The effectiveness of infertility treatment involving transfer of cryopreserved embryos obtained after autologous embryo-cumulus cells co-culture in women with repeated inplantation failure
Objective: To evaluate the effectiveness of infertility treatment in the programs of cryo-embryo transfer, when embryos were obtained after autologous co-culture with cumulus cells in assisted reproductive technology (ART) programs.Asfarova G.R., Smolnikova V.Yu., Makarova N.P., Zingerenko B.V., Kalinina E.A.
Materials and methods: 198 married couples with repeated implantation failure (at least 2 attempts) were examined during the period of the study. The couples underwent IVF treatment with frozen-thawed embryo transfer into the uterine cavity. 114 couples underwent the program of autologous embryo-cumulus cells co-culture, and 84 couples underwent IVF treatment with frozen-thawed embryo transfer without using this program. Preparation of all women for cryo-transfer included cyclic hormonal treatment. Only one embryo was thawed and transferred into the uterine cavity. Pregnancy and birth rates were assessed.
Results: The study showed that autologous embryo-cumulus cells co-culture increased pregnancy rate in the group of women aged ≤35 years from 26.3% to 48.2% (OR 1.83; 95% CI 1.00–3.32) and significantly dicreases pregnancy rate in women of later reproductive age from 41.3% to 20.6% (OR 0.37; 95% CI 0.15–0.88). There was no significant difference in birth rates between the groups.
Conclusion: The results obtained in the study make it possible to recommend using autologous embryo-cumulus cells co-culture in women aged ≤35 years, who had repeated failed attempts of ART in history, to improve the effectiveness of infertility treatment.
Autors’ contributions: Asfarova G.R. – design of the study, collection and analysis of literature data, clinical and experimental data processing, article writing; Smolnikova V.Yu. – design of the study, patient selection, article editing; Makarova N.P. – analysis of the obtained results, article writing; Zingerenko B.V. – performance of co-culture at embryonic stage of ART program, collection of biological material; Kalinina E.A. – article editing and authorization for publication.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was conducted with financial support of the State Assignment of the Ministry of Health of the Russian Federation No. № 121040600410-7 "Solution of the problem of infertility in modern conditions by development of a clinical and diagnostic model of infertile marriage and the use of innovative technologies in assisted reproduction programs”.
Ethical Approval: This study was approved by the local Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov of the Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Asfarova G.R., Smolnikova V.Yu., Makarova N.P., Zingerenko B.V., Kalinina E.A. The effectiveness of infertility treatment involving transfer of cryopreserved embryos obtained after autologous embryo-cumulus cells co-culture in women with repeated inplantation failure.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2023; (9): 115-120 (in Russian)
https://dx.doi.org/10.18565/aig.2023.204
Keywords
One of the most acute problems facing the patients in assisted reproductive technology (ART) programs is recurrent implantation failure (RIF) [1]. There is no unique formal definition of RIF. Bashiri A. et al. suggest that the term “recurrent implantation failure” can be used when there are 3 consecutive unsuccessful implantation attempts after good quality embryo transfer [2]. Some authors state that at least 2 good quality embryos are enough [3]. Other authors suggest that it is necessary to take into account not only embryo quality, but also endometrial thickness [4]. Coughlan С. et al define this term as implantation failure after 4 good quality embryo transfer at least in 3 IVF cycles (native or thawed) in women under 40 years of age [5]. The guidelines (2023) of European Society of Human Reproduction and Embryology (ESHRE) describe RIF with calculation of likelihood of biochemical pregnancy in a couple undergoing ART program using the formula: [probability of implantation]n=1-[(1-PR)]n, where PR is pregnancy rate (or birth rate ×1.16). The consensus is that it is always advisable to consider the couple's anamnesis, age, and other clinical characteristics that may influence embryo implantation failure in a particular couple.
There is a whole range of possible causes and risk factors for RIF. One of the major factors is maternal age [5, 6], since it influences embryo quality and endometrial receptivity. With increasing age, the incidence of biochemical pregnancy and embryo-endometrium asynchrony increases [7]. For this reason, it is appropriate to pay attention to the woman’s age in development of new approaches to optimize infertility treatment programs using ART techniques.
One of therapeutic options for treatment of RIF is to improve embryo culture conditions in vitro, in particular, the use of co-culture techniques. Various cells of reproductive tissues (cumulus, endometrium, fallopian tube epithelium, etc.) were proposed as a “substrate” for embryos and zygotes. In this regard, the choice of cumulus cells is due to availability and ease of isolation for subsequent use in infertility treatment programs using ART techniques.
As is known, the ability of the oocyte to utilize glucose, cholesterol and some amino acids is limited due to insufficient expression of the transporters necessary for their uptake [8]. Moreover, the main source of adenosine triphosphate (ATP) production in the oocyte is oxidative phosphorylation; however, the oocyte has significantly low capacity for glucose absorption and metabolism. The oocyte depends on cumulus cells, where glucose is absorbed and metabolized through glycolysis to pyruvate, which then enters the oocyte for metabolism through oxidative phosphorylation. Pyruvate, which is produced as the end-product of glycolysis in cumulus cells is required to maintain oocyte meiotic maturation to metaphase II (MII) [8]. At the same time, the mechanism of communication between cumulus cells and the oocyte is bidirectional: the oocyte regulates the metabolism of cumulus cells, secreting soluble growth factors that target cumulus cells [9].
Autologous embryo-cumulus cells co-culture in ART programs began to be used as one of the methods for treatment of RIF, since previously the difference in the rate of the growth and development of embryos was shown on animal models, as well as embryo morphology between the groups of classical embryo culture and embryo-cumulus cells co-culture [10].
In literature, there are currently conflicting data on the effect of embryo-cumulus cells co-culture on likelihood of implantation and occurrence of pregnancy in infertility treatment programs using ART techniques [11]. Our previous study of autologous embryo-cumulus cells co-culture showed significant increase in the number of embryos of better morphologic quality in the general cohort of women with RIF [12]. It was shown that autologous embryo-cumulus cells co-culture increases likelihood of implantation by 2.5 times in women aged 23–30 years. In women of other age groups autologous co-culture is not advisable.
The purpose of this study was to evaluate the effectiveness of infertility treatment in the programs of cryo-embryo transfer, when embryos were obtained after autologous co-culture with cumulus cells in assisted reproductive technology (ART) programs.
Materials and methods
This study was carried out in Prof. B.V. Leonov Department of Assisted Technologies in Infertility Treatment (Head of the Department – Kalinina E.A., Dr. Med. Sci., Professor) of the Institute of Reproductive Medicine (Director – Nazarenko T.A., Dr. Med. Sci., Professor), Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Director of the Center – Sukhikh G.T., Academician of the Russian Academy of Sciences). Infertile patients in IVF programs were recruited in the period from March 2017 to January 2019 in accordance with the goals. The outcomes of 198 cryotransfer programs were evaluated. Of them, 114 programs were blastocysts-cumulus cells co-culture and 84 programs without co-culture. Inclusion criteria were: no contraindications for infertility treatment using ART techniques in accordance with the Order No. 107n of the Ministry of Health of Russia of August, 2012 “On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use”, the patients aged 18–40 years; regular periods, anatomically normal uterus, endometrial preparation in addition to hormone replacement therapy cycle; at least 2 unsuccessful transfers of morphologically normal blastocysts into the uterine cavity (both native and cryopreserved). Non-inclusion criteria were contraindications for infertility treatment using ATY techniques in accordance with the Order No. 107n of the Ministry of Health of Russia of August, 2012 “On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use”; lesss that 2 failed IVF attempts in anamnesis of married couples; congenital malformations of geni tal tract (including conditions after surgical correction) or acquired uterine deformation, when there are no chances for embryo implantation or pregnancy; pronounced impairment of spermatogenesis, including IVF after testicular sperm extraction. All couples signed informed consent to participate in the study.
Embryos were co-cultured during ovarian stimulation phase in accordance with previously described method [12]. In short, on the day of transvaginal puncture, cumulus cells were cut off with a sterile microinstrument and placed into a separate well cell culture Petri dish in a culture medium (G-IVF, Vitrolife, Sweden) and kept for 24 hours before use. Normally fertilized zygotes were placed in a well with cumulus cells and cultured for up to 5 days. Embryo quality assessment was performed in accordance to Recommendations of the Russian Association of Human Reproduction (RAHR) (2020). Vitrification was performed according to the manufacturer’s instruction using Kitazato media (Japan). Embryos were stored in liquid nitrogen until cryotransfer.
Endometrial preparation in cryo cycles was performed with administration of estradiol and progesterone. On day 2-3 of the menstrual cycle, cervical assessment by ultrasound was done. Estradiol valerate was administered at a dosage of 2 mg daily, and endometrial thickness and structure was subsequently monitored on day 6–8 and 10–12 of the menstrual cycle. When endometrial thickness measured by ultrasound, reached more than 7–8 mm, micronized progesterone was administered at a dose of 200 mg 3 times a day according to the manufacturer’s instruction. Embryos were thawed in Kitazato culture media (Japan). Ultrasound guided embryo transfer was performed using single-use atraumatic COOK catheters (Ireland). The diagnosis of pregnancy was based on the results of β-chorionic gonadotropin (β-hCG) blood test on day 14 after embryo transfer. When β-hCG blood test results were positive, pelvic ultrasound was performed on day 21 to visualize the fertilized egg in the uterine cavity. The results of the cryotransfer program were assessed 9 months after pregnancy registration.
Statistical analysis
SPSS software program was used for statistical analysis of the obtained data on the clinical stage of the study. Statistical significance was checked by Fisher’s exact test and Chi-square test. Evaluation of statistical significance of the relationship between the outcome and risk factor was based on calculation of odds ratio (OR) with 95 confidence interval (CI). The threshold for statistical significance was at p=0.05.
Results
The first stage of the study was evaluation of the outcomes in the programs of thawed embryo transfer using different culture methods. After autologous embryo-cumulus cells co-culture, 114 embryos were transferred into the uterine cavity, and 84 embryos were transferred without co-culture. Each patient underwent only single embryo transfer. The clinical outcomes are shown in Table 1.
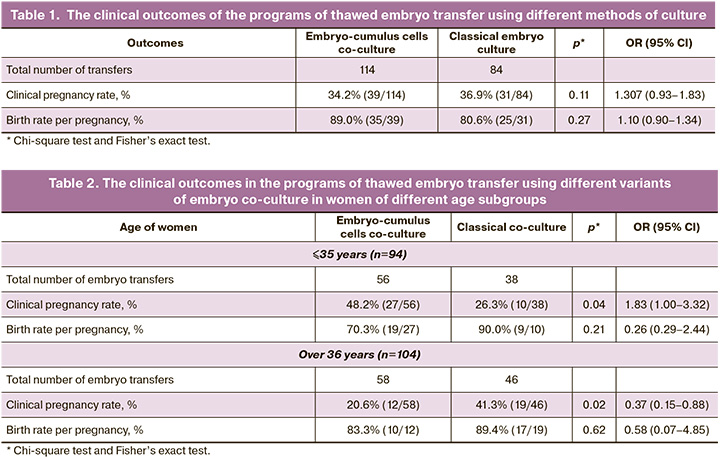
As can be seen from the presented data, there was no significant difference between pregnancy rates in cryo cycles between the groups – 34.2% in the group of women with embryo-cumulus cells co-culture and 36.9% in the group with classical embryo culture. Also, there was no significant difference in the birth rates (89.0% and 80.6%, p=0.27). The next stage was stratification of patients by age. The women were divided into age groups ≤35 years and >36 years. The same parameters of the outcomes in infertility treatment programs were estimated. The results are shown in Table 2.
The group of young women of ≤35 years underwent 56 embryo transfers after autologous embryo-cumulus cells co-culture and 38 transfers without co-culture. Embryo implantation into the uterine cavity occurred in 39.3% of cases (37/94). Of them, with autologous embryo-cumulus cells co-culture in 48.2% of women of the group (27/56), and in 26.3% (10/38) in the group without embryo co-culture. These data are statistically significant, р=0.04 (OR=1.83, 95% CI 1.00–3.32). It can be said that autologous embryo-cumulus cells co-culture increases the chances for young patients (≤35 years) with RIF to get pregnant, and this technique can be used to improve the effectiveness of infertility treatment programs by using ART. At the same time, the birth rate did not change significantly in these patients and was 70.3% in the group with autologous embryo-cumulus cells co-culture, and 90% without embryo co-culture.
Assessment of the outcomes in the programs of embryo cryotransfer in women of late reproductive age showed the opposite dynamics. A total of 104 cryotransfers were performed in women over 35 years, in 58 of them after autologous co-culture and in 46 without it. Pregnancy rate following embryo transfer after co-culture was 20.6% and 41.3% without co-culture (р=0.02; OR 0.37, 95% CI 0.15–0.88). As can be seen from the data presented, implantation rate in women of older age group with RIF depends on embryo co-culture technique, and autologous embryo-cumulus cells co-culture significantly worsens treatment results. At the same time, there was no statistically significant change in the birth rate per pregnancy (Table 2).
The data obtained in the study showed that there was statistically significant increase in pregnancy rate in young patients with application of autologous embryo-cumulus cells co-culture technique. At the same time, pregnancy rate significantly declined in women of older age group in cryotransfer programs.
Discussion
Autologous embryo-cumulus cells co-culture was suggested as one of the methods to overcome RIF in infertility treatment programs. However, scientific evidence was conflicting, and the patient contingent, in whom this technique could be recommended for application to improve treatment effectiveness, was not specified. In this study, we assessed the clinical effectiveness of thawed embryo transfer after using different co-culture techniques. The analysis of obtained data showed that application of autologous embryo-cumulus cells co-culture technique in the group of women with RIF under 35 years can significantly improve the effectiveness of treatment. Pregnancy rate in these patients increased from 26.3% to 48.2%. Cumulus cells increased the ability for embryo implantation and facilitated overcoming implantation failure.
The opposite effect of cumulus cells was in women of late reproductive age. With autologous co-culture, pregnancy rate decreased by 2 times – from 41.3% to 20.6%. Namely, it can be said that cumulus cells have a “toxic” effect on “older” patients when they are added to embryo culture. The obtained result can be explained by the general mechanisms of ovarian aging, which is observed with increasing of woman’s reproductive age. The scientific data show that impairments occur in all ovarian cells, especially in the growing cumulus-oocyte complexes [13]. In addition, there is a time frame of so-called postovulatory aging of cumulus-oocyte complexes [14]. Using omics technologies, the authors of this study identified the mechanism by which cumulus cells accelerate postovulatory aging through IL1-IL1R1 in cytokine–cytokine receptor interaction. After long-term co-culture of mouse oocytes with cumulus cells in vitro, many abnormalities were found, including abnormal distribution of aggregated mitochondria, an abnormal increase in ROS levels, early occurrence of apoptosis, and impairment of preimplantation embryo development. In other words, age-associated changes in cumulus cells impair both the morphology and genetic status of embryos [14].
Other authors reported that cumulus cells accelerate aging of mouse oocytes by secreting soluble and heat-sensitive factors during long-term culture [15]. Even if cumulus cells are not connected to oocytes through gap junctions, their distant interaction occurs in a drop of culture medium, where a zygote and autologous cumulus cells are placed.
With aging, irreversible changes occur in cumulus cells in the form of dysfunction of mitochondria, apoptosis and abnormal accumulation of ATP [14]. Placement of these “senescent” cells in embryo culture may impair embryo development, or at least does not facilitate its implantation after transfer into the uterine cavity. That is why the obtained results indicate inadvisability of autologous embryo-cumulus cells co-culture in women over 35 years.
The data obtained by us correlate with the results that were reported by Virant-Klun А. et al., who compared the groups of oocytes which were cultured in vitro using classical method, and in vitro using co-culture in young women. They found that gene expression profile of oocytes during co-culture is most similar to expression profile during natural oocyte maturation in vivo. Additionally, these two groups of samples had the greatest overlapping of expressed genes [16]. In young women, cumulus cells can facilitate “maturation” of intracellular structures and embryo fragmentation, and good-quality blastocyst formation.
The birth rates in both age groups did not change significantly. This may indicate that there are other important parameters that influence pregnancy progression to delivery in women with RIF.
Conclusion
Personification approach to infertility treatment plays an important role in increasing the effectiveness of ART programs, particularly with regard to the group of patients with RIF. This contingent of married couples is one of the most difficult in clinical practice in terms of applying techniques with proven effectiveness. In most cases, ART specialists use empirical approaches, offering modified infertility treatment options at the embryonic stage. One of the method can be autologous embryo-cumulus cells co-culture. However, the results of this study showed that this technique is effective only in young women under the age of 35. Autologous co-culture increases pregnancy rate by 1.83 times only in young patients with RIF, and this technique can be recommended for application in them.
References
- Cutting R. Single embryo transfer for all. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 53: 30-7. https://dx.doi.org/10.1016/j.bpobgyn.2018.07.001.
- Bashiri A., Halper K.I., Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018; 16(1): 121. https://dx.doi.org/10.1186/s12958-018-0414-2.
- Orvieto R., Brengauz M., Feldman B. A novel approach to normal responder patient with repeated implantation failures--a case report. Gynecol. Endocrinol. 2015; 31(6): 435-7. https://dx.doi.org/10.3109/09513590.2015.1005595.
- Zeyneloglu H.B., Onalan G. Remedies for recurrent implantation failure. Semin. Reprod. Med. 2014; 32(4): 297-305. https://dx.doi.org/10.1055/s-0034-1375182.
- Coughlan C., Ledger W., Wang Q., Liu F., Demirol A., Gurgan T. et al. Recurrent implantation failure: definition and management. Reprod. Biomed. Online. 2014; 28(1): 14-38. https://dx.doi.org/10.1016/j.rbmo.2013.08.011.
- Zeadna A., Son W.Y., Moon J.H., Dahan M.H. A comparison of biochemical pregnancy rates between women who underwent IVF and fertile controls who conceived spontaneously†. Hum. Reprod. 2015; 30(4): 783-8.https://dx.doi.org/10.1093/humrep/dev024.
- Larsen E.C., Christiansen O.B., Kolte A.M., Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013; 11: 154.https://dx.doi.org/10.1186/1741-7015-11-154.
- Richani D., Dunning K.R., Thompson J.G., Gilchrist R.B. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum. Reprod. Update. 2021; 27(1): 27-47. https://dx.doi.org/10.1093/humupd/dmaa043.
- Gilchrist R.B., Lane M., Thompson J.G. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update. 2008; 14(2): 159-77. https://dx.doi.org/10.1093/humupd/dmm040.
- Krisher R.L., Heuberger A.L., Paczkowski M., Stevens J., Pospisil C., Prather R.S. et al. Applying metabolomic analyses to the practice of embryology: physiology, development and assisted reproductive technology. Reprod. Fertil. Dev. 2015; 27(4): 602-20. https://dx.doi.org/10.1071/RD14359.
- Mansour R.T., Aboulghar M.A., Serour G.I., Abbass A.M. Co-culture of human pronucleate oocytes with their cumulus cells. Hum. Reprod. 1994; 9(9): 1727-9. https://dx.doi.org/10.1093/oxfordjournals.humrep.a138782.
- Асфарова Г.Р., Смольникова В.Ю., Макарова Н.П., Бобров М.Ю., Эльдаров Ч.М., Зингеренко Б.В., Калинина Е.А. Клинические и молекулярные аспекты аутологичного сокультивирования эмбрионов с клетками кумулюса в программах экстракорпорального оплодотворения. Акушерство и гинекология. 2023; 4: 97-110. [Asfarova G.R., Smolnikova V.Yu., Makarova N.P., Bobrov M.Yu., Eldarov Ch.M., Zingerenko B.V., Kalinina E.A. Clinical and molecular aspects of autologous embryo cumulus cells co culture in IVF programs. Obstetrics and Gynecology. 2023; (4): 97-110. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.306.
- Zhao H., Wang Y., Yang Y. Follicular development and ovary aging: single-cell studies. Biol. Reprod. 2023 Jul 28: ioad080. https://dx.doi.org/10.1093/biolre/ioad080.
- Wen X., Yang Q., Sun D., Jiang Z.Y., Wang T., Liu H.R. et al. Cumulus cells accelerate postovulatory oocyte aging through IL1-IL1R1 interaction in mice. Int. J. Mol. Sci. 2023; 24(4): 3530. https://dx.doi.org/10.3390/ijms24043530.
- Qiao T.W., Liu N., Miao D.Q., Zhang X., Han D., Ge L., Tan J.H. Cumulus cells accelerate aging of mouse oocytes by secreting a soluble factor(s). Mol. Reprod. Dev. 2008; 75(3): 521-8. https://dx.doi.org/10.1002/mrd.20779.
- Virant-Klun I., Bauer C., Ståhlberg A., Kubista M., Skutella T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online. 2018; 36(5): 508-23.https://dx.doi.org/10.1016/j.rbmo.2018.01.011.
Received 23.08.2023
Accepted 13.09.2023
About the Authors
Gunay R. Asfarova, postgraduate student, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(926)262-11-13, asfarovag@gmail.com,117997, Russia, Moscow, Academician Oparin str., 4.
Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, v_smolnikova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, np_makarova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Boris V. Zingerenko, Junior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, b_zingerenko@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, e_kalinina@oparina4.ru, 117997, Russia, Moscow,
Academician Oparin str., 4.



