Plasma levels of transforming growth factor β isoforms in women with preeclampsia
Objective. To investigate the role of transforming growth factor (TGF) -β in the development of preeclampsia.Vtorushina V.V., Kharchenko D.K., Krechetova L.V., Astashkin E.I., Kan N.E.
Material and methods. The study comprised 30 women with preeclampsia and 20 women with uncomplicated pregnancy. Plasma concentrations of TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) were determined by a standard test system on a Bio-Plex 200 analyzer (Bio-Rad, USA) with subsequent processing using Bio-Plex Manager 6.0 Properties.
Results. Plasma levels of TGF-β3 in women with preeclampsia were lower than in the control subjects (p = 0.034). Women with and without PE had similar plasma levels of TGF-β1 and TGF-β2 isoforms. Patients with early preeclampsia had significantly lower plasma levels of TGF-β3 than women in the control group [(38.71 (8.00) vs. 48.42 (10.60), p = 0.008].
Conclusion. The study findings suggest that the expression of TGF-β3 may be considered as a promising early biomarker for preeclampsia.
Financing. The work was supported by the grant of Russian Federation President for governmental support for the leading scientific schools № НШ-4566.2018.7 Contract № 075-02-2018-519.
Keywords
Preeclampsia (PE) is a pregnancy-specific hypertensive disease with multisystem involvement. It usually occurs in the second half of pregnancy and is accompanied by proteinuria (> 0.3 g/l in a 24-hour urine collection), edema, and the manifestation of multiple organ failure [1, 2].
The pathogenesis of PE involves impairment of the interaction between decidual immune cells and paternal antigens expressed by trophoblast cells. It is assumed that during normal pregnancy, once trophoblast antigens are recognized, stimulated decidual natural killer cells and T-lymphocytes produce cytokines and growth factors that contribute to placentation. Dysregulation of this process is associated with the insufficient transformation of the spiral arteries resulting in decidual ischemia, hypoxia of decidual cells, which leads to oxidative stress. Reduced oxidative activity is an integral part of the normal inflammatory response. However, in patients with PE, intense oxidative activity inhibits antioxidant defense systems, resulting in oxidative stress, which causes intracellular and intercellular space biochemical disturbances. Various components of the systemic inflammatory response are interconnected by numerous secreted proteins, such as cytokines, chemokines and growth factors [3].
One of the key factors is the transforming growth factor β (TGF-β) that is a multifunctional cytokine involved in the regulation of trophoblast invasion, proliferation, and differentiation.
An inactive form of TGF-β, which is synthesized by many cells, mainly macrophages, is linked to two other polypeptides — the latent TGF-β-binding protein (LTBP) and leucine aminopeptidase (LAP) [4]. There are three isoforms of TGF-β: TGF-β1, TGF-β2, and TGF-β3 [5]. Recent evidence suggests the anti-invasive effect of TGF (mainly the TGF-β1 isoform) in various cell and tissue systems, for example, in colon and thyroid cancer [6-8]. There is also evidence that the TGF-β3 isoform plays a particularly important role in the trophoblast differentiation and its overexpression can be detected in placentas of women with PE [9]. However, the role of individual TGF-β isoforms in the pathogenesis of PE is not well understood.
This study aimed to investigate plasma levels of TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) in pregnant women with and without PE.
Material and methods
The study comprised 50 pregnant women at 27 to 41 weeks’ gestation, who were hospitalized for delivery at V.I. Kulakov NMRC for OG&P of Minzdrav of Russia from September 2016 to September 2018. Clinical and laboratory examination of patients was carried out in full by order of Minzdrav of Russia of 01.11.2012 N 572n. The study cohort was divided into two groups: a study group consisting of 30 patients with PE and a control group of 20 conditionally healthy pregnant women with uncomplicated obstetric and gynecological history and normal current pregnancy.
Both groups were comparable for age, parity, and clinical characteristics. Baseline clinical evaluation consisted of detailed medical history, including age, gynecological and non-gynecological comorbidities, a history of surgery, menstrual and reproductive dysfunctions, the course and outcome of the current pregnancy, treatment in Group I, and perinatal outcomes.
All patients signed informed consent to participate in this study, which was approved by the local ethical committee of V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. The inclusion criteria in the groups were as follows: singleton pregnancy, delivery at 27 to 40 weeks’ gestation, the presence of PE in the study group. Exclusion criteria were severe extragenital pathology, multiple pregnancy, pregnancy resulting from assisted reproductive technology, congenital anomalies incompatible with life, maternal genetic disorders and acute infectious and inflammatory diseases.
The concentrations of TGF-β isoforms (TGF-β1, TGF-β2, TGF β3) were measured in peripheral venous blood samples obtained from women before delivery. Blood was collected into vacutainers containing ethylenediaminetetraacetic acid (EDTA).
The EDTA plasma concentrations of TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) were determined by the standard 3-plex test system Bio-Plex Pro TGF-β Panel 3-Plex (Bio-Rad, USA) using a flow-based laser immuno-analyzer Bio-Plex 200 (Bio-Rad, USA) with subsequent processing using Bio-Plex Manager 6.0 Properties (Bio-Rad, USA). According to the manufacturer’s (Bio-Rad) instructions, EDTA plasma samples were prepared by double centrifugation at 1,000 x g for 15 min at 40°C and at 1,000 x g for 10 min at 40°C to eliminate platelets and sediments. Plasma samples were frozen and stored at -80°C until analyzed.
The study results are presented as mean and standard deviation M (SD). The normality of the distribution was tested by the Shapiro-Wilk test. Continuous variables were compared with Student’s t-test and categorical variables with the Pearson Chi-square test. Differences between the groups were considered statistically significant at p < 0.05. The diagnostic performance of the model was assessed by ROC curve analysis that was performed by evaluating the area under the ROC curve with a 95% confidence interval. Statistical analysis and graphing were performed using Attestat (Russia) and OriginPro 8.5 (USA) software.
Results and discussion
Characteristics of the study participants regarding the somatic and gynecological history are presented in Table 1.

As seen in Table 1, the two groups were similar in age, rates of upper respiratory tract infections, cervical diseases, uterine fibroids, and genital infections. However, compared to the control subjects, the patients in the study group had 3-fold higher rate of chronic pelvic inflammatory diseases and 10% more cases (n = 3) with a history of PE including 2 severe PE during previous pregnancies.
Characteristics of the course of current pregnancies are presented in Table 2.
Compared to the controls, the patients with PE were 1.5, 6.5 and three times more likely to have threatened miscarriage in the second trimester, fetal growth restriction, and impaired fetoplacental and uteroplacental blood flow according to ultrasound and Doppler sonography, respectively. Most of the patients (n = 15) in the study group gave birth before 37 weeks of gestation, which was caused by an increase in PE severity; they underwent emergency surgical delivery four times more often than women in the control group.
There were no significant differences in the postpartum period. The median of first- and fifth-minute Apgar score was 8 [7: 8] and 8 [8: 9 ] in the study group and 8 [8: 8] and 9 [9: 9] in the control group, respectively. Newborn birth weight in the control group was 3404 (317.83) g, which was 1.5-fold higher than in the study group (2501 (463.18) g, p = 0.001).
Peripheral blood plasma levels of TGF-β isoforms in the study participants are presented in Table 3.
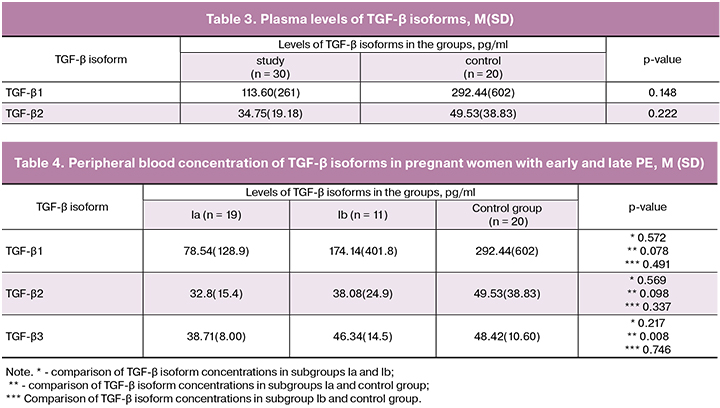
In women with PE, plasma concentrations of TGF-β3 were lower than in the control group (p = 0.034), and the levels of TGF-β1 and TGF-β2 were similar in both groups.
 ROC analysis (Fig. 1) showed a high diagnostic performance of plasma levels of TGF-β3 of women with PE (sensitivity 78%, specificity 66.67%, AUC = 0.80). This implies that the TGF-β3 isoform plays a significant role in the pathogenesis of PE and may be a predictor for the development of the disease.
ROC analysis (Fig. 1) showed a high diagnostic performance of plasma levels of TGF-β3 of women with PE (sensitivity 78%, specificity 66.67%, AUC = 0.80). This implies that the TGF-β3 isoform plays a significant role in the pathogenesis of PE and may be a predictor for the development of the disease.
According to generally accepted criteria [10], the study group was divided into two subgroups: subgroup Ia – patients with early PE (n = 19), occurring before 34 weeks’ gestation and subgroup Ib – patients with late PE (n = 11). Plasma concentrations of the three TGF-β isoforms in subgroup Ia was significantly lower than in the control subjects (Table 4). No other statistical differences were found.
ROC analysis showed a high diagnostic performance of plasma levels of TGF-β3 (sensitivity 94%, specificity 60%, AUC = 0.81) in early prediction of PE (Fig. 2).
To date, there have been no studies investigating peripheral blood levels of all three TGF-β isoforms [11-14] though some researchers reported the levels of TGF-β3 isoform in PE and normal pregnancy. Ganigia et al. [15] examined placental tissue and found that in normal pregnancy, TGF-β3 expression by placental cells grows until about 7–8 weeks gestation with a gradual decrease after that. The expression of TGF-β3 mRNA in chorionic villi is also high during the first 7 weeks, which then decreases by about 10 weeks. According to published experimental data, the TGF-β3 isoform is involved in the inhibition of trophoblast invasion [16]. The detected changes in the TGF-β3 isoform level during normal pregnancy may reflect its involvement in controlling the degree of trophoblast invasion.
Analysis of different TGF-β isoforms in the placental explant culture of women with PE, who gave birth at 30–32 weeks’ gestation, placentas of women with normal pregnancy and specimens of miscarriage tissues at 5–13 weeks’ gestation showed that only TGF-β3 inhibits trophoblast differentiation in relation to the invasive phenotype in placental explants of the first trimester.
The cells of the placental tissue of women with PE overexpress TGF-β3 and exhibit a hypoinvasive phenotype in vitro. Differentiation of the invasive phenotype can be restored in these explants using antibodies to TGF-β3. Chorionic villus cells of placentas of women with normal pregnancy have weak TGF-β3 immunoreactivity, while in samples of placental tissue of women with PE it is strong. Inhibition of TGF-β3 expression in the placentae of women with PE restores trophoblast invasiveness, and the treatment of samples of the placental tissue of women with PE with antibodies to TGF-β1 or TGF-β2 does not affect the formation of the trophoblast invasiveness. The authors conclude that the TGF-β3 isoform is a regulator of human trophoblast differentiation concerning the invasive phenotype, which is necessary for adequate invasion and remodeling of the spiral arteries [15].
Some authors emphasize the role of other TGF-β isoforms in the pathogenesis of PE. For example, in women with PE, the expression and activity of cyclooxygenase-2 (COX-2) in trophoblast cells were higher than in healthy pregnant women. TGF-β1 increases COX-2 expression by activating the SMAD2/3-SMAD4 signaling pathways, and an increase in the COX-2 level subsequently contributes to the suppression of the invasion of human trophoblast cells by TGF-β1 [17]. Thus, it has been shown that the use of a TGF-β1 receptor inhibitor blocks its stimulating effects on COX-2 expression.
Conclusion
In our work, we have not found significant differences of plasma levels of TGF-β1 and TGF-β2 isoforms between women with and without PE. Low plasma levels of TGF-β3 in women with PE may reflect the accumulation of this isoform in the placental tissue and indicate its possible role in the pathogenesis of this pregnancy complication, which is consistent with the data reported by Caniggia et al. [15]. Therefore, the expression of TGF-β3 may be considered as a promising early biomarker for PE.
References
- Серов В.Н., Баранов И.И., Пекарев О.Г., Пырегов А.В., Тютюнник В.Л., Шмаков Р.Г. Неотложная помощь в акушерстве и гинекологии. М.: ГЭОТАР-Медиа; 2017. 240с. [Serov V.N., Baranov I.I., Pekarev OG, Pyregov AV, Tyutyunnik V.L., Shmakov R.G. Emergency care in obstetrics and gynecology. M.: GEOTAR-Media; 2017. 240 s. (in Russian)]
- Сидорова И.С., Никитина Н.А., Унанян А.Л. Преэклампсия и снижение материнской смертности в России. Акушерство и гинекология. 2018; 1: 107-12. [Sidorova I.S., Nikitina N.A., Unanyan A.L. Preeclampsia and reduction of maternal mortality in Russia. Obstetrics and gynecology, 2018; 1: 107-112. (in Russian)].
- Кетлинский С.А., Симбирцев А.С. Цитокины. М.: Фолиант; 2008: 369-78. [Ketlinsky S. A., Simbirtsev A. S. Cytokines. M.: Foliant; 2008. 369-378 (in Russian)].
- Guller S. Role of the syncytium in placenta-mediated complications of preeclampsia. Thromb. Res. 2009; 124(4): 389-92. doi: 10.1016/j.thromres.2009.05.016.
- Li X., Shen L., Tan H. Polymorphisms and plasma level of transforming growth factor-Beta 1 and risk for preeclampsia: a systematic review. PLoS One. 2014; 9(5): e97230. doi: 10.1371/journal.pone.0097230. eCollection 2014.
- Prunier C., Baker D., Ten Dijke P., Ritsma L. TGF-β family signaling pathways in cellular dormancy. Trends Cancer. 2019; 5(1): 66-78. doi: 10.1016/j.trecan.2018.10.010.
- Cantelli G., Crosas-Molist E., Georgouli M., Sanz-Moreno V. TGF-β -induced transcription in cancer. Semin. Cancer Biol. 2017; 42: 60-9. doi: 10.1016/j.semcancer.2016.08.009.
- Zhong J., Liu C., Zhang Q.H., Chen L., Shen Y.Y., Chen Y.J. et al. TGF-β1 induces HMGA1 expression: The role of HMGA1 in thyroid cancer proliferation and invasion. Int. J. Oncol. 2017; 50(5): 1567-78. doi: 10.3892/ijo.2017.3958.
- Lee S.B., Wong A.P., Kanasaki K., Xu Y., Shenoy V.K., McElrath T.F. et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am. J. Pathol. 2010; 176(2): 710-20. doi: 10.2353/ajpath.2010.090513.
- Ходжаева З.С., Холин А.М., Вихляева Е.М. Ранняя и поздняя преэклампсия: парадигмы патобиологии и клиническая практика. Акушерство и гинекология. 2013; 10: 4-11. [Khodzhaeva Z.S., Kholin A.M, Vikhlyaeva E.M. Early and late preeclampsia: paradigms of pathobiology and clinical practice. Obstetrics and gynecology, 2013; 10: 4-11.(in Russian)].
- Li X., Tan H., Chen M., Zhou S. Transforming growth factor beta 1 related gene polymorphisms in gestational hypertension and preeclampsia: A case-control candidate gene association study. Pregnancy Hypertens. 2018; 12: 155-60. doi: 10.1016/j.preghy.2017.11.010.
- Darmochwal-Kolarz D., Michalak M., Kolarz B., Przegalinska-Kalamucka M., Bojarska-Junak A., Sliwa D., Oleszczuk J. The role of interleukin-17, interleukin-23, and transforming growth factor-β in pregnancy complicated by placental insufficiency. Biomed. Res. Int. 2017; 2017: 6904325. doi: 10.1155/2017/6904325.
- Xu Y.T., Shen M.H., Jin A.Y., Li H., Zhu R. Maternal circulating levels of transforming growth factor-β superfamily and its soluble receptors in hypertensive disorders of pregnancy. Int. J. Gynaecol. Obstet. 2017; 137(3): 246-52.doi: 10.1002/ijgo.12142.
- Zhang J., Dunk C.E., Shynlova O., Caniggia I., Lye S.J. TGFb1 suppresses the activation of distinct dNK subpopulations in preeclampsia. EBioMedicine. 2019; 39: 531-9. doi: 10.1016/j.ebiom.2018.12.015.
- Caniggia I., Grisaru-Gravnosky S., Kuliszewsky M., Post M., Lye S.J. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J. Clin. Invest. 1999; 103(12): 1641-50. doi: 10.1172/JCI6380
- Kar M. Role of biomarkers in early detection of preeclampsia. J. Clin. Diagn. Res. 2014; 8(4): BE01-4. doi: 10.7860/JCDR/2014/7969.4261.
- Yi Y., Cheng J.C., Klausen C., Leung P.C.K. TGF-β1 inhibits human trophoblast cell invasion by upregulating cyclooxygenase-2. Placenta. 2018; 68: 44-51. doi: 10.1016/j.placenta.2018.06.313.
Received 30.11.2018
Accepted 07.12.2018
About the Authors
Vtorushina, Valentina V., PhD, doctor of the highest category of the Clinical Immunology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str.4. Tel.: +79169807895. E-mail: vtorushina@inbox.ruKharchenko, Daria K., postgraduate student, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79151658700. E-mail: drkharchenko@mail.ru
Krechetova, Lyubov V., PhD, head of the Clinical Immunology Laboratory, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79166473929. E-mail: l_krechetova@oparina4.ru .
Researcher ID Y-4837-208.
Astashkin, Evgeny I., MD, professor of the Department of Pathology, I.M. Sechenov First Moscow State Medical University of Minzdrav of Russia (Sechenov University). 119991, Russia, Moscow, Trubetskaya str. 8/2. Tel.: +79160620530. E-mail: ast-med@mail.ru
Kan, Natalia E., MD, professor, the Chief Doctor of the Perinatal Center EMC. 125040, Russia, Moscow, ul. Pravdy str. 15/1. Tel.: +79262208655. E-mail: kan-med@mail.ru. Number Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946.
For citation: Vtorushina V.V., Kharchenko D.K., Krechetova L.V., Astashkin E.I., Kan N.E. Plasma levels of transforming growth factor β isoforms in women with preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 68-73. (in Russian)
http://dx.doi.org/10.18565/aig.2019.2.68-73



