Peripheral blood cytokine levels in women according to the phase of the first period of labor
Objective. To study peripheral blood cytokine levels in primiparous women according to the phase of the first period of labor.Tysyachnyy O.V., Pavlova O.A., Vtorushina V.V., Krechetova L.V., Baev O.R.
Subjects and methods. Peripheral blood samples were collected from 38 primiparous women in the latent and active phases of labor. Serum cytokine levels were determined using the standard Bio-Plex Pro Human Cytokine 17-plex Assay (Bio-Rad, USA).
Results. The active phase of labor was found to be characterized by increased IL-6 levels and decreased TNF-α, IFN-γ, IL-12, IL-4, and IL-13.
Conclusion. Cytokine balance is important in the dynamics of delivery and in the mechanisms of its abnormal development.
Keywords
As of today, the factors causing the onset of labor remain understudied, however, we generally recognize the role of hormonal, neurohumoral and immune components of both maternal organism and fetoplacental complex [1].
The number of leucocytes in the uterine cervix is known to increase at the onset and during the course of labor. The leucocytes in their turn serve as the main source of cytokine synthesis [2]. One study revealed a significant increase in the pro-inflammatory cytokines and chemokines that ensure activation of endothelium and migration of leukocytes and fibroblasts, as well as increased growth factors expression; in this study tissue samples of the lower uterine segment were obtained during spontaneous labor at term [3]. These сytokines also stimulate the production of prostaglandins, such as prostaglandin F2α by activating prostaglandin-H-synthase-2, which contributes to the process of cervical ripening.
In previous studies, the increased cytokine production was considered solely as a sign of preterm labor triggered by an infection. Further studies demonstrated an association of the labor onset (both term and preterm) with the change in cytokines levels within fetoplacental complex [4].
Researchers have recently emphasized the study of cytokines levels in the peripheral blood of parturient women during labor. However, there is only a limited number of publications related to this subject [5]. The interest in evaluation of cytokines levels in peripheral blood of parturient women may be of crucial diagnostic value, reflecting the labor progress and development of complications, since the availability of peripheral blood obtained directly during labor is incomparable with the availability of reproductive organ tissues obtained after delivery.
The objective of the study is to evaluate peripheral blood cytokine levels during the first stage of labor in the peripheral blood of primiparous women.
Materials and Methods
The study included 38 healthy nulliparous patients delivered at the National Medical Research Center for Obstretrics, Gynecology and Perintology in 2017. Inclusion criteria were maternal age 18-40 years, spontaneous singleton pregnancy, cephalic presentation, ≥ 37 weeks gestation, first uncomplicated spontaneous vaginal delivery, written informed consent of the patients. The study was approved by the local ethics committee.
Exclusion criteria were somatic pathology, pregnancy and labor complications, uterine malformations, fetal abnormalities, clinical and laboratory signs of intrauterine or neonatal infection.
Peripheral blood samples were obtained in latent phase of labor (cervical dilatation 3–4 cm) and active phase of labor (cervical dilatation 5 cm and more). Samples of peripheral blood were collected from each patient into 9-milliliter polypropylene vacutainer tubes (S-Monovette, Germany). Samples were centrifuged at 23 °C for 10 min at 3000 ×g and the blood serum stored at −80 °C until later cytokine analysis.
Frozen blood serum was thawed and analyzed for levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-γ using a cytokine multiplexed immunoassay. All assays were performed in 17-well flat bottom plates (Bio-Rad, USA). The plates were measured on the Bio-Plex 200 system (Bio-Rad, USA), and analyzed using the Bio-Plex Manager 6.0 software (Bio-Rad, USA) according to the manufacturer’s instruction. Cytokine levels in blood serum were expressed in picograms per milliliter (pg/ml). The measurement range was 0.1–5.0 pg/ml.
Statistical analysis was performed with the MedCalc Statistical Version 12.1, «IBM SPSS Statistics 22 for Windows». Shapiro–Wilk test was used to determine the normality of the data distribution. Data are presented as medians; minimum and maximum distribution, (Mean±SD) and frequencies/percentages. Comparison of data was performed according to each case with the Mann–Whitney U test (independent nonnormally distributed samples). For all calculations, a p value of <0.05 was considered as statistically significant.
Results
Table 1 shows main characteristics of patients and their newborns, findings of the obstetric and gynecological history, complications during pregnancy and labor. All newborns had Apgar score from 7 to 9 points.
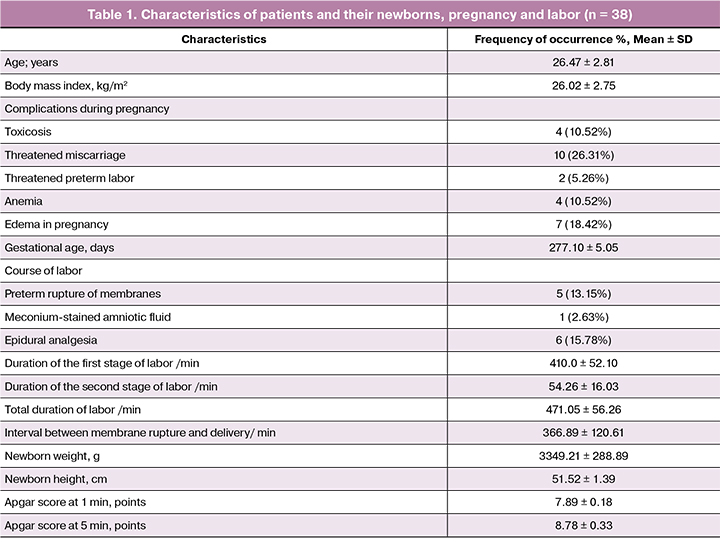
The analysis of the incidence of extragenital diseases showed that the diseases of urinary system (cystitis, pyelonephritis, urolithiasis) were most common, 21.05% cases. No significant differences were found in rates of eye diseases (myopia), gastrointestinal diseases (gastritis, cholecystitis, peptic ulcer, pancreatitis), ENT disorders (rhinitis, tonsillitis, sinusitis) and cardiovascular diseases (vegetative-vascular dystonia, mitral valve prolapse, varicose veins of the lower extremities), 13.15%, 10.52%, 7.89% and 2.63%, respectively.
Gynecological history showed that benign diseases of the uterine cervix (ectopia), ovulatory dysfunction, ovarian cysts and uterine fibroids were detected in 34.21%, 7.89%, 5.26%, 2.63% of cases, respectively.
The study of the course of pregnancy revealed that the most frequent complications were threatened miscarriage and edema in pregnant women, 26.31% and 18.42%, respectively (Table 1).
Labor in 13.15% of cases was complicated by preterm rupture of membranes. The mean interval between membrane rupture and delivery was 366.89 ± 120.61 min. Meconium-stained amniotic fluid occurred during labor in 2.63% of cases.
We found that the duration of the first stage of labor was 410.0 ± 52.10 min, second stage lasted 54.26 ± 16.03 min, total duration of labor was 471.05 ± 56.26 min. Epidural analgesia was used in 15.78% of labor. All newborns had Apgar score from 7 to 9 points. The mean values of weight and length of newborn was 3349.21 ± 288.89 g and 51.52 ± 1.39 cm, respectively.
To determine changes in the levels of peripheral blood serum cytokines, we evaluated them in the latent and active phases of labor.
Table 2 shows serum cytokine levels in the latent and active phases of labor.
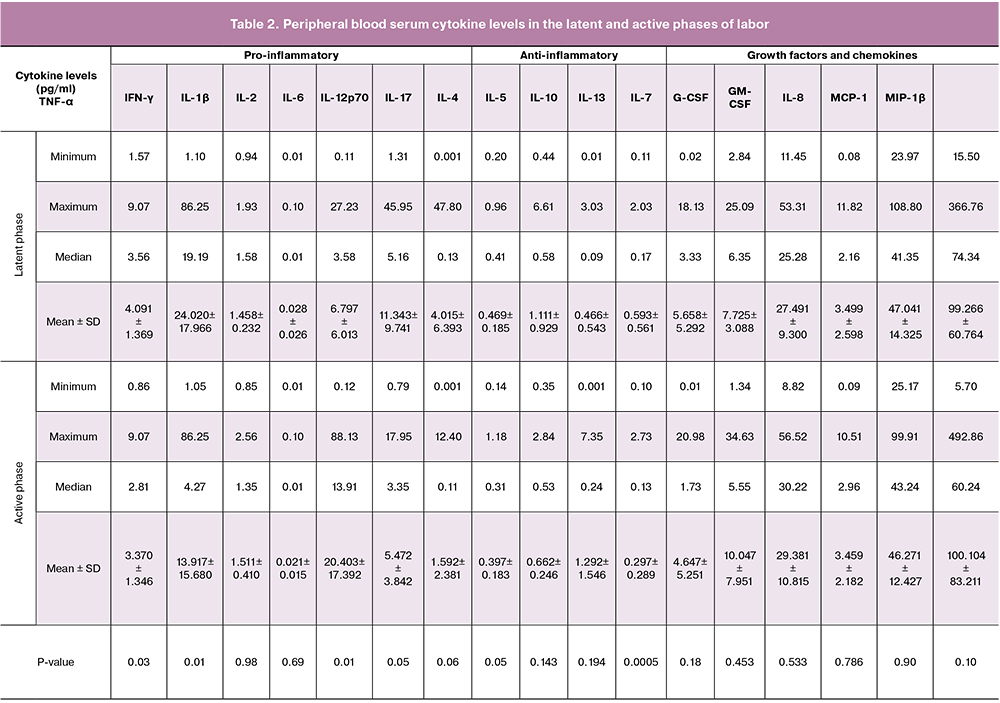
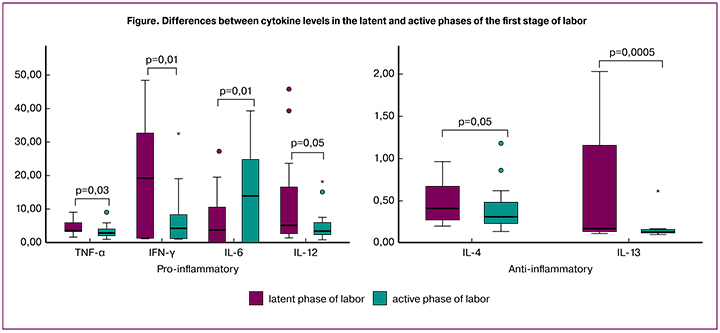
Our study demonstrates that in comparison with latent phase of labor, active phase of labor is associated with higher level of IL-6 and lower levels of TNF-α, IFN-γ, IL-12, IL-4 and IL-13. Moreover, IL-6 had a 3-fold increase (р = 0.01); there was a decrease in TNF-α level by 1.2 times (р = 0.03), IFN-γ level by 1.7 times (р = 0.01), IL-12 level was twice lower (р = 0.05), IL-4 level decreased by 1.1 times (р = 0.05) and IL-13 by 1.9 times (р = 0.0005). There were no differences found between other cytokines levels.
The Figure 1 shows the significant differences between the cytokine levels in the latent and active phases of labor.
Discussion
We compared cytokine levels in serum samples obtained during latent and active phases of the first stage of labor in primiparous women. As a result of our research, an increase in levels of IL-6 and a decrease in TNF-α, IFN-γ, IL-12, IL-4, IL-13 were detected in the active phase as compared to the latent one.
Selkov et al. revealed that at different pregnancy stages diverse cytokines can play a central role in the regulation of gestational processes and the initiation of labor. Furthermore, a change in the local concentrations of pro-inflammatory cytokines and, as a result, the disruption in mechanisms of the auto- and paracrine regulation may be one of the causes leading to preterm termination of pregnancy [6].
Pro-inflammatory cytokine IL-6 is known to be an essential predictor of preterm labor, premature rupture of membranes and a diagnostic marker of the fetal inflammatory response syndrome. Data showed that the progression of labor is associated with increasing IL-6 plasma levels in parturient women. Our results correspond to the existing literature claiming that the IL-6 serum levels in the peripheral blood of parturient women increase with higher frequency of uterine contractions and correlate with cervix dilatation. Women with increased labor activity have higher blood levels of this cytokine even two hours post-delivery compared to samples obtained before the onset of labor (p <0.001) [7]. Cierny et al. claim that IL-6 activates oxytocin receptors in myocytes and its levels have a positive correlation with the intensity of uterine contractions [8]. At the same time, an excessive amount of this cytokine can also have a negative effect. Mittal et al. suggest that over-expression of IL-6 correlates with the decreased frequency of uterine contractions, which may be related to the depletion of glycogen stores in uterus myocytes [9]. A significant increase of IL-6 level during the active labor phase may indicate the important role of this cytokine in the development and progression of labor. Supporting this theory, data from Alvarez-de-la-Rosa et al. showed that during threatened preterm labor, women with a successful tocolytic therapy had significantly lower serum IL-6 levels as compared to the ineffective treatment group [10]. The effect of IL-6 on the labor activity can be explained by its ability to potentiate the secretion of vasopressin and oxytocin, it has been proved in vitro [11].
Common knowledge suggests that the normal course of pregnancy is impossible without the production of two necessary hormones - progesterone and relaxin. Progesterone is a powerful inducer of the Th2-type cytokine synthesis. It promotes the production of pro-inflammatory cytokines IL-4, IL-13, while relaxin increases the pro-inflammatory cytokine IFN-γ production by the T-cells [12]. Earlier studies have shown that the plasma levels of progesterone rise along with the increased duration of pregnancy, but it decreases to the moment of labor [13]. The decrease in progesterone levels during labor is accompanied by the decrease in relaxin. However, relaxin level may increase on the day before and during labor in women with poor progress in labor [14]. Decreased levels of IL-4, IL-13, IFN-γ observed in our study during the latent phase when compared to the active phase, are probably associated with lower levels of the above-mentioned hormones during the onset and progression of labor. Hanna et al. claim that decreased IFN-γ blood plasma level is associated with the development of spontaneous preterm and term labor due to the increased activity of cyclooxygenase and the production of prostaglandin E2 [15]. Reyes-Lagos et al. showed in their study that onset of labor is accompanied by a decrease in plasma of IFN-γ levels when compared to the third trimester of pregnancy that indicates its active role in the process of labor [16]. Our findings correspond to the hypothesis of cellular immunity suppression occurring during pregnancy, which is accompanied by a decrease in IFN-γ levels in plasma. A further decrease in IFN-γ plasma concentration is associated with the onset and progression of labor. Along with the listed cytokines, we also discovered a decrease in levels of TNF-α and IL-12 during the active phase. Thus, TNF-α, that is one of the pro-inflammatory cytokines produced by activated macrophages of the decidua and trophoblast, is one of the key mediators of the inflammatory process and leukocyte activation. As a biomarker of inflammation it contributes to the labor progression by influencing the production of prostaglandins [17]. According to the existing literature, TNF-α concentration in amniotic fluid is significantly higher in cases of preterm labor compared to term labor [18]. Preterm labor is also associated with higher TNF-α levels in serum in comparison with normal course of pregnancy at the same gestation [19]. Cierny et al. state that an increase in TNF-α levels in blood serum is accompanied by the prolonged first stage of labor [20]. M. Winkler in his study showed that TNF-α concentration in lower uterine segment tissue samples rises at the onset of labor and continues to increase until cervix opens by 4–6 cm, after which it starts to decrease, however, not significantly [21]. TNF-α, as a biomarker of inflammation, contributes to the development and progression of labor by altering the production of prostaglandins. We found a significantly lower level of TNF-α during the latent phase as compared to the active phase of the first stage of labor, which may indicate the weakening of the role of prostaglandins and the activation of other factors during labor when the cervix is dilated by 5 cm or more.
IL-12 is a pro-inflammatory cytokine that plays an important role in the regulation of the activity of natural killer cells during early pregnancy and is considered to be vital for the reproductive outcome. Changes in its concentration can have a negative effect since its increase in maternal serum has been described in women with preeclampsia [22]. Ekelund et al. suggest that a decrease of IL-18 (below the 25th percentile) levels in combination with an increase of IL-12 (above 75th percentile) doubles the risk of preterm labor before 34 weeks of gestation [23].
Conclusion
Our data revealed an increase in level of pro-inflammatory cytokine IL-6 and a decrease in levels of TNF-α, IFN-γ, IL-12, as well as in anti-inflammatory cytokines IL-4 and IL-13 in peripheral blood plasma of parturient women from latent to the active phase of spontaneous labor. These findings suggest that the cytokine balance is an essential component of the physiology of labor and further research would allow us to detect patterns associated with development of disorders.
References
- Ravanos K., Dagklis T., Petousis S., Margioula-Siarkou C., Prapas Y., Prapas N. Factors implicated in the initiation of human parturition in term and preterm labor: a review. Gynecol. Endocrinol. 2015; 31(9): 679-83. doi: 10.3109/09513590.2015.1076783.
- Dubicke A., Fransson E., Centini G., Andersson E., Byström B., Malmström A. et al. Pro-inflammatory and anti-inflammatory cytokines in human preterm and term cervical ripening. J. Reprod. Immunol. 2010; 84(2): 176-85. doi: 10.1016/j.jri.2009.12.004.
- Павлов Р.В., Сельков С.А., Телегина И.В. Особенности морфологии и содержания цитокинов в ткани нижнего маточного сегмента в зависимости от родовой деятельности. Журнал акушерства и женских болезней. 2011; 60(6): 57-61. [Pavlov R.V., Selkov S.A., Telegina I.V. Features of the morphology and content of cytokines in the tissue of the lower uterine segment, depending on the generic activity. Journal of Obstetrics and Women’s Diseases. 2011; 60 (6): 57-61. (in Russian)]
- Степанова О.И., Сафронова Н.Ю., Фураева К.Н., Львова Т.Ю., Соколов Д.И., Сельков С.А. Влияние секреторных факторов плаценты на продукцию цитокинов эндотелиальными клетками. Бюллетень экспериментальной биологии и медицины. 2012; 154(9): 361-4. [Stepanova OI, Safronova N.Yu., Furaeva KN, Lvova T.Yu., Sokolov D.I., Selkov S.A. Influence of placental secretion factors on cytokine production by endothelial cells. Bulletin of experimental biology and medicine. 2012; 154 (9): 361-4. (in Russian)]
- Bukowski R., Sadovsky Y., Goodarzi H., Zhang H., Biggio J.R., Varner M. et al. Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ. 2017; 5: e3685. doi: 10.7717/peerj.3685. eCollection 2017.
- Сельков С.А., Павлов О.В., Лалаян Д.В. Цитокиновая сеть плаценты. Возможная роль в инициации родовой деятельности. Медицинская иммунология. 2003; 5(3-4): 341. [Selkov S.A., Pavlov O.V., Lalayan D.V. Placenta cytokine network. Possible role in the initiation of labor. Medical immunology. 2003; 5 (3-4): 341. (in Russian)]
- Osanyin G.E., Adegbola O. Maternal serum interleukin 6 levels and fetomaternal outcomes in women with preterm premature rupture of membranes in Lagos, South-western Nigeria. J. Matern. Fetal Neonatal Med. 2016; 29(21): 3506-10. doi: 10.3109/14767058.2015.1135123.
- Cierny J.T., Unal E.R., Flood P., Rhee K.Y., Praktish A., Olson T.H., Goetzl L. Maternal inflammatory markers and term labor performance. Am. J. Obstet. Gynecol. 2014; 210(5): 447. e1-6. doi: 10.1016/j.ajog.2013.11.038.
- Mittal P., Romero R., Tarca A.L., Draghici S., Nhan-Chang C.L., Chaiworapongsa T. et al. A molecular signature of an arrest of descent in human parturition. Am. J. Obstet. Gynecol. 2011; 204(2): 177. e15-33. doi: 10.1016/j.ajog.2010.09.025.
- Shahshahan Z., Hashemi L., Rasouli O. Maternal serum interleukin 6 and 8 and C-reactive protein in predicting the tocolytic therapy in preterm labor. J. Res. Med. Sci. 2014; 19(6): 537-41.
- Renner U., De Santana E.C., Gerez J., Fröhlich B., Haedo M., Pereda M.P. et al. Intrapituitary expression and regulation of the gp130 cytokine interleukin-6 and its implication in pituitary physiology and pathophysiology. Ann. N. Y. Acad. Sci. 2009; 1153: 89-97.
- Lissauer D., Kilby M.D., Moss P. Maternal effector T cells within decidua: The adaptive immune response to pregnancy? Placenta. 2017; 60: 140-4. doi: 10.1016/j.placenta.2017.09.003.
- Guo C.M., Sun G. The three mechanisms underlying progesterone withdrawal in late pregnancy in mammals. Sheng Li Xue Bao. 2010; 62(2):171-8.
- Некрасова М.Г., Орлов А.В., Погорелова Т.Н., Друккер Н.А. Динамика продукции релаксантов при слабости родовой деятельности. Современные проблемы науки и образования. 2011; 6: 43. [Nekrasova M.G., Orlov A.V., Pogorelova T.N., Drukker N.A. The dynamics of the production of relaxants with weak labor. Modern problems of science and education. 2011; 6:43. (in Russian)]
- Frascoli M., Coniglio L., Witt R., Jeanty C., Fleck-Derderian S., Myers D.E. et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-γ and TNF-α. Sci. Transl. Med. 2018; 10(438). pii: eaan2263. doi: 10.1126/scitranslmed.aan2263.
- Reyes-Lagos J.J., Peña-Castillo M.Á., Echeverría J.C., Pérez-Sánchez G., Álvarez-Herrera S., Becerril-Villanueva E. et al. Women serum concentrations of the IL-10 family of cytokines and IFN-γ decrease from the third trimester of pregnancy to active labor. Neuroimmunomodulation. 2017; 24(3): 162-70. doi: 10.1159/000480734.
- Keelan J.A., Blumenstein M., Helliwell R.J., Sato T.A., Marvin K.W., Mitchell M.D. Cytokines, prostaglandins and parturition--a review. Placenta. 2003; 24(Suppl. A): S33-46.
- Lim R., Barker G., Lappas M. TRADD, TRAF2, RIP1 and TAK1 are required for TNF-α-induced pro-labour mediators in human primary myometrial cells. Am. J. Reprod. Immunol. 2017; 78(1): e12664. doi: 10.1111/aji.12664.
- Thomakos N., Daskalakis G., Papapanagiotou A., Papantoniou N., Mesogitis S., Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010; 148(2): 147‐51.
- Herrera-Muñoz A., Fernández-Alonso A.M., Fischer-Suárez N., Chedraui P., Pérez-López F.R. Maternal serum cytokine levels in pregnancies complicated with threatened preterm labour. Gynecol. Endocrinol. 2017; 33(5): 408-12. doi: 10.1080/09513590.2017.1284786.
- Cierny J.T., Unal E.R., Flood P., Rhee K.Y., Praktish A., Olson T.H., Goetzl L. Maternal inflammatory markers and term labor performance. Am. J. Obstet. Gynecol. 2014; 210(5): 447. e1-6. doi: 10.1016/j.ajog.2013.11.038.
- Winkler M. Role of cytokines and other inflammatory mediators. BJOG. 2003; 110(Suppl. 20): 118-23.
- El-Kabarity R.H., Naguib A.H. Serum levels of IL-18, IL-12 and TH-1/TH-2 ratio in patients with pre-eclampsia. Egypt. J. Immunol. 2011; 18(1): 1-8.
Received 24.05.2018
Accepted 22.06.2018
About the Authors
Tysyachnyy, Oleg V., PhD., junior scientific researcher of the first maternity departments, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381188. E-mail: olti23@mail.ruPavlova, Olga A., postgraduate student of the first maternity departments, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381188. E-mail: olgandrevna@mail.ru
Vtorushina, Valentina V., PhD, doctor of the Laboratory of Clinical Immunology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381183. E-mail: vtorushina@inbox.ru
Krechetova, Lyubov V., PhD., head of Laboratory of Clinical Immunology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381183. E-mail: l_krechetova@oparina4.ru
Baev, Oleg R., MD, professor, head of the first Maternity Department, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381188. E-mail: o_baev@oparina4.ru
I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), Department of Obstetrics, Gynecology, Perinatology and Reproduction.
For reference: Tysyachnyy O.V., Pavlova O.A., Vtorushina V.V., Krechetova L.V., Baev O.R. Peripheral blood cytokine levels in women according to the phase of the first period of labor. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 86-92. (in Russian)
http://dx.doi.org/10.18565/aig.2019.2.86-92



