K., postgraduate student of the Surgery Department, National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79054357317. E-mail: www.gold11@mail.ruKhilkevich Elena G., MD, obstetrician-gynecologist of the Surgery Department, National Medical Research Center of Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954387783. E-mail: e_khilkevich@oparina4.ru.
ORCID ID: 0000-0001-8826-8439
Chuprynin Vladimir D., PhD, Head of the Surgery Department, National Medical Research Center of Obstetrics, Gynecology, and Perinatology named
after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954383575. E-mail: v_chuprynin@oparina4.ru
Balashov Ivan S. – Researcher of Biostatistics Laboratory of National Medical Research Center for Obstetrics, Gynecology and Perinatology named after V.I. Kulakov, 4 Academica Oparina str., Moscow, Russia, 117997, e-mail: fiordmaster@gmail.com
Gus Alexander I. , MD, Head of Functional Diagnostics at the Department of Diagnostic Imaging, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. E-mail: a_gus@oparina4.ru; eLibrary SPIN. code: 1464-2786.
17997, Russia, Moscow, Ac. Oparina str. 4.
Bychenko Vladimir G. , M.D., Ph.D., Head of the Radiology Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation. 4 Oparin str., 117997, Moscow, Russia. E-mail: vbychenko@yandex.ru
Matronitskiy Roman B. , endoscopist doctor of the Surgery Department, National Medical Research Center of Obstetrics, Gynecology, and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.:+7(495)438-77-83. E-mail: r_matronitskiy@oparina4.ru
Serov Vladimir N., MD, Professor, Academician of the Russian Academy of Sciences, Principal Researcher, Research Center for Obstetrics, Gynecology, and Perinatology; President of Russian Society of Obstetricians and Gynecologists. Тel.: +7(495) 438-72-87. E-mail: v_serov@oparina4.ru
For citation: Almova I.K., Khilkevich E.G., Chuprynin V.D., Balashov I.S., Gus A.S., Bychenko V.A,. Matronitsky R.B, Serov V.N. Comparative analysis of techniques for diagnosing retrocervical endometriosis
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (10):129-39. (in Russian).
http://dx.doi.org/10.18565/aig.2019.10.129-139



