Эндометриоз занимает ведущее место среди пролиферативных заболеваний органов малого таза [1].
Около 10% женщин репродуктивного возраста страдают эндометриозом, что составляет 176 млн женщин во всем мире, к тому же эндометриоз выявляется у 30–50% женщин с бесплодием и/или хронической тазовой болью [2].
Эндометриоз – процесс, при котором за пределами полости матки происходит доброкачественное разрастание ткани, по морфологическим и функциональным свойствам подобной эндометрию [3].
В основе патофизиологии заболевания лежит воспалительный процесс, вызванный распространением эндометриальных клеток за пределы слизистой оболочки матки, а также изменения в других физиологических процессах, в том числе повышение клеточной адгезии, пролиферации, инвазии и ангиогенеза, деградация внеклеточного матрикса, нарушение апоптоза, увеличение окислительного стресса, повышение биосинтеза стероидных гормонов [4, 5]. Считается, что в результате данных патофизиологических процессов клетки эндометрия могут выживать и размножаться в эктопических участках [5].
«Золотым» стандартом диагностики наружного генитального эндометриоза (НГЭ) является лапароскопия с последующим гистологическим исследованием удаленных биоптатов [6]. Это связано с тем, что консервативные методы постановки диагноза эндометриоза имеют ограничения, например, для гинекологического осмотра характерно слабое прогнозирующее значение, а визуализационные технологии не позволяют выявить поверхностный перитонеальный эндометриоз [1, 6]. В среднем задержка в постановке диагноза НГЭ составляет 7–11 лет после появления первых клинических симптомов заболевания [4]. В соответствии с консенсусом Всемирного общества по эндометриозу (World Endometriosis Society), создание надежной неинвазивной диагностики НГЭ является одной из приоритетных задач научных исследований в области гинекологии [7].
Несмотря на то, что было предложено более 100 потенциальных биомаркеров эндометриоза, ни один из них не показал диагностической значимости и возможности клинического применения [5, 7].
Недавно группой наших исследователей было проведено метаболомное исследование эндометриоидных тканей различной локализации [8]. Как выяснилось, в эктопическом эндометрии, в отличие от эутопического, уровень липидов 5 различных классов оказался достоверно различным: фосфатидилхолинов (PC 32:1, PC O-36:3, PC 38:7, PC 38:6, PC 40:8, PC 40:7, PC 40:6, PC O-42:1), фосфоэтаноламина (PE O-20:0), сфингомиелина (SM 34:1), диглицерида (DG 44:9) и триглицеридов (TG 41:2, TG 49:4, TG 52:3) [8]. Фосфатидилхолины и сфингомиелин могут быть потенциальными маркерами эндометриоидного процесса, поскольку эти липиды тесно связаны с подавлением апоптоза, окислительным стрессом и малигнизацией клеток [9]. Данные результаты подтверждаются исследованиями иностранных коллег, так Dutta и соавт. [10] также выявили увеличение концентрации фосфатидилхолинов в образцах сыворотки у пациентов с эндометриозом. Кроме того, участие сфинголипидов в патофизиологии эндометриоза было продемонстрировано в работе Lee с соавт. [11], которые выявили изменения метаболизма сфинголипидов в эндометриоидных тканях, сыворотке крови и перитонеальной жидкости больных НГЭ.
В настоящем исследовании был проведен анализ содержания липидов в перитонеальной жидкости и плазме крови больных эндометриозом. Перитонеальная жидкость при эндометриозе имеет свои особенности вследствие непосредственного контакта с очагами и является перспективной биологической средой для поиска различных биомаркеров. Привлекательность использования плазмы крови для диагностики различных заболеваний человека обусловлена главным образом тем обстоятельством, что она наиболее полно отражает фенотип организма и его состояние в конкретный момент времени.
В данном исследовании проанализирован ряд метаболитов, выявленных в эктопическом эндометрии. Рассмотрена их роль в патофизиологии эндометриоза и возможность создания диагностической модели, включающей комбинацию найденных липидов.
Цель данной работы – повышение эффективности диагностики НГЭ методом масс-спектрометрии.
Материал и методы исследования
В исследование включены 100 пациенток с НГЭ и 50 пациенток контрольной группы с миомой матки, которые были обследованы и прооперированы в ФГБУ НЦАГиП им. В.И. Кулакова МЗ РФ. Включение пациенток в клиническое исследование проводилось после получения информированного согласия и протоколировалось по стандартам Этического комитета Российской Федерации.
Критериями включения были: фертильный возраст женщины, гистологически подтвержденный диагноз НГЭ. Для женщин группы сравнения критериями включения были: фертильный возраст пациенток и отсутствие НГЭ по данным лапароскопии.
Критерии исключения были общими для обеих групп: прием гормональных препаратов в течение 6 месяцев до операции, воспалительные заболевания органов малого таза, наличие инфекций передающихся половым путем, сопутствующих гинекологических заболеваний, тяжелой соматической, онкологической и эндокринной патологии (в том числе ожирения).
Возраст больных с эндометриозом варьировал от 22 до 41 года (среднее значение 31±6 лет). Средний возраст женщин группы сравнения (с миомой матки) составил 33±5 лет. Ведущей жалобой больных НГЭ были боли внизу живота как циклического, так и ациклического характера (89% больных). Из всех вариантов болевого синдрома при НГЭ наиболее часто встречалась дисменорея (82% больных). Тазовые боли, не связанные с менструацией, отмечали 75% больных, иррадиация боли в крестец и поясницу имела место у 18% пациенток. Жалобы на бесплодие предъявляли 72% больных НГЭ, причем первичное бесплодие наблюдалось почти в 2 раза чаще (49%), чем вторичное (23%).
Индекс массы тела (ИМТ) женщин с эндометриозом колебался от 18,2 до 28,6 (среднее значение 23,43±1,06), а у женщин контрольной группы варьировал между 18,8 и 29,8 (в среднем 24,1±1,2).
Диагноз НГЭ был выставлен на основании клинико-инструментального обследования, лечебно-диагностической лапароскопии и подтвержден гистологически. Наиболее часто диагностировались эндометриоидные кисты яичников (66% случаев), эндометриоз крестцово-маточных связок (75%), брюшины Дугласова пространства (66%), поверхностный эндометриоз яичников (42%), эндометриоз пузырно-маточной складки (42%), широкой маточной связки (40%), ректовагинальной перегородки (28%). У большинства больных отмечалась сочетанная локализация эндометриоидных очагов (в 80% случаев). В ходе лапароскопической операции оценивалась степень распространения патологического процесса, локализация и размер эндометриоидных гетеротопий, глубина инвазии, а также выраженность спаечного процесса. Стадию наружного НГЭ определяли, используя классификацию Американского общества фертильности (r-AFS) 1996 г. Согласно данной классификации все пациентки были разделены на 3 клинические группы для сравнительного анализа:
- I группа – 50 больных с I–II стадией НГЭ;
- II группа – 50 больных с III–IV стадией НГЭ;
- III группа – 50 женщин без НГЭ, которым была выполнена миомэктомия.
На момент включения в исследование 72% женщин находились в пролиферативной и 28% – в секреторной фазах менструального цикла.
Кровь из вены забирали перед проведением наркоза на операционном столе. Пункцию проводили одноразовым шприцом вместимостью 3 мл, натощак, после 12-часовой голодной диеты. Кровь забирали в вакуумную стерильную пробирку с ЭДТА-натрий (0,5 мл 1,5% раствора на 10 мл крови) и центрифугировали в течение 10 мин при 2500 оборотах для получения плазмы. После чего плазма отбиралась специальной пипеткой и переливалась в стерильную пробирку типа Эппендорф 1 мл.
Аспират перитонеальной жидкости забирали сразу же после вхождения в брюшной полость (до проведения гистероскопии) и также переливали в отдельные стерильные пробирки типа Эппендорф 1 мл. Далее пробирки с биологическими жидкостями немедленно (в операционном блоке) погружались в жидкий азот для предотвращения окисления липидов и транспортировались в лабораторию, где хранились в морозильной камере при температуре -80° С до анализа.
Экстракты липидов получали в соответствии с модифицированным методом Фолча [12]. В ходе данного эксперимента к 200 мл образца добавляли 4 мл смеси хлороформ-метанол (2:1, об./об.), смесь инкубировали в течение 10 мин, фильтровали с использованием фильтровальной бумаги и в полученных раствор добавляли 800 мкл водного раствора NaCl (1 моль/л). Смесь центрифугировали при 3000 об./мин. в течение 5 минут при температуре окружающей среды. Органический нижний слой, содержащий липиды, отбирали и высушивали в потоке азота, затем повторно растворяли в смеси ацетонитрил-2-пропанол (1:1, об./об.) для последующего масс-спектрометрического анализа.
Состав образцов проводили с помощью метода масс-спектрометрии с ионизацией электрораспыления на масс-спектрометре Maxis Impact qTOF (Bruker Daltonics, Бремен, Германия). Масс-спектры получали в режиме положительных ионов в диапазоне m/z 400–1000 со следующими настройками: напряжение на капилляре 4,1 кВ, давление распыляющего газа 0,7 бар, скорость потока осушающего газа 6 л/мин, температура осушающего газа 200oC.
Полученные масс-спектрометрические данные обрабатывали многофакторным методом PLS-DA (дискриминантный анализ с помощью частных наименьших квадратов), осуществляющим классификацию исследуемых образцов [13]. Этот метод позволяет построить статистическую модель на некоторой обучающей выборке, для которой принадлежность каждого образца к той или иной группе известна. Затем каждый новый образец встраивается в уже существующую модель и оценивается принадлежность этого неизвестного образца к какой-либо группе из рассматриваемых. Такой подход дает возможность не только классифицировать образцы, но и выяснить, какие именно ионы, отраженные в масс-спектрах, ответственны за различия в группах.
Липиды, вносящие наибольший вклад в классификацию образцов, идентифицировали по точной массе с помощью базы данных Lipid Maps [14] и по характерным тандемным масс-спектрам.
Результаты исследования
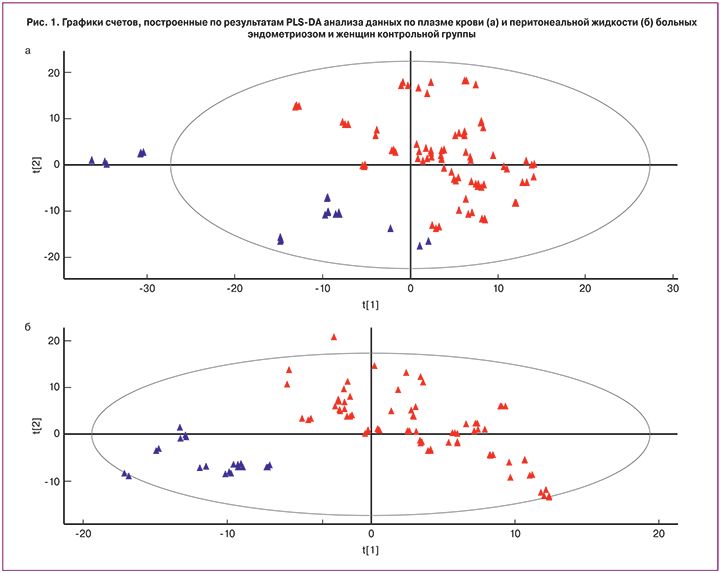
В масс-спектрах положительных ионов образцов плазмы крови и перитонеальной жидкости наиболее интенсивные пики соответствовали фосфатидилхолинам. Из 148 найденных метаболитов были идентифицированы 53 значимых липидных пиков, относящихся к пяти различным классам: фосфатидилхолины, фосфатидилэтаноламины, сфингомиелины, ди- и триглицериды. Полученные данные проанализировали с помощью метода PLS-DA. На рис. 1 представлены графики счетов, построенные по результатам этого анализа. Как в случае плазмы (рис. 1а), так и в случае перитонеальной жидкости (рис. 1б) точки, соответствующие эндометриозу и контролю, сгруппировались в отдельные кластеры, что свидетельствует о том, что с помощью предлагаемой платформы можно классифицировать образцы по признаку случай-контроль и отличать плазму и перитонеальную жидкость больных эндометриозом от образцов контрольной группы. При этом данные результаты не зависели от степени распространения эндометриоидного процесса. Качество созданных моделей проверяли методом перекрестной валидации. Оказалось, что модель для плазмы крови характеризуется чувствительностью 93% и специфичностью 95%. Модель для перитонеальной жидкости показала чувствительность 90% и специфичность – 95%.
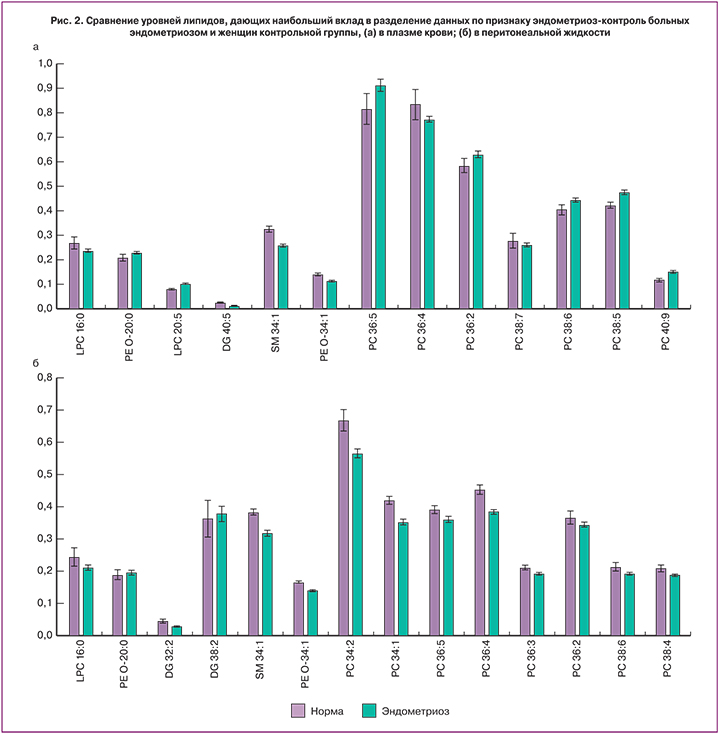
Для созданной методом PLS-DA модели были проанализированы переменные, дающие наибольший вклад в проекции на скрытые структуры (VIP-переменные). Это те переменные, которые могут выступать в качестве биомаркеров, и были использованы для построения диагностической панели. Уровни переменных, характеризующихся наибольшими значениями VIP-параметра, показаны на рис. 2. Наиболее значимыми в перитонеальной жидкости оказались переменные, соответствующие нескольким фосфатидилхолинам, лизофосфатидилхолинам, фосфатидилэтаноламинам, сфингомиелинам и диглицеридам: лизофосфатидилхолин LPC 16:0, фосфоэтаноламины PE O-20:0, PE O 34:1, диглицериды DG 32:2, DG 38:2, фосфатидилхолины PC 34:1, PC 34:2, PC 36:2, PC 36:3, PC 36:4, PC 36:5, PC 38:4, PС 38:6 и сфингомиелин SM 34:1.
Для плазмы крови наиболее важными оказались 13 следующих липидов: лизофосфатидилхолины LPC 16:0, LPC 20:5, фосфоэтаноламины PE O-20:0, PE O-34:1, диглицерид DG 40:5, фосфатидилхолины PC 36:2, PC 36:4, PC 36:5, PC 38:5, PC 38:6, PC 38:7, PС 40:9 и сфингомиелин SM 34:1. Интересно, что уровень фосфоэтаноламина PE O-20:0 оказался повышенным не только в крови и перитонеальной жидкости, но и в эндометриоидных тканях [8], что свидетельствует о диагностической ценности данного липида. Сфингомиелин SM 34:1, почти в 2 раза повышенный в эндометриоидных тканях [8–11], оказался сниженным в плазме крови и перитонеальной жидкости больных эндометриозом по сравнению с образцами женщин с отсутствием НГЭ, наряду с лизофосфатидилхолином LPС 16:0, фосфоэтаноламином PE O-34:1 и фосфатидилхолином PC 36:4.
Следует отметить, что в плазме крови больных НГЭ уровень фосфолипидов оказался выше, чем в перитонеальной жидкости этих пациентов, так, среди них отмечались высокие уровни лизофосфатидилхолина LPC 20:5 и фосфатидилхолинов PC 36:5, PC 36:2, PC 38:5, PC 38:6, PC 40:9. Снижение общего уровня липидов в перитонеальной жидкости может быть вследствие перекисного окисления липидов, характерного для больных эндометриозом [15]. Значительное повышение интенсивности свободно радикального окисления при одновременном снижении активности антиоксидантной защиты приводит к накоплению токсичных продуктов перекисного окисления липидов, которые нарушают целостность клеточных мембран, ионную проницаемость, биохимические и энергетические механизмы регуляции функций клеток и, вероятно, являются провоцирующим фактором в распространении и прогрессировании НГЭ [16].
Обсуждение
Развитие метаболомных технологий, в том числе применяемых в липидомике и основанных на применении масс-спектрометрии, привело к появлению новых методов, позволяющих быстро и с высокой точностью исследовать молекулярный состав любого биологического образца [17]. Метаболиты формируют молекулярный фенотип организма, так как являются субстратами, интермедиатами и конечными продуктами биохимических реакций [15].
Поэтому в метаболоме, как в «молекулярном зеркале», отражаются все изменения, происходящие в организме [17]. Липиды и жирные кислоты, как первичные метаболиты, вовлечены практически во все процессы, протекающие в организме человека, в том числе являются структурным элементом мембран клеток, выполняют транспортную функцию для гидрофобных и амфифильных веществ, являются гормонами и вторичными мессенджерами [17, 18].
В данном исследовании проведен липидомный анализ крови и перитонеальной жидкости больных эндометриозом по сравнению с контрольной группой. Как выяснилось, уровень некоторых сфинго- и фосфолипидов был идентично изменен в биологических жидкостях больных НГЭ.
Фосфолипиды
Фосфоэтаноламин (РЕ) является наиболее важным и pacпpoстраненным представителем липидов цитоплазматической мембраны, который вовлечен в различные биологические процессы, такие как жизненный цикл клеток, слияние липидных мембран, аутофагия, апоптоз [19]. Биосинтез фосфоэтаноламина является жизненно важным для роста клеток и последовательности смены периодов клеточного цикла. Повышение уровня этого липида приводит к нарушению регуляции роста и дифференцировки клеток, что характерно для эндометриоидного процесса [20].
PE выступает в качестве субстрата для многих клеточных процессов [19]. Фосфоэтаноламин-цитидилтрансфераза (Pcyt2) является основным регуляторным ферментом в de novo биосинтезе PE из этаноламина и диацилглицерина по пути Кеннеди [21]. Pcyt2 является геном, который участвует в регуляции роста клеток и метаболического гомеостаза, а также может играть важную роль в пересечении этих процессов [19, 20]. Считается, что de novo синтез РЕ зависит от экспрессии этаноламина и диацилглицерида (DAG) [21]. Сверхэкспрессия Pcyt2 не повышает уровень PE при низком уровне DAG [22]. Кроме того, вазопрессин (антидиуретический гормон) стимулирует превращение этаноламина в PE [21].
Фосфоэтаноламин-цитидилтрансфераза (Pcyt2) также служит основным компонентом в синтезе плазмалогенов фосфоэтаноламина [22]. Было показано, что в крови циркулирующие уровни лизофосфатидилхолина (LPC) и плазменил-фосфоэтаноламина снижаются у пациентов с раком яичников и печени [19]. Несколько исследований показывают, что экспрессия Pcyt2 обычно снижается в эпителии раковых клеток по сравнению с аналогами нормальных клеток [23, 24]. Таким образом, большинство исследования показали, что синтез Pcyt2 и PE обычно подавляется при раковых заболеваниях, что открывает важный вопрос о том, какое воздействие оказывает биогенез этих веществ на рост раковых клеток и развитие гиперпролиферативных состояний. Как ген, который является важным для роста клеток и регуляции гомеостаза липидов, Pcyt2 следует учитывать при разработке новых подходов к лечению метаболических нарушений, рака и, по всей видимости, эндометриоза.
Интересно, что фосфатидилхолины (PC) в перитонеальной жидкости идентифицированы в низких концентрациях (4–5 двойных связей в ацильных боковых цепях), что может свидетельствовать о том, что они служат источником для повышения производства простагландинов [15]. Эта гипотеза подтверждается увеличением экспрессии фермента фосфолипазы A2 (PLA2G2F, PLA2G1B, PLA2G5, PLA2G2D, PLA2G2A), которая, согласно ряду исследований, наблюдается в эктопическом эндометрии [8, 9, 25]. Несмотря на то, что концентрация лизофосфатидилхолина (LPC) в перитонеальной жидкости больных эндометриозом оказалась ниже, чем у женщин группы сравнения, было выявлено также более низкое соотношение между содержанием общего фосфатидилхолина и лизофосфатидилхолина, что также подтверждает нашу гипотезу. Кроме того, фосфатидилхолин может служить источником для синтеза сфингомиелина путем передачи сигнала с фосфохолина на церамиды. Эта реакция катализируется сфингомиелинсинтазой 1 (SGMS1), которая также была повышена у больных эндометриозом [9–11], что приводит к снижению общего уровня PC в перитонеальной жидкости. С другой стороны, сниженные уровни PC могут иметь место вследствие низкой экспрессией фермента алкилглицерон-фосфатсинтазы (AGPS), который катализирует первый шаг в синтезе РС и также обнаружен в эутопическом эндометрии больных эндометриозом [26].
Сфинголипиды
Класс сфинголипидов представляет собой высокоактивные биологические соединения, которые участвуют в регулировании клеточной пролиферации, дифференцировки, миграции, внеклеточной и внутриклеточной передаче сигналов, а также в гибели клеток [11]. Сфингомиелины (SM) являются ключевыми компонентом в сфингомиелиновом пути передачи сигнала [9]. Некоторые метаболиты этого цикла, такие как церамиды и сфингозин, индуцируют апоптоз, в то время как сфингозин-1-фосфат (S-1P) способствует выживанию клеток в ответ на стимулы апоптоза [15].
Многочисленные опытные исследования показывают, что перитонеальная жидкость у пациентов с эндометриозом характеризуется повышенным окислительным стрессом [5, 14, 16], что подтверждается нашими экспериментальными данными в снижении уровня SM. Окислительный стресс способствует ускоренному превращению сфингомиелинов до церамида путем транслокации кислой сфингомиелиназы из лизосом на наружный слой клеточной мембраны [27].
Усиленному образованию церамидов способствует также повышение уровня сфингомиелин фосфодиэстеразы 3 (SMPD3), которое, как было показано недавно группой иностранных коллег [11, 26], наблюдалось в ткани эндометрия у пациентов с эндометриозом. Таким образом, церамиды могут быть преобразованы в сфингозин-1-фосфат (S-1P), который является биоактивным липидом, участвующем в ангиогенезе, миграции клеток и апоптозе, а также регулирует иммуномодуляцию и дифференциацию клеток [28]. В эндометриоидных тканях отмечена дисрегуляция в пути сфингозина, которая приводит к снижению катаболизма сфингозин-1-фосфата (S-1P) и, таким образом, происходит усиление его действия через соответствующие рецепторы (S1PR-1 и S1PR-2) [28].
Многие ученые также полагают, что непрерывные процессы денервации и реиннервации, проходящие в эктопической эндометрии и вызывающие болевой синдром у пациенток с эндометриозом, также связаны с повышенным уровнем сфингомиелина и изменением экспрессии генов, способствующих преобразованию сфингомиелина и его метаболитов в S-1P [7, 9, 29].
Заключение
Анализ липидного состава плазмы крови и перитонеальной жидкости больных НГЭ выявил ряд глицерофосфолипидов, сфинголипидов и глицеролипидов, уровни которых существенно отличаются в биологических жидкостях женщин с эндометриозом от контрольной группы (чувствительность метода для плазмы крови составила 93%, специфичность – 95%; для перитонеальной жидкости чувствительность – 90%, специфичность – 95%).
Валидация этих липидов в качестве серологических биомаркеров в последующих исследованиях может открыть новые диагностические возможности у пациентов с подозрением на НГЭ и снизить необходимость в проведении диагностической лапароскопии.
Дальнейшее изучение молекулярных механизмов, регулирующих повышенную пролиферацию, снижение апоптоза, имплантацию и инвазию эндометриоидных клеток может привести к созданию новых прогностических стратегий и методов терапии НГЭ.



