Comparative effectiveness of different laparoscopic metroplasty techniques in patients with significant uterine scar defects after cesarean section
Martynov S.A., Sukhareva T.A., Adamyan L.V.
Objective: To improve the effectiveness of surgical management in patients with significant uterine scar defects (USD) after cesarean section (CS). Materials and methods: This comparative prospective study included 113 patients with a diagnosis of significant USD after CS who were interested in repeat pregnancies. All the patients underwent laparoscopic metroplasty. In group 1 (n=61), patients underwent standard laparoscopy, and in group 2 (n=52), laparoscopy was performed using a technique with shortening and plication of the uterine round ligament. Baseline clinical evaluation included clinical data and medical history, index of undifferentiated connective tissue dysplasia (uCTD), preoperative and postoperative scar condition according to expert ultrasound, and analysis of reproductive outcomes. A comparative analysis of the results of two methods of surgical treatment was performed. Results: In USD patients after CS, the most common complaints were menstrual cycle disorders, including postmenstrual bleeding (70.8%), intermenstrual bleeding (12.4%), heavy menstrual bleeding (3.5%), painful menstrual bleeding (8%), pain syndrome (16.8%), and secondary infertility (31%). There was a high incidence of two or more previous CS (36.3%) and the emergency nature of the previous CS (68.1%). Clinical features of USD included retroflexio uteri (75.2%) and concomitant uCTD (46%). As a result of standard laparoscopic metroplasty, clinical manifestations disappeared in 90.2% of patients and the mean minimal scar thickness (mST) increased significantly to 4.8 (1.8) mm (p=0.00001). The use of round ligament shortening and plication as an additional stage allowed the elimination of clinical manifestations in 100% of cases, and the mean mST as a result of the operation significantly increased and amounted to 5.3 (1.2) mm (p=0.00001). In addition, the increase in mST was significantly greater in the round ligament shortening group than in the standard technique group (3.16 (1.21) and 2.5 (2.07) mm, respectively) (p=0.049). In addition, there were no unsatisfactory results in the round ligament shortening group, while the incidence of unsatisfactory results in the metroplasty without round ligament shortening group was 6/61 (9.8%) (p=0.036). According to the statistical analysis, the main risk factors for unsatisfactory results of metroplasty were three or more previous CS and the presence of uCTD with a diagnostic coefficient (DC) ≥17 points. Conclusion: Shortening and plication of the round ligament can improve the results of laparoscopic metroplasty. The risk factors for unsatisfactory results of metroplasty are the presence of three or more CS in the past history and uCTD (DC uCTD ≥17 points).
Authors' contributions: All listed authors made substantial, direct and intellectual contribution to the work and approved it for submission. Adamyan L.V., Martynov S.A., Sukhareva T.A. – conception and design of the study; Adamyan L.V., Martynov S.A., Sukhareva T.A. – carrying out surgical treatment; Sukhareva T.A., Martynov S.A. – data collection and analysis, statistical analysis; Martynov S.A., Adamyan L.V., Sukhareva T.A. – drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Martynov S.A., Sukhareva T.A., Adamyan L.V. Comparative effectiveness of different laparoscopic metroplasty techniques in patients with significant uterine scar defects after cesarean section. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (10): 126-136 (in Russian) https://dx.doi.org/10.18565/aig.2023.163
Keywords
The incidence of cesarean section (CS) has been increasing globally, with rates reaching 40.5% in certain regions [1, 2]. In Russia, the rate stands at 30.3% [3].
With the rise in CS procedures, a new issue has emerged: the formation of uterine scar defects (USD) following cesarean deliveries, which have become quite common. While most scar defects are asymptomatic and are discovered incidentally during ultrasound examinations (USI), they can cause menstrual disorders (MD) and pain syndrome in some cases. This can significantly affect the quality of life for patients and potentially lead to complications during subsequent pregnancies and childbirth [4]. Due to these complications, there is increasing interest in studying the risk factors associated with USD formation, as well as exploring potential treatment options.
Conservative treatment is generally recommended for patients who do not plan to have any more pregnancies. On the other hand, surgical treatment is recommended for patients who are interested in getting pregnant and have a significant USD.
Metroplasty is the most common surgical treatment for significant USD. It involves the excision of thinned fibrous tissue in the USD area, suturing of the uterus, and peritonization using the peritoneum of the vesicouterine fold.
However, it is worth noting that even skilled surgeons encounter approximately 10% of unsatisfactory metroplasty outcomes, regardless of the approach used [5-8]. Thus, there is an ongoing search for new surgical treatment methods to decrease the percentage of unsatisfactory results in women with USD following a CS.
The objective of this study was to improve the effectiveness of surgical management in patients with significant USD after CS.
Materials and methods
This prospective observational comparative study was conducted at V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The study included 113 patients with significant USD after CS in the lower uterine segment (minimal scar thickness (mST) < 2.5 mm according to echo hysterosalpingography (eHSG) or mST < 3 mm according to magnetic resonance imaging (MRI) [9, 10]) who were interested in repeat pregnancy; more than one year had passed since the last CS.
The inclusion criteria were age 18–45 years, pregnancy planning, USD after CS in the lower uterine segment, scar location in the projection of the internal os or in the lower third of the uterine body according to hysteroscopy, and signed informed consent for participation in the study.
Exclusion criteria were age <18 and >45 years, acute inflammatory pelvic diseases, severe somatic comorbidities, malignant neoplasms, and scar location in the cervical area (below the internal os) according to hysteroscopy.
Before inclusion in the study, all patients were examined according to the examination algorithm accepted by the department. They underwent expert ultrasound (eUS) and eHSG or MRI, and confirmed USD (mST<2.5 mm according to eHSG or mST<3 mm according to MRI), which required surgical correction [9, 10].
In the first stage of the preoperative period, all 113 patients were evaluated for clinical and anamnestic data, signs of undifferentiated connective tissue dysplasia (uCTD), calculation of the uCTD diagnostic coefficient (DC uCTD) [11], and assessment of scar status using eUS, eHSG, or MRI [9, 10].
In the second stage, all 113 patients underwent a metroplasty. In group 1 61 patients underwent laparoscopic metroplasty according to the standard technique. In group 2, 52 patients underwent laparoscopic metroplasty with an additional step of shortening and plication of the uterine ligaments.
Technique of the operation
The operation was performed under endotracheal anesthesia. Initially, liquid hysteroscopy was performed, during which the condition of the uterine cavity was investigated, and the scar area was revised. A rigid Karl Storz hysteroscope with an external diameter of 5 mm was used. There was no need to dilate the cervical canal. Sterile 0.9% sodium chloride solution was used as a medium for uterine cavity dilation. First of all, the uterine cavity was examined, its shape was determined, as well as the presence of pathological formations and the condition of the fallopian tube openings. The scar zone was carefully evaluated, including the location of the defect relative to the internal os, the presence of the niche and its size (transverse, longitudinal, depth), branches and pockets, the shape of the niche edges (smoothed or undermined), and its bottom (the presence of dilated vessels, foci of endometriosis, and endocervical cysts). After hysteroscopy, Hegar dilators were used to dilate the cervical canal up to 10 mm; dilator No. 10 was not removed, and it was further used as a manipulator.
Subsequently, laparoscopy and revision of the pelvic organs and the abdominal cavity were performed. The position, contour, and size of the uterus, the condition of the uterine appendages, and the presence of adhesions in the area of the post-CS uterine scar were evaluated.
Metroplasty consisted of several stages.
Stage I: Separation of adhesions with sharp and blunt instruments (if adhesions are present) (Fig. 1a).
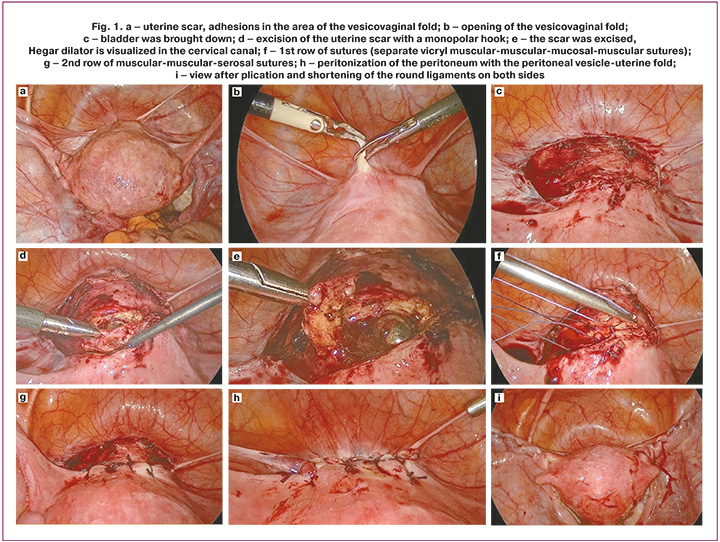
Stage II: opening of the vesicouterine fold and lowering of the bladder to the middle third of the cervix or lower (10–15 mm away from the lower edge of the defect), and isolation of the scar area (Fig. 1b–c).
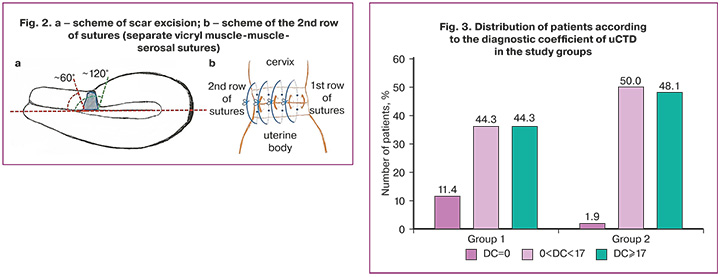
Stage III: The thinned myometrium and fibrous tissue in the scar area are removed using a monopolar hook in cutting mode (power not exceeding 80 W, which provides a low coagulation effect on the tissue). For better visualization of the defect edges, the uterine manipulator was partially removed from the uterine cavity, leaving the upper pole at the level of the excised scar and tilted forward or backward, opening access to the upper and lower edges of the incision (Fig. 1d–e). Excision of the thinned scar in the lower part (closer to the cervix) was performed at an angle of approximately 60° to the longitudinal axis of the uterus and in the upper part (closer to the uterine body) at an angle of approximately 120° to the longitudinal axis of the uterus to increase the contact area of the subsequent apposition of wound edges (Fig. 2a) [12]. After removal of the excised tissue, selective bipolar hemostasis was performed if necessary.
Stage IV: Uterine suturing was performed layer-by-layer in two rows. The first row was performed using separate interrupted extracorporeal muscular, mucosal, muscular "Vicryl 2-0" sutures at a distance of 5 mm from each other (Fig. 1f). The needle was inserted and withdrawn in the middle of the thickness of the juxtaposed edges of the myometrium on both sides, carrying it out with capture of the mucous layer. At the same time, using the intrauterine dilator Hegar, capture of the posterior wall of the cervix and uterine body into the suture was prevented. The second row of separate interrupted muscular-muscular-serosal sutures was sutured extracorporeally with "Vicryl 0" sutures in a chequer-wise order in relation to the sutures of the first row (Fig. 1h). The needle was inserted and withdrawn 4 mm from the incision edges on both sides, passing it through the middle of the thickness of the apposed myometrial edges (Fig. 2b).
Stage V: peritonization of the suture line with the peritoneum of the vesicoureteral fold using "monocryl 2-0" sutures (Fig. 1i).
Fifty-two patients (group 2) additionally underwent plication and shortening of the round ligaments of the uterus using a monofilament sutures with a long resorption period "PDS*II 0". Endoscopic suturing of the uterine round ligaments along their entire length with "gathering" continuous sutures was performed starting from the place of their entry into the internal opening of the inguinal canal, towards the uterus with myometrium capture and back, the needle was cut. Extraction of the free ends of the " PDS*II 0" sutures from the abdominal cavity was performed as follows: at a point 1 cm above the projection of the internal ring of the inguinal canal, a 3-mm long skin incision was made with a scalpel, and a scorning needle was passed through it into the abdominal cavity. One suture end was grasped and brought out. The second puncture of the skinning needle was made 3 mm away from the first one, leaving an aponeurotic bridge between the punctures, and the second end of the sutures was similarly withdrawn. This procedure was repeated on the opposite side. The ends of the sutures on both sides were tied over the aponeurosis under laparoscopic control until the uterus reached the anteflexed position (Fig. 1).
During the postoperative period, all patients underwent nonspecific measures to prevent thrombotic complications, including early activation and elastic compression of the lower limbs. Low-molecular-weight heparin was administered to patients at risk for thromboembolic complications under the control of coagulation tests. All patients were treated with antibacterial therapy for five days (broad-spectrum antibiotics: semisynthetic penicillins or cephalosporins). Symptomatic and infusion therapies were administered, as indicated.
The efficacy of metroplasty was monitored 6 months after surgery using a questionnaire to assess complaints and eUS to assess parameters of the new scar due to the noninvasive nature of the study. The new scar parameters obtained were compared with similar preoperative eUS parameters. For eUS measurements, the standardized technique adopted in the Delphi study was used [13]. A value of mST<3 mm was considered the ultrasound criterion for the ineffectiveness of the performed metroplasty. After obtaining satisfactory results according to the follow-up eUS, pregnancy preparation was initiated 6 months after surgery.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2010 and STATISTICA 10. The normality of the distribution was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Variables showing a normal distribution are expressed as the mean (standard deviation). Student's t-test was used to compare the data obtained. Qualitative variables were compared using the nonparametric Mann–Whitney test. The critical level of significance when testing the statistical hypotheses was set at p<0.05. The assessment of the influence of individual qualitative factors on the success of the operation was performed using contingency tables, with the calculation of the chi-square criterion, which makes it possible to determine whether the relationship between the values of a factor and the success of the operation is statistically significant, and the relative risk with a 95% confidence interval (CI). To compensate for the small number of observations, a bootstrap estimation procedure based on 1,000 and 2,000 samples was applied when calculating the parameters from the contingency tables.
Results
The mean age of the USD patients was 34.1 (3.9) years (group 1 – 33.9 (3.5) years, group 2 – 34.6 (4.2) years, p=0.379). At the time of the study, all women had more than one year since their last CS. When eUS data were evaluated, patients in group 1 had an mST of 2.3 (0.9) mm, and patients in group 2 had an mST of 2.1 (0.6) mm.
The most frequent clinical manifestations of USD were MD, including bloody discharge before menstruation, which was noted in 2/61 (3.3%) patients in group 1, after menstruation in 80/113 (70.8%) patients (41/61 (67.2%) in group 1, 39/52 (75%) in group 2), intermenstrual blood discharge in 14/113 (12.4%) patients (8/61 (13.1%) in group 1, 6/52 (11.5%) in group 2), and heavy menstrual bleeding in 4/113 (3.5%) patients (2/61 (3.3%) in group 1, 2/52 (3.8%) in group 2), p=0.897). Intermittent pulling pain in the lower abdomen, occurring after the last CS (pain syndrome), was reported by 19/113 (16.8%) patients (13/61 (21.3%) in group 1 and 6/52 (11.5%) in group 2; (p=0.169). Painful menstruation was reported by 9/113 (8%) patients (7/61 (11.5%) in group 1 and 2/52 (3.8%) in group 2, p=0.139).
Secondary infertility occurred in 35/113 (31%) patients and was less frequent in group 1 than in group 2 (13/61 (21.2 %) and 22/52 (42.3 %) patients, respectively; p=0.017). The duration of infertility in the groups averaged 4.8 (2.9) and 4.0 (1.9) years, respectively, p=0.334.
When assessing somatic comorbidities in patients with USD, the following diseases were the most common:
- Musculoskeletal system diseases (scoliosis, flat feet, joint hypermobility) – 54/113 (47.8%): 25/61 (41%) and 29/52 (55.8%) in groups 1 and 2, respectively; p=0.139.
- Varicose veins of the lower limbs – 52/113 (46%): 24/61 (39,3%) and 28/52 (53,8%) in groups 1 and 2, respectively; p=0,184;
- Visual disorders (myopia, astigmatism) – 42/113 (37.2%): 23/61 (37.7%) and 19/52 (36.5%) in groups 1 and 2, respectively; p=0.821.
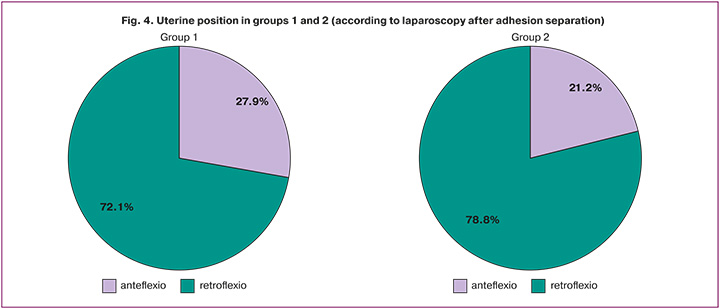
When uCTD signs were assessed in the groups according to clinical guidelines [11], the diagnosis was established when the DC of uCTD reached a value of 17 or more. In group 1, the diagnosis of uCTD was established in 27/61 (44.3%) patients (DC=19.2 (1.6)) and in group 2, in 25/52 (48.1%) patients (DC=19.0 (1.7)) (p=0.917) (Fig. 3).
No differences were found in the analysis of other somatic comorbidities.
Among the extragenital surgical interventions performed, appendectomy was the most frequent (group 1 – 11/61 (18%); group 2 – 11/52 (21.1%]). Several patients underwent surgeries related to CTD manifestations: in group 1, one operation for umbilical hernia and one repair of divergence of the rectus abdominis of the anterior abdominal wall; in group 2, one operation for inguinal hernia.
Importantly, only 31/113 (27.4%) patients were able to provide a postpartum case report, and only 9/113 (8%) patients had a CS case report, making it difficult to assess the impact of the technique and duration of surgery, suture material, intraoperative blood loss, level of incision during CS, and cervical opening at the time of CS on USD formation.
Analysis obstetric history showed that the total number of pregnancies per patient in groups 1 and 2 was 2.1 (1.3) and 2.0 (1.1), respectively, and the total number of CS per patient was 1.5 (0.6) and 1.4 (0.6), respectively. Two or more CS groups, 1 and 2, were reported by 26/61 (42.3%) and 15/52 (28.8%) patients, respectively. Analysis of the course of the last pregnancy ending in CS showed a significant prevalence of emergency CS in both groups (77/113 [68.1 %] patients).
Cervical insufficiency was observed in 11/113 (9.7%) patients, including 6/61 (9.8%) and 5/52 (9.6%) in groups 1 and 2, respectively (p=0.973).
Analysis of the postpartum period showed that after the last CS, hematometra was diagnosed in 3/61 (4.9%) patients in group 1, which required vacuum aspiration and emptying of the hematometra in all cases. In group 2, hematometra was diagnosed in six (11.5%) patients (p=0.218) and required medical treatment in three cases and hysteroscopy and emptying of the hematometra in three cases. Also, one patient from group 1 underwent relaparotomy, emptying the hematoma in the area of the uterine suture after CS on the 10th day after delivery.
Results of surgical treatment
The comparative evaluation of the two methods of metroplasty showed no statistically significant differences in intraoperative blood loss (group 1 – 57.0 (26.3) ml; group 2 – 54.2 (28.2) ml; p=0.586) and duration of surgery (group 1 – 94.1 (23.2) min; group 2 – 101.3 (20.5) min, p=0.084). On average, the operation with shortening and plication of the round ligaments took 7 min longer due to the low complexity of this stage.
Hysteroscopy enabled determination of the exact location of the scar defect, the presence of a niche, its borders, and the presence of endocervical cysts and atypically dilated vessels. In group 1, the scar was located in the area of the internal os in 50/61 (82%) cases, in the area of the lower third of the uterine body in 11/61 (18%) cases; in group 2, in the area of the internal os in 46/52 (88.5%) cases, in the area of the lower third of the uterine body in 6/52 (11.5%) cases (p=0.340).
During laparoscopy, pelvic adhesions were detected in the form of bladder fixation to the anterior wall of the uterus in the scar area after CS in 51/61 (83.6%) patients in group 1 and in 40/52 (76.9%) patients in group 2 (p=0.376).
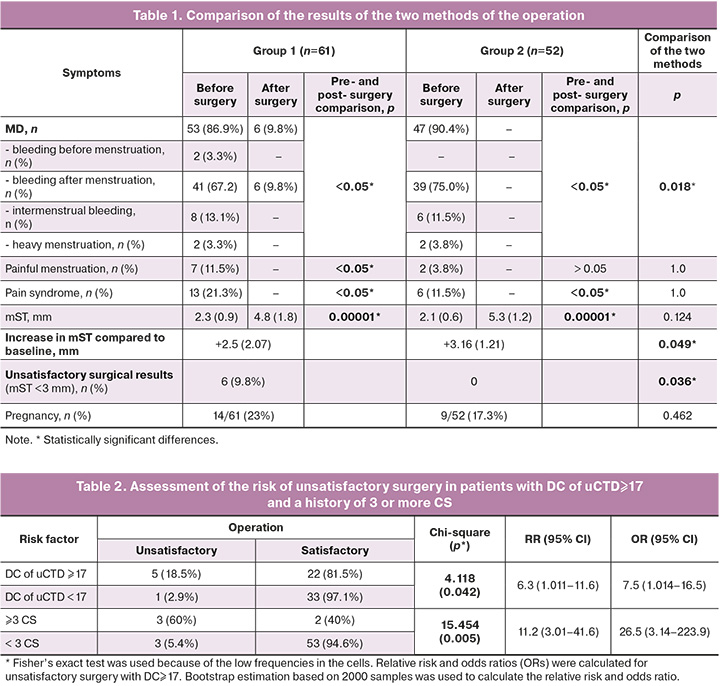
Thus, after separation of adhesions, the final evaluation of uterine position revealed that anteflexed uterine position was determined in 28/113 (24.8%) patients (in group 1 – 17/61 (27.9%); group 2 – 11/52 (21.2%)) and anteflexed uterine position in 85/113 (75.2%) patients (in group 1 – 44/61 (72.1%); group 2, 41/52 (76.9%); p=0,414 (Fig. 4).
All operations were performed without intra- or postoperative complications, and the patients were discharged in satisfactory condition on the 6-7th day after surgery. One patient in group 2 had paresthesia along the right genitofemoral nerve, which resolved spontaneously within 3 weeks.
At repeated questioning of the patients 6 months after surgery, a significant reduction in complaints in both groups was noted. MDs resolved in 94/100 (94%) patients in both groups, and in group 2, the resolution of the complaints was significantly more frequent than in group 1 (47/47 (100%) patients from group 2 and 47/53 (88.7%) from group 1, p=0.018). Pain syndrome was absent in both groups (p<0.05) (Table 1). Thus, the resolution of clinical manifestations was noted in 90.2% (55/61) of patients in group 1 and 100% (52/52) of cases in group 2 (p=0.021).
Follow-up eUS 6 months after surgery to evaluate the effectiveness of the metroplasty showed that mST in group 1 was slightly smaller than in group 2 and amounted to 4.8 (1.8) and 5.3 (1.2) mm, respectively (p=0.124). However, the increase in mST compared to preoperative values in group 2 was statistically significantly greater compared to group 1 and amounted to +3.16 (1.21) and +2.5 (2.07) mm, respectively, p=0.049. In addition, group 1 had 6 (9.8%) cases of unsatisfactory outcomes (mST<3 mm), 4 (7.7%) cases had an mST of 3 mm, and the rest (82.7%) had an mST greater than 3 mm; group 2 had no unsatisfactory outcomes, and 100% of cases had an mST greater than 3 mm, which constituted a statistically significant difference (p=0.036).
Evaluation of the risk factors for unsatisfactory surgical outcomes identified two parameters that significantly influenced the outcome of surgery (Table 2).
A DC of uCTD ≥17 increased the risk (likelihood) of unsatisfactory surgery by 6.3-fold and the odds of an unfavorable outcome by 7.5-fold compared to patients with a DC of uCTD<17.
In patients with a history of three or more CS, the risk (likelihood) of an unsatisfactory surgical outcome was 11.2 times higher, and the odds of an unfavorable outcome were 26.5 times higher than in patients with a history of less than three CS events.
Evaluation of reproductive outcomes showed that in group 1, pregnancy occurred in of 14/61 (23%) patients:4 patients were currently pregnant, 3 pregnancies ended in missed miscarriage in early pregnancy, 2 patients ended in spontaneous abortion, 1 patient had an ectopic (tubal) pregnancy requiring laparoscopy and tubectomy, and 4 patients ended in operative delivery at term. Repeat CS was routinely performed at a mean gestational age of 37.2 (1.1) weeks, with placenta accreta in two cases.
In the 2nd group, pregnancy occurred in 9/52 (17.3%) patients: in three patients, the pregnancy ended in missed miscarriage in early pregnancy, two patients were currently pregnant, and in four pregnancies ended in operative delivery at term. Repeat CS was routinely performed at a mean gestational age of 38.1 (0.2) weeks, with 1 case of dichorionic diamniotic twins.
In both groups, there were no signs of scar defects during pregnancy or childbirth.
Discussion
Today, an important task is not only to optimize the diagnosis of USD but also to identify risk factors for atypical reparative processes in the uterus. In addition, it is crucial to develop new surgical treatment methods that can effectively promote optimal conditions for de novo scar formation.
Recognized risk factors for USD formation include the level of incision and suturing technique during CS, condition of the lower uterine segment during CS, retroflexed uterus, adhesion formation in the scar area, number of previous CSs, presence of postpartum inflammatory diseases, and somatic comorbidities [4, 14–31]. However, it remains unclear which factor has the most significant influence.
In our opinion, one often underestimated risk factor for USD formation is uCTD. Data from multiple studies indicate that the incidence of scar defects during pregnancy in patients with uCTD ranges from 28.6% to 42.4% [32, 33]. It is believed that impaired repair processes caused by defects in cytokines and hormonal regulation of the inflammatory response and neoangiogenesis contribute to the formation of defective uterine scars after CS in uCTD. Histological examination of excised tissue in the scar zone with uCTD revealed increased stromal content, reduced vascularization, myocyte damage in the form of hydropic degeneration, and apoptosis of smooth muscle cells [32].
According to our results, connective tissue dysplasia was detected in 52/113 (46%) patients with USD. The high incidence of this condition is consistent with the results of a study by Shchukina N.A. et al. [33] where this figure was 48%. The presence of uCTD in patients was confirmed by the high incidence of pathogenetically homogeneous diseases: varicose veins of the lower extremities in 46% (52/113), musculoskeletal disorders in 47.8% (54/113), visual organ disorders in 37.2% (42/113), and CI in 9.7% (11/113).
The role of uCTD in the inadequate course of the reparative process was confirmed by the high frequency of retroflexed uteri in patients with USD. According to the literature, the incidence of this condition in the population is approximately 18–20% among nulliparous women and approximately 30% among patients with a history of childbirth (both vaginally and via CS) [34-36]. Among the patients in our study, this figure was 75.2% (85/113), which was more than double that of the general population. In our opinion, the high frequency of retroflexed uterus in patients with USD may be associated with a high probability of its formation after CS because of overstretching of the uterine isthmus in the area of an incompletely formed scar (secondary retroflexion).
The course of the reparative process is influenced by many factors including tissue ischemia due to incorrect apposition or the use of blocking sutures (Reverdin suture), the presence of endocervical cysts in the projection of the scar when it is located in the cervix, a large number of previous CSs, the inflammatory process, and somatic comorbidities. This has been demonstrated in a recent review [4]. An additional influence, in addition to the factors mentioned above, can be a mechanical effect on the tissue in the form of excessive tension. Ofili-Yebovi D. et al. suggested that in patients with a retroflexed uterus, tissue tension in the scar area leads to decreased perfusion, ischemia, and slower collagen production [21]. Thus, uCTD, retroflexed uterus and the resulting mechanical tension of tissues in the area of the developing scar create a kind of “vicious circle” leading to impairment of reparative process.
Features of the course of the reparative process influence not only the formation of the scar after CS but also the effectiveness of metroplasty performed outside of pregnancy. According to the literature, the frequency of unsatisfactory outcomes of this operation remains high, reaching 10.5% with laparoscopic access [5–8] and 10.9% with vaginal access [37]. At the same time, data on the analysis of possible causes of surgical failures are rarely found in the literature, although in published works, the authors noted uCTD and endometriosis of the scar zone [38]. According to our data, in 9.8% (6/61) of patients, standard metroplasty also did not lead to a satisfactory result, and the mST 6 months after surgery was less than 3 mm. According to our statistical analysis, two parameters had a significant impact on the frequency of unsatisfactory outcomes of surgical treatment: repeated trauma of the uterine isthmus (three or more previous CSs), increasing the probability of failure by 11.2 times, as well as the presence of uCTD (DC uCTD≥17), increasing the frequency of failure by 6.3 times.
Thus, the totality of our data allowed us to assume the important role of uCTD not only in the pathogenesis of USD formation after CS but also in the ineffectiveness of surgical correction of this condition and to take this mechanism into account when performing metroplasty in such patients.
One obvious method to eliminate excessive tissue tension and create more favorable conditions for de novo scar formation is the shortening and plication of the round ligaments of the uterus. This surgery is performed using suture materials or special equipment [6, 38, 39]. Although this method has been mentioned in the literature for metroplasty in patients with USD, the authors did not evaluate its efficacy compared with standard surgery [6, 40], which was the purpose of this study.
When assessing the effectiveness of laparoscopic metroplasty, it was noted that when using the standard technique, clinical manifestations in the form of MD resolved in 47/53 (88.7%) patients and in the group with shortening and plication of the round ligaments in 47/47 (100%) patients (p=0.018). Pain syndrome was completely eliminated in both the groups (p<0.001). Thus, both methods were highly effective in correcting clinical manifestations of USD. Our data are consistent with other studies. Thus, according to Vervoort A. [7], laparoscopic correction of the niche led to a statistically significant decrease in the duration of postmenstrual flow and an increase in the mST in 79.2% of patients. According to Zang Y. et al., clinical manifestations resolved in 86% of patients [41], according to Ciebiera M. in 90% [42], and according to Donnez O. in 91% [6].
However, the parameters of scars formed de novo were different between the two groups, which may indicate a different quality of the reparative process. At control eUS after 6 months in group 1, the average mST was 4.8 (1.8) mm, which was significantly higher than before surgery – 2.3 (0.9) mm, p=0.00001. In group 2, this figure was 5.3 (1.2) mm, which was also significantly higher than before surgery – 2.1 (0.6) mm, p=0.00001. However, the difference in the average mST values in each group six months after surgery was statistically insignificant (p=0.124). However, when comparing the degree of increase in mST compared to the initial values, a statistically significant difference was noted: in the group with shortening of the round ligament of the uterus, the increase in mST was greater than that in the group without it (+3.16 (1.23) and +2.5 (2.07) mm, respectively; p=0.049). In addition, in the group with shortening and plication of the round ligament of the uterus, no single unsatisfactory outcome of metroplasty (mST <3 mm) was detected, whereas in the group without this stage, this figure was 9.8% (p=0.036).
When assessing reproductive outcomes, both methods allowed achieving reproductive function in 21/113 (18.6%) cases (14/61 (23%) in group 1 and 7/52 (13.5%) in group 2, p=0.120). However, given the short observation period of the results of the surgery and the fact that the majority of patients currently planning a pregnancy, both through assisted reproductive technology programs and spontaneously, this indicator requires further evaluation.
Conclusion
Given the rising incidence of cesarean sections, the issue of uterine scar defects is likely to remain relevant for a long time. As it is challenging to significantly alter the birth structure in developed countries, our primary focus should be on preventing the development of USDs and enhancing the methods used for their correction.
Metroplasty, a form of corrective plastic surgery, is designed to facilitate a subsequent pregnancy free from complications. Therefore, it is critical to achieve a near 100% success rate when performing this procedure. However, a considerable rate of unsatisfactory outcomes (approximately 10%), as documented in medical literature and our study, highlights the necessity to explore advanced methods of USD correction.
Based on our findings regarding the role of uCTD in the pathogenesis of USD, an effective approach to promote favorable conditions for de novo scar formation may involve additional shortening of the uterine round ligament. This technique could potentially reduce tissue tension in the scar area and enhance the effectiveness of the metroplasty
References
- Robson S.J., de Costa C.M. Thirty years of the World Health Organization's target caesarean section rate: time to move on. Med. J. Aust. 2017; 206(4): 181-5. https://dx.doi.org/10.5694/mja16.00832.
- Betrán A.P., Ye J., Moller A.B., Zhang J., Gülmezoglu A.M., Torloni M.R. The increasing trend in Caesarean section rates: global, regional and national estimates: 1990-2014. PLoS One. 2016; 11(2): e0148343. https://dx.doi.org/10.1371/journal.pone.0148343.
- Федеральная служба государственной статистики Российской Федерации. Здравоохранение в России. Статистический сборник. 2021. 171с. [Federal State Statistics Service of the Russian Federation. Health care in Russia. 2021. 171 p. (in Russian)].
- Сидорова Т.А., Мартынов С.А. Факторы риска и механизмы формирования дефектов рубца на матке после операции кесарева сечения. Гинекология. 2022; 24(1): 11-7. [Sidorova T.A., Martynov S.A. Risk factors and mechanisms of uterine scar defects formation after caesarean section: A review. Gynecology. 2022; 24(1): 11-7. (in Russian)]. https://dx.doi.org/10.26442/20795696.2022.1.201356.
- Vervoort A.J., Uittenbogaard L.B., Hehenkamp W.J., Brölmann H.A., Mol B.W., Huirne J.A. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum. Reprod. 2015; 30(12): 2695-702. https://dx.doi.org/10.1093/humrep/dev240.
- Donnez O., Donnez J., Orellana R., Dolmans M.M. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil. Steril. 2017;107(1): 289-96.e2. https://dx.doi.org/10.1016/j.fertnstert.2016.09.033.
- Vervoort A., Vissers J., Hehenkamp W., Brölmann H., Huirne J. The effect of laparoscopic resection of large niches in the uterine caesarean scar on symptoms, ultrasound findings and quality of life: a prospective cohort study. BJOG. 2018; 125(3): 317-25. https://dx.doi.org/10.1111/1471-0528.14822.
- Малышева А.А., Матухин В.И., Резник В.А., Рухляда Н.Н., Тайц А.Н. Опыт оперативной коррекции несостоятельного рубца на матке после кесарева сечения на этапе прегравидарной подготовки. Проблемы репродукции. 2018; 24(6): 46-50. [Malysheva A.A., Matukhin V.I., Reznik V.A., Rukhliada N.N., Taits A.N. Experience of the surgical correction of the scar on the uterus after cesarian section at the pre-conceptional preparation.
- Russian Journal of Human Reproduction. 2018; 24(6): 46-50. (in Russian)]. https://dx.doi.org/10.17116/repro20182406146.
- Сидорова Т.А., Мартынов С.А., Адамян Л.В., Летуновская А.Б., Бойкова Ю.В. Сравнение эффективности ультразвуковых методов диагностики в оценке дефектов рубца на матке после операции кесарева сечения. Акушерство и гинекология. 2022; 4: 132-40. [Sidorova T.A., Martynov S.A., Adamyan L.V., Letunovskaya A.B., Boykova Yu.V. Comparison of the effectiveness of ultrasound diagnosis in assessment of uterine scar defets after cesarean section. Obstetrics and Gynecology. 2022; (4): 132-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.4.132-140.
- Сухарева Т.А., Мартынов С.А., Адамян Л.В., Кулабухова Е.А., Учеваткина П.В., Летуновская А.Б., Бойкова Ю.В. Сравнение эффективности ультразвуковых методов диагностики и магнитно‑резонансной томографии в оценке дефектов рубца на матке после кесарева сечения. Акушерство и гинекология. 2023; 4: 78-86. [Sukhareva T.A., Martynov S.A., Adamyan L.V., Kulabukhova E.A., Uchevatkina P.V., Letunovskaya A.B., Boykova Yu.V. Comparing the effectiveness of ultrasound and MRI in assessing cesarean uterine scar defects. Obstetrics and Gynecology. 2023; (4): 78-86. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.264.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации «Дисплазии соединительной ткани». 2017. [Ministry of Health of the Russian Federation. Clinical Guidelines «Connective Tissue Dysplasia». 2017. (in Russian)].
- Мартынов С.А., Адамян Л.В., Сухарева Т.А. Способ хирургической коррекции дефектов рубца на матке после кесарева сечения: пат. 2795080 Российская Федерация: МПК А61В 17/42 (2006.01); заявитель и патентообладатель ФГБУ «Национальный медицинский исследовательский центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова» МЗ РФ. № 2022122483; заявл. 19.08.2022; опубл. 28.04.2023 Бюл. № 13. [Martynov S.A., Adamyan L.V., Sukhareva T.A. Method of surgical correction of scar defects in the uterus after caesarean section: pat. 2795080 Russian Federation: IPC A61V 17/42 (2006.01); applicant and patent holder of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. № 2022122483; declared. 19.08.2022; publ. 28.04.2023 Bul. № 13. (in Russian)].
- Jordans I.P.M., de Leeuw R.A., Stegwee S.I., Amso N.N., Barri-Soldevia P.N., van den Bosch T. et al. Sonographic examination of uterine niche in non-pregnant women: a modified Delphi procedure. Ultrasound Obstet. Gynecol. 2019; 53(1): 107-15. https://dx.doi.org/10.1002/uog.19049.
- Roberge S., Demers S., Girard M., Vikhareva O., Markey S., Chaillet N. et al. Impact of uterine closure on residual myometrial thickness after cesarean: a randomized controlled trial. Am. J. Obstet. Gynecol. 2016; 214(4): 507.e1-507.e6. https://dx.doi.org/10.1016/j.ajog.2015.10.916.
- Hayakawa H., Itakura A., Mitsui T., Okada M., Suzuki M., Tamakoshi K., Kikkawa F. Methods for myometrium closure and other factors impacting effects on cesarean section scars of the uterine segment detected by the ultrasonography. Acta Obstet. Gynecol. Scand. 2006; 85(4): 429-34. https://dx.doi.org/10.1080/00016340500430436.
- Abalos E., Addo V., Brocklehurst P., El Sheikh M., Farrell B., Gray S. et al.; CORONIS collaborative group. Caesarean section surgical techniques: 3 year follow-up of the CORONIS fractional, factorial, unmasked, randomised controlled trial. Lancet. 2016; 388(10039): 62-72. https://dx.doi.org/10.1016/S0140-6736(13)60441-9.
- Bamberg C., Hinkson L., Dudenhausen J.W., Bujak V., Kalache K.D., Henrich W. Longitudinal transvaginal ultrasound evaluation of cesarean scar niche incidence and depth in the first two years after single- or double-layer uterotomy closure: a randomized controlled trial. Acta Obstet. Gynecol. Scand. 2017; 96(12): 1484-9. https://dx.doi.org/10.1111/aogs.13213.
- Vikhareva Osser O., Valentin L. Risk factors for incomplete healing of the uterine incision after caesarean section. BJOG. 2010; 117(9): 1119-26. 10.1111/j.1471-0528.2010.02631.x.
- Chen Y., Han P., Wang Y.J., Li Y.X. Risk factors for incomplete healing of the uterine incision after cesarean section. Arch. Gynecol. Obstet. 2017; 296(2): 355-61. https://dx.doi.org/10.1007/s00404-017-4417-6.
- Vikhareva O., Rickle G.S., Lavesson T., Nedopekina E., Brandell K., Salvesen K.Å. Hysterotomy level at Cesarean section and occurrence of large scar defects: a randomized single-blind trial. Ultrasound Obstet. Gynecol. 2019; 53(4): 438-42. https://dx.doi.org/10.1002/uog.20184.
- Ofili-Yebovi D., Ben-Nagi J., Sawyer E., Yazbek J., Lee C., Gonzalez J., Jurkovic D. Deficient lower-segment Cesarean section scars: prevalence and risk factors. Ultrasound Obstet. Gynecol. 2008; 31(1): 72-7. https://dx.doi.org/10.1002/uog.5200.
- Han G., Ceilley R. Chronic wound healing: a review of current management and treatments. Adv. Ther. 2017; 34(3): 599-610. https://dx.doi.org/10.1007/s12325-017-0478-y.
- Wang C.B., Chiu W.W., Lee C.Y., Sun Y.L., Lin Y.H., Tseng C.J. Cesarean scar defect: correlation between Cesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet. Gynecol. 2009; 34(1): 85-9. https://dx.doi.org/10.1002/uog.6405.
- Ryo E., Sakurai R., Kamata H., Seto M., Morita M., Ayabe T. Changes in uterine flexion caused by cesarean section: correlation between post-flexion and deficient cesarean section scars. J. Med. Ultrason (2001). 2016; 43(2): 237-42. https://dx.doi.org/10.1007/s10396-015-0678-5.
- Woodd S.L., Montoya A., Barreix M., Pi L., Calvert C., Rehman A.M. et al. Incidence of maternal peripartum infection: a systematic review and meta-analysis. PLoS Med. 2019; 16(12): e1002984. https://dx.doi.org/10.1371/journal.pmed.1002984.
- Taylor M., Pillarisetty L.S. Endometritis. StatPearls. Treasure Island (FL): StatPearls, 2020 Jan.
- Walfisch A., Beloosesky R., Shrim A., Hallak M. Adhesion prevention after cesarean delivery: evidence, and lack of it. Am. J. Obstet. Gynecol. 2014; 211(5): 446-52. https://dx.doi.org/10.1016/j.ajog.2014.05.027.
- Клеменов А.В., Ткачева О.Н., Верткин А.Л. Дисплазия соединительной ткани и беременность (обзор). Терапевтический архив. 2004; 76(11): 80-3. [Klemenov A.V., Tkacheva O.N., Vertkin A.L. Dysplasia of connective tissue and pregnancy (review). Therapeutic Archive. 2004; 76(11): 80-3. (in Russian)].
- Комиссарова Л.М., Карачаева А.Н., Кесова М.И. Течение беременности и родов при дисплазии соединительной ткани. Акушерство и гинекология. 2012; 3: 4-8. [Komissarova L.M., Karachayeva A.N., Kesova M.I. The course of pregnancy and labor in connective tissue dysplasia. Obsteterics and Gynecology. 2012; (3): 4-8. (in Russian)].
- Armstrong V., Hansen W.F., Van Voorhis B.J., Syrop C.H. Detection of cesarean scars by transvaginal ultrasound. Obstet. Gynecol. 2003; 101(1): 61-5. https://dx.doi.org/10.1016/s0029-7844(02)02450-x.
- Краснопольский В.И., Буянова С.Н., Щукина Н.А., Логутова Л.С. Несостоятельность шва (рубца) на матке после кесарева сечения: проблемы и решения (редакционная статья). Российский вестник акушера-гинеколога. 2015; 15(3): 4-8. [Krasnopol'skiĭ V.I., Buianova S.N., Shchukina N.A., Logutova L.S. Uterine suture (scar) incompetence after cesarean section: Problems and solutions (an editorial). Russian Bulletin of Obstetrician-Gynecologist. 2015; 15(3): 4-8. (in Russian)]. https://dx.doi.org/10.17116/rosakush20151534-8.
- Кесова М.И. Течение беременности и родов у пациенток с дисплазией соединительной ткани. Вестник Национального медико-хирургического центра им. Н.И. Пирогова. 2011; 6(2): 81-4. [Kesova M.I. Pregnancy and labor in women with connective tissue disorders. Bulletin of the N.I. Pirogov National Medical and Surgical Center. 2011; 6(2): 81-4. (in Russian)].
- Щукина Н.А., Буянова С.Н., Чечнева М.А., Земскова Н.Ю., Пучкова Н.В., Барто Р.А., Баринова И.В., Благина Е.И. Причины формирования несостоятельного рубца на матке после кесарева сечения, роль дисплазии соединительной ткани. Российский вестник акушера-гинеколога. 2018; 18(5): 4‑11. [Shchukina N.A., Buianova S.N., Chechneva M.A., Zemskova N.Iu., Puchkova N.V., Barto R.A., Barinova I.V., Blagina E.I. Causes of a postcesarean incompetent uterine scar: a role of connective tissue dysplasia. Russian Bulletin of Obstetrician-Gynecologist. 2018; 18(5): 4-11. (in Russian)]. https://dx.doi.org/10.17116/rosakush2018180514.
- Ameer M.A., Fagan S.E., Sosa-Stanley J.N., Peterson D.C. Anatomy, abdomen and pelvis: uterus. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan.
- Haylen B.T., McNally G., Ramsay P., Birrell W., Logan V. A standardized ultrasonic diagnosis and an accurate prevalence for the retroverted uterus in general gynaecology patients. Aust. N. Z. J. Obstet. Gynaecol. 2007; 47(4): 326-8. https://dx.doi.org/10.1111/j.1479-828X.2007.00745.x.
- Fidan U., Keskin U., Ulubay M., Öztürk M., Bodur S. Value of vaginal cervical position in estimating uterine anatomy. Clin. Anat. 2017; 30(3): 404-8. https://dx.doi.org/10.1002/ca.22854.
- Гарифуллова Ю.В., Журавлева В.И. Пластика несостоятельного рубца на матке влагалищным доступом при сопутствующей дисплазии соединительной ткани. Практическая медицина. 2019; 17(4): 85-7. [Garifullova Yu.V., Zhuravleva V.I. Vaginal access repair of uterine scar dehiscence with concomitant connective tissue dysplasia. Practical Medicine. 2019; 17(4): 85-7. (in Russian)].
- Ножницева О.Н., Беженарь В.Ф. Комбинированный способ коррекции локальной несостоятельности рубца на матке после кесарева сечения. Проблемы репродукции. 2018; 24(5): 45‑52. [Nozhnitseva O.N., Bezhenar' V.F. Combined correction of the local post cesarean scar insufficiency. Russian Journal of Human Reproduction. 2018; 24(5): 45-52. (in Russian)]. https://dx.doi.org/10.17116/repro20182405145.
- Гасанова М.А. Патент 2 234 271 Российская Федерация, МПК A 61 B 17/42. Способ укорачивания круглых маточных связок и устройство для его осуществления; заявитель и патентообладатель Дагестанская государственная медицинская академия. № 2001134491/14; заявл. 17.12.01; опубл. 20.08.04, Бюл. № 10. [Gasanova M.A. Patent 2,234,271 Russian Federation, IPC A 61 B 17/42. Method for shortening round uterine ligaments and device for its implementation; applicant and patent holder Dagestan State Medical Academy. № 2001134491/14; declared. 17.12.01; publ. 20.08.04, Bul. № 10. (in Russian)].
- Sipahi S., Sasaki K., Miller C.E. The minimally invasive approach to the symptomatic isthmocele – what does the literature say? A step-by-step primer on laparoscopic isthmocele – excision and repair. Curr. Opin. Obste.t Gynecol. 2017; 29(4): 257-65. 1 https://dx.doi.org/0.1097/GCO.0000000000000380.
- Zhang Y. A comparative study of transvaginal repair and laparoscopic repair in the management of patients with previous Cesarean scar defect. J. Minim. Invasive Gynecol. 2016; 23(4): 535-41. https://dx.doi.org/10.1016/j.jmig.2016.01.007.
- Ciebiera M., Ciebiera M., Czekanska-Rawska M., Jakiel G. Laparoscopic isthmocele treatment – single center experience. Videochir. Inne Tech. Maloinwazyjne. 2017; 12(1): 88-95. https://dx.doi.org/10.5114/wiitm.2017.66025.
Received 27.06.2023
Accepted 25.07.2023
About the Authors
Sergey A. Martynov, Dr. Med. Sci., Leading Researcher at the Gynecological Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-77-83, s_martynov@oparina4.ru, https://orcid.org/0000-0002-6795-1033,117997, Russia, Moscow, Ac. Oparina str., 4.
Tatyana A. Sukhareva, Graduate Student at the Gynecological Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-77-83, t_sidorova@oparina4.ru, https://orcid.org/0000-0002-5508-3611, 117997, Russia, Moscow, Ac. Oparina str., 4.
Leyla V. Adamyan, Dr. Med. Sci., Professor, Academician of RAS, Deputy Director for Science, Head of the Gynecological Department, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(495)438-77-83, l_adamyan@oparina4.ru,
https://orcid.org/0000-0002-3253-4512, 117997, Russia, Moscow, Ac. Oparina str., 4.



