Comparison of active and expectant management of pregnant women with non-immune fetal hydrops
Aim. To compare perinatal outcomes of women with non-immune hydrops fetalis undergoing active and expectant management.Kadyberdieva F.Z., Shmakov R.G., Bockeria E.L., Kostyukov K.V., Tetruashvili N.K.
Materials and methods. The study comprised all pregnant women with non-immune fetal hydrops, who were managed at the V.I. Kulakov NMRC for OG&P from 2015 to 2020 (n=45). Group 1 (n=30) included pregnant women with non-immune fetal hydrops, who were examined antenatally according to the developed protocol, and some of them received intrauterine treatment (prospective group from 2018 to 2020). Group 2 included 15 pregnant women who received no antenatal examination and treatment (retrospective group from 2015 to 2018).
Results. In group 1, the causes of non-immune hydrops fetalis were identified antenatally in 83.3% (25/30) of patients, and in more than a half of them (56.7%), pregnancy management was based on these findings. In group 2, causes of non-immune hydrops fetalis were identified antenatally in 60% (9/15) of patients, and they received no intrauterine treatment. Patients with an active management approach had higher rates of antenatal resolution of non-immune hydrops and higher perinatal survival rates; their newborns had higher Apgar scores and a lower need for resuscitation, mechanical ventilation, and cardiotonic agents.
Conclusion. Antenatal identification of non-immune fetal hydrops causes helps guide active pregnancy management, leading to improved perinatal outcomes.
Keywords
Non-immune hydrops fetalis is a severe fetal condition defined as the excessive accumulation of fetal fluid within the fetal extravascular compartments and body cavities. Non-immune hydrops fetalis can be caused by many underlining pathologies leading to an imbalance in the regulation of fluid movement between the vascular and interstitial spaces, with an increase in the interstitial fluid production or a decrease in lymphatic return [1].
The prognosis in non-immune fetal hydrops is related to the underlying etiology, gestational age at its onset during pregnancy, and delivery [1–3]. Aneuploidies, gestational age less than 24 weeks, and fetal malformations are associated with a poor prognosis [4]. Despite progress in perinatal care, perinatal mortality and neonatal morbidity in non-immune hydrops fetalis are still high. However, fetal therapy and surgical interventions have proven to improve perinatal outcomes [5-8].
Since the etiology of non-immune fetal hydrops is multifactorial, it remains a subject of debate whether to prolong pregnancy and perform fetal intervention at the time of non-immune fetal hydrops detection. In previous studies, we have shown that antenatal examination, according to our protocol, allows significant improvement in identifying causes and structure them and considering the possibility and methods of fetal therapeutic interventions [9].
The study aimed to compare perinatal outcomes of women with non-immune hydrops fetalis undergoing active and expectant management.
Materials and methods
The study comprised all pregnant women with non-immune fetal hydrops, who were managed at the V.I. Kulakov NMRC for OG&P from 2015 to 2020 (n=45). Group 1 (n=30) included pregnant women with non-immune fetal hydrops, who were examined antenatally according to the developed protocol, and some of them received intrauterine treatment (prospective group from 2018 to 2020). Group 2 included 15 pregnant women who received no antenatal examination and treatment (retrospective group from 2015 to 2018).
Patients of group 1 were administered pathogenetically relevant therapy including antiarrhythmic agents, intrauterine packed erythrocytes transfusions, antiviral and immunoglobulin therapy, antibiotics, interstitial laser coagulation of the vessels supplying the sacrococcygeal teratoma), thoracocentesis, paracentesis, thoracoamniotic shunt, transplacental cardiotonic treatment with digoxin, administration of corticosteroids).
Group 2 included pregnant women admitted to the Center only for delivery and who did not receive the above intrauterine treatment.
We compared the two groups by the following parameters: ultrasound findings before delivery, pregnancy outcomes, morphological and functional characteristics at birth, the need for mechanical ventilation, factors and complications of the neonatal period, and neonatal mortality.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistica 22 (USA), StatTech v1.0.0 (Russia) software. The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Quantitative variables are presented as the median (Me) and interquartile range (Q1; Q3). The statistical significance of between-group differences for continuous variables was tested with Mann–Whitney test. Categorical variables were compared by the Fisher's exact test, Pearson χ2 test, and Pearson χ2 test with Yates' correction. Differences were considered statistically significant at p≤0.05.
Results
In earlier studies, we presented a non-immune fetal hydrops examination algorithm and the results of its implementation in clinical practice [9]. This protocol helps assess fetal morphological and functional state in more detail, provide therapeutic measures, and evaluate their effectiveness in dynamics. In group 1, the causes of non-immune hydrops fetalis were identified antenatally in 83.3% (25/30) of patients, and in 56.7% (17/30) of them, various intrauterine treatments were used based on these findings. In group 2, causes of non-immune hydrops fetalis were identified antenatally only by fetal ultrasound in 60% (9/15) of patients, and they received no intrauterine treatment.
Table 1 summarizes the fetal surgical interventions used in group 1.
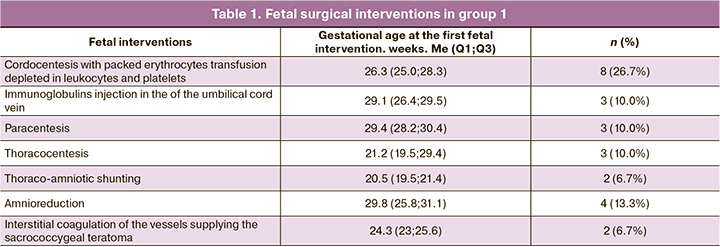
Cordocentesis with intrauterine transfusion of donor erythrocytes was required in 8 (26.7%) cases, in 3 (10%) of which immunoglobulin was additionally injected into the umbilical cord vein for antiviral purposes. The fetal survival rate after intrauterine anemia correction was 75% (6/8). There was one antenatal fetal and one early neonatal death, despite a pronounced regression of fetal hydrops. Two cases were excluded from the study. They were prenatally diagnosed as non-immune hydrops (due to intrauterine anemia) and received pathogenetically oriented therapy. However, a post-natal comprehensive examination revealed fetal hemolytic disease due to Kell antibodies.
Non-immune hydrops due to fetal tachyarrhythmia, which is the most successfully corrected antenatally, was identified in 5 cases. Transplacental antiarrhythmic therapy involved a combination of digoxin and sotalol, with concomitant administration of potassium supplements. The route of administration, dosage, and frequency was selected depending on the clinical situation, the woman's tolerance to therapy. By the time of delivery, in all 5 cases, the non-immune fetal hydrops was resolved. After birth, antiarrhythmic treatment was required for two children; the other three children had no arrhythmia recurrence during the entire follow-up.
Decompression of fetal serous cavities by paracentesis and thoracocentesis was only temporary and transudate accumulated over the next 24–72 hours. Some researchers recommend thoracoamniotic shunt placement in case of repeat fluid accumulation [10]. In this study, a thoracoamniotic shunt was placed in two cases due to the presence of a cystic-adenomatous lung malformation with a large cyst. In one case, thoracoamniotic shunt placement resulted in a complete hydrops regression with reaching full-term pregnancy. However, on day 4, neonatal death occurred due to sudden heart failure. In another case, antenatal fetal death occurred.
Interstitial laser coagulation of the vessels supplying the sacrococcygeal teratoma (n=2) did not give a significant effect, both in terms of reducing the size of the tumor and in terms of hydrops regression. In both cases, intrauterine fetal death occurred.
Fetal cardiac decompensation was an indication for transplacental digoxin therapy, which was administered in 13 cases (43.3%). The median grade of tricuspid valve insufficiency before the start of digoxin therapy was +3 (2.5; 3), while against the background of complex intrauterine treatment, before delivery, it was +1.5 (1; 3).
Amnioreduction for severe polyhydramnios was performed in 4 cases (13.3%).
In other cases (13/30 – 43.3%), there were no indications for intrauterine interventions. Dynamic ultrasound and echocardiographic observation were performed to diagnose and correct manifestations and complications of non-immune fetal hydrops.
Placentomegaly and polyhydramnios often accompany non-immune hydrops fetalis [11]. In this study, placentomegaly and polyhydramnios were observed in 15/45 (33.3%) and 20/45 (44.4%), respectively. However, no statistically significant differences were found between the groups: 10/30 (33.3%) versus 5/15 (33.3%) (p=0.95) and 11/30 (36.7%) versus 9/15 (60%) (p=0.13), respectively.
Fetal growth restriction was observed in 6 cases including 4/30 (13.3%) in group 1 and 2/15 (13.3%) in group 2 (p=0.95). Isolated shortening of tubular bones, according to ultrasound data, not associated with aneuploidies, was detected in 11 (24.4%) cases and was statistically significantly higher in group 2 [7/15 (46.7%)] versus 4/30 (13.3%)] (p=0.01).
At the time of delivery, soft tissue edema and severe bilateral hydrothorax in group 2 were statistically significantly more common [12/15 (80%) and 9/15 (60%)] than in group 1 [14/30 (46.7%) and 6/30 (20%)], (p=0.03) and (p=0.007), respectively. Table 2 presents fetal ultrasound findings before delivery

In group 1, intrauterine treatment was associated with a regression of non-immune fetal hydrops by the time of delivery, and 11/21 (52.4%) children born alive had no signs of hydrops (p=0.008). In group 2, all 11/11 (100%) children were born alive and had signs of fetal hydrops. The most successful intrauterine fetal hydrops regression was associated with the treatment of tachyarrhythmias and intrauterine anemias.
Most patients [31/45 (68.8%)] underwent cesarean section, mainly as an emergency cesarean delivery [26/31 (83.8%)]. Vaginal births occurred in 14/45 (31.2%) cases, of which 11/14 (78.5%) required labor induction mainly due to antenatal fetal death.
The structure of the outcomes of pregnancies with non-immune fetal hydrops is presented in Table 3.
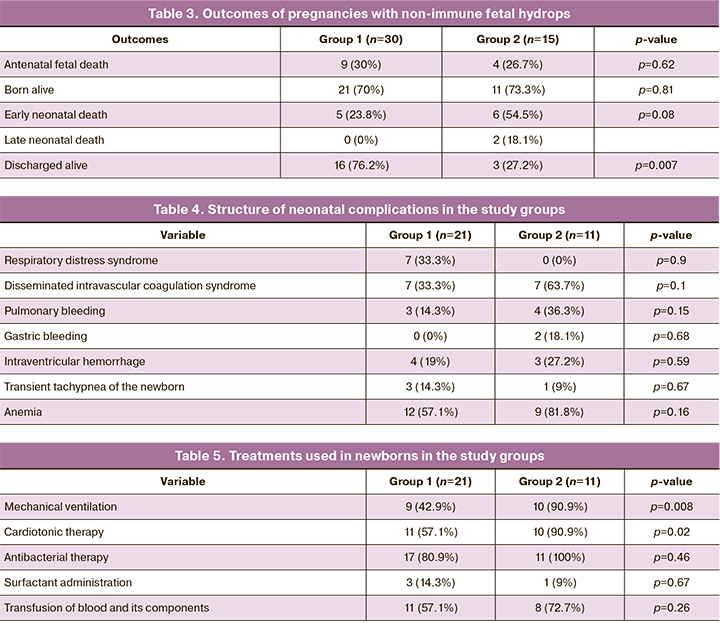
The median gestational age at delivery among babies born alive was 35 (32.1; 37.2) and 34.6 (32.1; 36.2) weeks in groups 1 and 2, respectively (p=0.88). There were no statistically significant differences in birth weight and body height of newborns. Median birth weight was 2545 (2330; 3126) g and 2425 (2209; 2808) g in groups 1 and 2, respectively (p=0.37). Body length was 47 (45; 51) cm versus 44 (42; 47.5) cm (p=0.07), respectively. The median Apgar score at the first-minute was 6 (5; 7) and (3.5; 5) (p=0.04), and 7 (7; 8) and 6 (5;7) at the fifth-minute (p=0.11) in groups 1 and 2, respectively.
Newborns from group 2 had a more severe condition at birth (p=0.04). They more often required resuscitation, mechanical ventilation (p=0.008), and cardiotonic therapy (p=0.02). Neonatal complications were observed in both groups and had no statistically significant differences. The structure of neonatal complications and treatments is presented in Tables 4 and 5, respectively.
Comparison of cases with favorable (discharged alive, n=19) and unfavorable (antenatal fetal and neonatal death, n=26) outcomes revealed significant factors that adversely affect outcomes. They included fetal congenital malformations (p=0.02), congenital heart defects (p=0.02), pronounced bilateral hydrothorax (p=0.004), soft tissue edema (p=0.001), secondary lung hypoplasia (p=0.001), mediastinal displacement (0.02). Postnatal factors included prematurity (p=0.002), low Apgar score (p=0.001), need for mechanical ventilation (p=0.001), cardiotonic therapy (p=0.002), blood transfusion and its components (p=0.03).
Conclusion
Identifying the hydrops cause is an essential aspect of developing a pregnancy management strategy that dictates the need for an antenatal examination. This study demonstrated an increase in the detection rate of non-immune fetal hydrops causes after introducing the examination protocol. Based on the study results, an active pregnancy management strategy was chosen in more than half of the cases (56.2%). The study findings confirm that proactive pregnancy management improves perinatal outcomes. Patients undergoing active pregnancy management had higher rates of antenatal regression of non-immune hydrops and higher perinatal survival rates; their newborns had higher Apgar scores and a lower need for resuscitation, mechanical ventilation, and cardiotonic agents.
Therefore, antenatal examination allows structuring the causes of non-immune fetal hydrops, identifying among them the most promising to guide the choice of antenatal interventions, which helps improve perinatal outcomes.
References
- Sileo F.G., Kulkarni A., Branescu I., Homfray T., Dempsey E., Mansour S. et al. Non-immune fetal hydrops: etiology and outcome according to gestational age at diagnosis. Ultrasound Obstet. Gynecol. 2020; 56(3): 416-21. https://dx.doi.org/10.1002/uog.22019.
- Huang H., Tsay P.K., Chiang M.C., Lien R., Chou Y.H. Prognostic factors and clinical features in liveborn neonates with hydrops fetalis. Am. J. Perinatol. 2007; 24(1): 33-8. https://dx.doi.org/10.1055/s-2006-958158.
- Ota S., Sahara J., Mabuchi A., Yamamoto R., Ishii K., Mitsuda N. Perinatal and one-year outcomes of non-immune hydrops fetalis by etiology and age at diagnosis. J. Obstet. Gynecol. Res. 2016; 42(4): 385-91. https://dx.doi.org/10.1111/jog.12922.
- Norton M.E., Chauhan S.P., Dashe J.S. Society for Maternal-Fetal Medicine (SMFM). Society for maternal-fetal medicine (SMFM) clinical guideline#7: nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2015; 212(2): 127-39. https://dx.doi.org/10.1016/j.ajog.2014.12.018.
- Bellini C., Hennekam R.C.M. Non-immune hydrops fetalis: a short review of etiology and pathophysiology. Am. J. Med. Genet. 2012; 158A(3): 597-605. https://dx.doi.org/10.1002/ajmg.a.34438.
- Santo S., Mansour S., Thilaganathan B., Homfray T., Papageorghiou A., Calvert S. et al. Prenatal diagnosis of non-immune hydrops fetalis: what do we tell the parents? Prenat. Diagn. 2011; 31(2): 186-95. https://dx.doi.org/10.1002/pd.2677.
- Bellini C., Donarini G., Paladini D., Calevo M.G., Bellini T., Ramenghi L.A. et al. Etiology of non-immune hydrops fetalis: an update. Am. J. Med. Genet. A. 2015; 167A(5): 1082-8. https://dx.doi.org/10.1002/ajmg.a.36988.
- Abrams M., Meredith K., Kinnard P., Clark R. Hydrops fetalis: a retrospective review of cases reported to a large national database and identification of risk factors associated with death. Pediatrics. 2007; 120(1): 84-9. https://dx.doi.org/10.1542/peds.2006-3680.
- Кадырбердиева Ф.З., Шмаков Р.Г., Бокерия Е.Л., Тетруашвили Н.К., Костюков К.В. Эффективность применения алгоритма обследования на антенатальном этапе при неиммунной водянке плода. Акушерство и гинекология. 2020; 7: 71-8. [Kadyrberdieva F.Z., Shmakov R.G., Bokeriya E.L., Kostyukov K.V., Tetruashvili N.K. The effectiveness of the antenatal examination algorithm for non immune hydrops fetalis. Obstetrics and Gynecology. 2020; 7: 71-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.7.71-78.
- Abbasi N., Ryan G. Fetal primary pleural effusions: prenatal diagnosis and management. Best Pract. Res. Clin. Obstet. Gynecol. 2019; 58: 66-77. https://dx.doi.org/10.1016/ j.bp.obgyn.2019.01.005.
- Berger V.K., Sparks T.N., Jelin A.C., Derderian C., Jeanty C., Gosnell K. et al. Non-immune hydrops fetalis. Do placentomegaly and polyhydramnios matter? J. Ultrasound Med. 2018; 37(5): 1185-91. https://dx.doi.org/10.1002/jum.14462.
Received 26.10.2020
Accepted 26.11.2020
About the Authors
Faina Z. Kadyrberdieva, Ph.D. Student, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(909)916-58-52. E-mail: f_kadyrberdieva@oparina4.ru.117997, Russia, Moscow, Ac. Oparina str., 4.
Roman G. Shmakov, Dr. Med. Sci., Professor of the RAS, Director of the Institute of Obstetrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-72-00. E-mail: r_shmakov@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Ekaterina L. Bockeria, Dr. Med. Sci., Professor, Head of the 2nd Department of Pathology of Newborns and Premature Babies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor at the Department of Neonatology, N.F. Filatov Clinical Institute of Children's Health, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). Tel.: +7(495)438-27-05. E-mail: e_bokeriya@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Kirill V. Kostyukov, Ph.D., Diagnostic Medical Sonographer at the Functional and Ultrasound Diagnostics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-25-29. E-mail: k_kostyukov@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Nana K. Tetruashvili, Dr. Med. Sci., Head of the 2nd Obstetrics Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Tel.: +7(495)438-14-77. E-mail: n_tetruashvili@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Kadyberdieva F.Z., Shmakov R.G., Bockeria E.L., Kostyukov K.V., Tetruashvili N.K. Comparison of active and expectant management of pregnant women with non-immune fetal hydrops.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 55-60 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.55-60



