Comparative study on the use of mineral oil in the embryological stage of assisted reproductive technology programs
Skorlupkina N.N., Apryshko V.P., Lebedeva E.B., Kirienko K.V., Mironova A.G., Yakovenko S.A.
Objective: To investigate the effect of mineral oil type on the development of human embryos during in vitro culture in the context of in vitro fertilization (IVF) programs.
Materials and methods: This study involved zygotes/sibling embryos (1198 embryos) obtained from 84 patients undergoing IVF treatment at the AltraVita Human Reproduction Clinic. After fertilization, the sibling embryos of each patient were cultured for 5–6 days in microdrops of medium, either under a layer of mineral oil or under a layer of paraffin oil. The parameters analyzed included fertilization and embryo cleavage rates, blastocyst formation rates, good-quality blastocyst formation rates, embryo implantation rates, and clinical pregnancy rates.
Results: The group of embryos cultured with paraffin oil showed a significantly higher total number of blastocysts and a higher rate of good-quality blastocyst formation compared to the group of embryos cultured under a layer of mineral oil. Fertilization and embryo cleavage rates, as well as implantation and clinical pregnancy rates, were not statistically significantly different between the two groups.
Conclusion: Coating droplets with medium using paraffin oil during in vitro embryo culture improves the efficiency of the embryological stage compared with the use of mineral oil for the same purpose.
Authors' contributions: Skorlupkina N.N., Apryshko V.P., Yakovenko S.A. – conception and design of the study; Skorlupkina N.N., Lebedeva E.B., Kirienko K.V., Mironova A.G. – data collection and processing; Skorlupkina N.N. – statistical analysis; Skorlupkina N.N., Kirienko K.V. – drafting of the manuscript; Kirienko K.V. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was conducted in accordance with the Standards of Good Clinical Practice and the tenets of the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of the AltraVita Human Reproduction Clinic, IVF CENTER LLC.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Skorlupkina N.N., Apryshko V.P., Lebedeva E.B., Kirienko K.V., Mironova A.G., Yakovenko S.A. Comparative study on the use of mineral oil in the embryological stage of assisted reproductive technology programs.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (8): 121-126 (in Russian)
https://dx.doi.org/10.18565/aig.2024.123
Keywords
Covering culture medium (hereafter "medium") with oil in embryo culture dishes has been an embryological method since the 1960s [1, 2]. The purpose of using oil is to prevent damage to oocytes and embryos that can occur during culture in a CO2 incubator or during routine manipulations outside the incubator [3, 4]. Additionally, oil-covered media are less prone to contamination by particles in the external environment and provide better protection against contamination by microorganisms [2, 5]. The standard technique for maintaining humidity in CO2 incubators can lead to medium evaporation and changes in the osmolarity [5]. Thus, an important benefit of using oil in in vitro embryo culture is safeguarding the medium from changes in osmolarity [3].
Culturing embryos in separate droplets is a common method in modern in vitro fertilization (IVF) laboratories [1, 6]. While mammalian embryos can tolerate a wide range of osmolarities, human embryos require a constant osmolarity of the culture medium [2, 3]. Maintaining consistent osmolarity is critical for blastocyst formation [2]. Deviations in media pH can affect embryo metabolism and osmotic stress can have a detrimental effect on embryo viability [2, 3]. Conversely, the use of oil in embryo cultures may have a positive impact on their development [4, 7]. However, during oil production, transportation, and storage, contamination with substances that are toxic to oocytes and embryos is possible [7–9]. These compounds can accumulate in oil [8, 9]. Studies on mouse, bovine, and human embryos have shown that toxic substances can leach from oil into the culture medium [3, 8–10]. Therefore, it is recommended to "wash" the oil by equilibrating it for 24 h or more with water and/or medium in which the embryos will subsequently be cultured [8].
Mineral oil from different manufacturers may vary in viscosity, density, and qualitative composition [4, 9]. Additionally, different batches of oils from the same manufacturer may differ in their physicochemical properties and subsequently affect embryo viability during in vitro culture. Thus, it is advisable to test oil on a biological sample before using it as part of the embryo culture system.
This study aimed to compare two versions of commercially available oil intended for in vitro embryo culture.
Materials and methods
A study on the culture of sibling embryos using mineral and paraffin oils was conducted at the AltraVita Human Reproduction Clinic. The study included 84 couples undergoing IVF infertility treatment, who received more than 10 mature MII oocytes as a result of superovulation induction. Patients who underwent preimplantation genetic testing were excluded from this study.
This study was approved by the Research Ethics Committee of AltraVita Human Reproduction Clinic. All patients enrolled in the study were informed about the procedure prior to the ovarian puncture and provided signed informed consent.
Superovulation was induced using a short protocol with gonadotropin-releasing hormone antagonists. From days 2 to 3 of the menstrual cycle, recombinant and/or urinary gonadotropins were administered at a daily dose of 150–300 IU. Ovulation was induced by the administration of human chorionic gonadotropin (hCG) at a dose of 10,000 IU 34–36 h prior to ovarian puncture. The oocyte-cumulus complexes obtained during puncture were washed twice in HTF media (IVF basics, Gyniotec, Netherlands) and awaited further denudation in HTF media (IVF basics, Gyniotec, Netherlands) with the addition of 10% Life Global Protein supplement (LGPS, Life Global, USA) under a layer of mineral oil. Denudation of the oocyte-cumulus complexes was performed 1 h after ovarian puncture. Oocyte-cumulus complexes were briefly placed in a medium containing hyaluronidase (Hyadase, Vitromed, Germany) and then mechanically purified by gentle pipetting in HTF HEPES media (IVF Basics, Gyniotec, Netherlands) with the addition of 10% Life Global Protein supplement (LGPS, Life Global, USA). Sperm preparation for fertilization was performed using density gradient centrifugation. Intracytoplasmic sperm injection (ICSI) was performed 2 h after denudation in HTF HEPES media (IVF Basics, Gyniotec, Netherlands) supplemented with 10% Life Global Protein supplement (LGPS, Life Global, USA). Distribution into split groups was performed after fertilization. Oocytes were randomly divided into two groups (1:1) and placed in two different wells of a four-well plate with Vitromed One Step medium (Vitromed, Germany) supplemented with 10% Life Global Protein Supplement (LGPS, Life Global, USA). The first well was covered with mineral oil (mineral oil, Sigma-Aldrich Inc., REF: M5310), and the second well was covered with paraffin oil (Oil for IVF culture (light paraffin oil), Vitromed GmbH, REF: V-OIL-P100). The results of oocyte fertilization were assessed 16–18 h after ICSI; zygotes with two pronuclei (2PN) were transferred for cultivation into microdroplets (20 μl) of Vitromed One Step medium (Vitromed, Germany) with the addition of 10% Life Global Protein supplement (LGPS, Life Global, USA) under a layer of the appropriate oil (mineral or paraffin) covering the dish in a volume of 6.0 ml. Culture dishes were prepared in advance and equilibrated for 12–14 h in a CO2 incubator. The embryos were cultured simultaneously in the same CO2 incubator (Thermo Scientific, USA) in an atmosphere containing 6% CO2 at 37°C and maximum humidity during the entire incubation period. Before use, both types of oils were incubated for 24 h with water (sterile water for cell culture solutions, BioloT, Russia) at 4°C, and then for 24 h with Vitromed One Step medium (Vitromed, Germany) in a CO2 incubator with 6% CO2 at 37°C. The embryo cleavage stage was analyzed on the 3rd day of development according to standard evaluation criteria based on morphological parameters (number and size of blastomeres and degree of fragmentation). Blastocysts were analyzed on days 5th–6th day of development by size (from 1 to 6), quality of the inner cell mass (A, B, C), and trophectoderm (A, B, C), according to the Gardner grading system. Blastocysts of BB quality and higher, and of size 3 or more, were either transferred in a stimulation cycle or vitrified. Embryo transfer in the stimulation cycle was performed on the 5th day of development. The blastocysts were vitrified according to the Cryotech protocol (Vitrification Kit 101; Cryotech, Tokyo, Japan). The blastocysts for transfer in the cryopreserved cycle were thawed according to the Cryotech protocol (Warming Kit 102, Cryotech, Tokyo, Japan) and kept in a CO2 incubator for 2 h after thawing, without covering the dishes with oil, before being transferred into the uterine cavity. To determine the effectiveness of using the two types of oils, the following parameters were assessed: the frequency of obtaining correctly fertilized 2PN zygotes (Fertilization rate, FR), the frequency of embryo cleavage (cleavage rate, ClR), the frequency of blastocyst formation (Blastocyst rate, BlR), and good quality blastocysts (utilization rate, UR), the frequency of embryo implantation (implantation rate, IR), and obtaining clinical pregnancies (clinical pregnancy rate, CR) after transfer in the stimulation cycle and cryopreserved cycle. The onset of clinical pregnancy was recorded based on the results of an ultrasound examination performed 14 days after receiving a positive β-hCG result.
Statistical analysis
The number of retrieved oocyte-cumulus complexes, number of mature oocytes, number of blastocysts and blastocysts of good quality, and number of embryos per transfer were described by the arithmetic mean (M) with standard deviation (SD). The non-parametric Mann–Whitney U test was used to compare the means of two independent samples. The calculation of the rates of correct fertilization, blastocyst formation, pregnancy with embryo transfer in the stimulation cycle, and pregnancy with cryopreserved transfer was performed for the whole group, and the chi-square test (χ2) was used to compare categorical data. Statistical significance was set at p<0.05.
Results
This study included 1,490 MII oocytes obtained from 84 patients in 84 superovulation induction cycles. The age of the patients ranged from 26 to 45 years at the time of the ovarian puncture, and the mean age of the women was 31.3 years (Table 1).
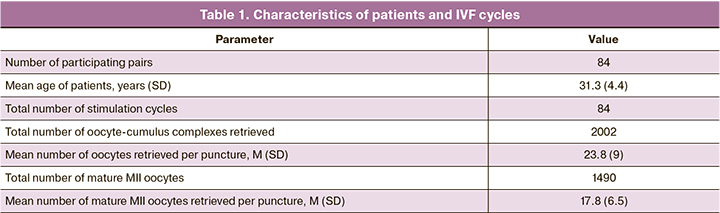
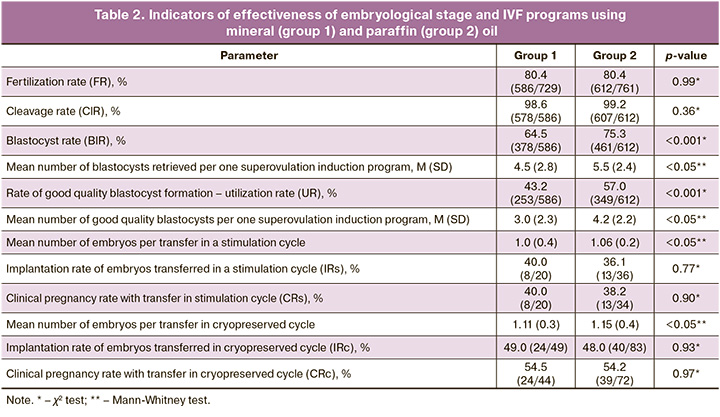
The normal fertilization rate (80.4% (586/729) and 80.4% (612/761), respectively) and proportion of cleavage embryos (98.6% (578/586) and 99.2% (607/612), respectively) showed no statistically significant differences between the mineral oil and paraffin oil groups. The blastocyst formation rate (64.5% (378/586) and 75.3% (461/612), respectively) and good-quality blastocyst formation rate (43.2% (253/586) and 57.0% (349/612), respectively) showed statistically significant differences and were lower in the mineral oil group than in the paraffin oil group. The mean number of blastocysts and good quality blastocysts obtained as a result of the embryological stage in the mineral oil and paraffin oil groups were 4.5 and 3.0 embryos, respectively, versus 5.5 and 4.2 embryos, respectively. No statistically significant differences were found between the groups using mineral and paraffin oils in embryo implantation and clinical pregnancy rates (during embryo transfer in the stimulation cycle and in the cryopreserved cycle) (Table 2).
Discussion
Oil is used in the embryo culture system to cover the microdroplets in the medium in which the embryos are located. This helps to stabilize the medium during the culture period in a CO2 incubator, as well as during manipulation of oocytes and embryos outside the incubator [2]. While the purpose of covering the medium with oil is to protect embryos from the environment and sudden changes in temperature and osmolarity, it can also potentially contain embryotoxic compounds that may negatively affect embryo viability [11]. Different manufacturers produce oils with varying viscosities, qualities, and purification levels, which can affect embryo development during in vitro culture [11, 12]. Although many commercial oils are available for human embryo culture, only a few studies have directly compared their efficacy and safety in terms of IVF cycle outcomes.
Initial studies on the effects of different types of oils used to cover microdroplets on human IVF outcomes were conducted in the late 2000s [11]. However, these studies did not find statistically significant differences in fertilization and pregnancy rates [11]. However, the average number of high-quality embryos on day 3 was significantly higher in the paraffin oil-treated group [10]. In the present study, the use of different types of oils for cultivation did not affect the frequency of embryo cleavage on day three of development. It should be noted that our study only monitored the presence or absence of cleavage, regardless of quality, whereas Sifer S. et al. compared the quality of embryos at the cleavage stage [10]. This difference, along with variations in oil manufacturers, may explain the disparity in the results. Additionally, the timing of embryo transfer differed between the two studies, with day 2–3 transfers in the study by Sifer S. et al. and day 5 transfers at the good-quality blastocyst stage in our study. Therefore, it is inappropriate to compare the pregnancy rates in our study with those in the aforementioned study.
Another study compared the effects of paraffin- and mineral-oil-covered culture media on in vitro development and utilization rates of day 3 human embryos. This study, which involved sibling splits in 1237 oocytes [12], found no difference in embryo development up to day 3 between the two groups, which is consistent with our results.
In our study, we observed that both the tested oils had a similar effect on the percentage of correct oocyte fertilization. This finding is consistent with those of previous studies [11, 12], in which we compared the results of embryo culture using two types of oils to reach the blastocyst stage. This allowed us to evaluate the viability and quality of the embryos at later stages of development. We found that using paraffin oil resulted in an increase in the total number of blastocysts and good-quality blastocysts compared to the use of mineral oil for culture. This difference may be due to the potential toxic effects of certain oils on embryonic development [2, 8, 13]. These effects could stem from variations in the manufacturing processes, such as different types of industrial purification, storage containers, and transportation methods. Additionally, interactions between the oil and culture media under current laboratory conditions may also play a role. Therefore, it is crucial to conduct preliminary testing of commercially available oils intended for in vitro embryo cultures in IVF laboratories. This will help improve the effectiveness of assisted reproductive technology programs by increasing the number of high-quality embryos suitable for transfer or vitrification, and subsequently enhancing pregnancy rates in IVF programs. This may also lead to a reduction in superovulation induction protocols for patients.
Conclusion
Using paraffin oil to cover droplets with the medium during in vitro embryo culture improves the efficiency of the embryological stage compared to using mineral oil for the same purpose.
References
- Fukui Y., Lee E.S., Araki N. Effect of medium renewal during culture in two different culture systems on development to blastocysts from in vitro produced early bovine embryos. J. Anim. Sci. 1996; 74(11): 2752-8. https://dx.doi.org/10.2527/1996.74112752x.
- Morbeck D.E., Khan Z., Barnidge D.R., Walker D.L. Washing mineral oil reduces contaminants and embryotoxicity. Fertil. Steril. 2010; 94(7): 2747-52. https://dx.doi.org/10.1016/j.fertnstert.2010.03.067.
- Ebrahimi M., Mara L., Parham A., Dattena M. Mineral oil for in vitro embryo production: what we should know? Arch. Razi Inst. 2022; 77(4): 1325-30. https://dx.doi.org/10.22092/ARI.2022.358955.2343.
- Scarica C., Monaco A., Borini A., Pontemezzo E., Bonanni V., De Santis L. et al.; SIERR, Società Italiana di Embriologia Riproduzione e Ricerca. Use of mineral oil in IVF culture systems: physico-chemical aspects, management, and safety. J. Assist. Reprod. Genet. 2022; 39(4): 883-92. https://dx.doi.org/10.1007/s10815-022-02479-z.
- Yoon J., Yoon S., Ko Y., Lim J. P-262: Effect of filtration of mineral oil on the mouse embryonic development in vitro. Fertil. Steril. 2006; 86(3): 232. https://dx.doi.org/10.1016/S0015-0282(06)03381-4.
- Labied S., Jouan C., Wenders F., Ravet S., Gaspard O., Thonon F. et al. Comparison between paraffin and mineral oil covering on early human embryo culture: a prospective randomized study. Syst. Biol. Reprod. Med. 2019; 65(1): 81-6. https://dx.doi.org/10.1080/19396368.2018.1492645.
- Gardner D.K., Reed L., Linck D., Sheehan C., Lane M. Quality control in human in vitro fertilization. Semin. Reprod. Med. 2005; 23(4): 319-24. https://dx.doi.org/10.1055/s-2005-923389.
- Mantikou E., Youssef M.A., van Wely M., van der Veen F., Al-Inany H.G., Repping S. et al. Embryo culture media and IVF/ICSI success rates: a systematic review. Hum. Reprod. Update. 2013; 19(3): 210-20. https://dx.doi.org/10.1093/humupd/dms061.
- Mestres E., Matia-Algué Q., Villamar A., Casals A., Acacio M., García-Jiménez M. et al. Characterization and comparison of commercial oils used for human embryo culture. Hum. Reprod. 2022; 37(2): 212-25. https://dx.doi.org/10.1093/humrep/deab245.
- Sifer C., Pont J.C., Porcher R., Martin-Pont B., Benzacken B., Wolf J.P. A prospective randomized study to compare four different mineral oils used to culture human embryos in IVF/ICSI treatments. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009; 147(1): 52-6. https://dx.doi.org/10.1016/j.ejogrb.2009.06.023.
- Morbeck D.E., Leonard P.H. Culture systems: mineral oil overlay. Methods Mol. Biol. 2012; 912: 325-31. https://dx.doi.org/10.1007/978-1-61779-971-6_18.
- Kane M.T. Culture media and culture of early embryos. Theriogenology. 1987; 27(1): 49-57. https://dx.doi.org/10.1016/0093-691X(87)90069-0.
- Van Soom A., Mahmoudzadeh A.R., Christophe A., Ysebaert M.T., de Kruif A. Silicone oil used in microdrop culture can affect bovine embryonic development and freezability. Reprod. Domest. Anim. 2001; 36(3-4): 169-76. https://dx.doi.org/10.1046/j.1439-0531.2001.00281.x.
Received 20.05.2024
Accepted 02.08.2024
About the Authors
Nadezhda N. Skorlupkina, Senior Embryologist, AltraVita Human Reproduction Clinic, IVF CENTER LLC, 4A Nagornaya str., 117186, Moscow, Russia, +7(968)536-16-80, nadezhdaskor@yandex.ruValentina P. Apryshko, PhD, Head of Embryology Department, AltraVita Human Reproduction Clinic, IVF CENTER LLC, 4A Nagornaya str., 117186, Moscow, Russia; Researcher at the Faculty of Biology, Lomonosov Moscow State University, 1/12 Leninskie gori, 119991, Moscow, Russia, +7(910)409-28-13, supermycolog@mail.ru
Elena B. Lebedeva, PhD, Coordinator of Medical Research, AltraVita Human Reproduction Clinic, IVF CENTER LLC, 4A, Nagornaya str., 117186, Moscow, Russia, +7(926)382-73-06, lebedester@gmail.com
Konstantin V. Kiriyenko, PhD, Leading Embryologist, AltraVita Human Reproduction Clinic, IVF CENTER LLC, 4A Nagornaya str., 117186, Moscow, Russia, +7(967)167-79-23, kkiriyenko@rambler.ru; https://orcid.org/0000-0001-8713-6231
Anna G. Mironova, Leading Embryologist, AltraVita Human Reproduction Clinic, IVF CENTER LLC, 4A Nagornaya str., 117186, Moscow, Russia, +7(916)167-00-88,
agm90@mail.ru
Sergey A. Yakovenko, PhD, CEO, AltraVita Human Reproduction Clinic, IVF CENTER LLC, 4A, Nagornaya str., 117186, Moscow, Russia; Researcher at Biophisics Department, Faculty of Physics, Lomonosov Moscow State University, 1/2 Leninskie gori, 119991, Moscow, Russia, +7(903)790-90-18, altravita@mail.ru
Corresponding author: Konstantin V. Kiriyenko, kkiriyenko@rambler.ru



