Cytokine levels and lipid peroxidation status in the blood, follicular fluid, and saliva of infertile women and their relationship with the effectiveness of in vitro fertilization
Romanova E.A., Sidorenkova K.A., Petrov I.A., Spirina L.V., Samoilova Yu.G., Stakheeva M.N., Merkulov E.D., Okkel Yu.V., Kubykina M.I., Arkhipova Ya.I.
Infertility affects approximately 17% of couples worldwide, representing approximately one-sixth of the adult population, underscoring the significance of this issue. The causes of infertility are diverse and affect human reproductive function. However, the role of immune disorders in the development of acute and chronic infectious and inflammatory diseases of the reproductive system in women has not yet been thoroughly investigated.
Objective: To determine the levels of TNF-α, interleukin-8, interleukin-10, VEGF, MCP-1, malondialdehyde, and the activity of catalase and superoxide dismutase (SOD) in the blood serum, follicular fluid, and saliva of infertile women undergoing in vitro fertilization (IVF), and to assess their impact on treatment effectiveness.
Materials and methods: This study included 32 women diagnosed with infertility who were undergoing IVF treatment. The participants were comparable in age (35.4 (4.4) years]). The study materials included blood serum, non-blood-contaminated follicular fluid samples, and saliva.
Cytokine levels (TNF-α, interleukin-8, interleukin-10, VEGF, and MCP-1) in the biological fluids were measured using enzyme immunoassays. Lipid peroxidation was assessed by measuring malondialdehyde levels and catalase and superoxide dismutase (SOD) activities.
Results: The cytokine levels in the follicular fluid were higher than those in the blood serum and saliva, regardless of IVF effectiveness. In women who successfully completed the IVF program (group 1), interleukin-8 levels increased 1.27-fold, MCP-1 levels increased 1.42-fold, and SOD activity decreased 2.54-fold in the follicular fluid. No significant differences were observed in the serum and saliva parameters.
Conclusion: The study found that follicular fluid contained the highest concentrations of cytokines, whereas TNF-α levels were nearly undetectable. In patients with favorable IVF outcomes, interleukin-8 and MCP-1 levels in the follicular fluid were higher than those in patients with negative IVF results. Distinct features of the cytokine profile were identified that may serve as potential predictors of ART program outcomes in patients diagnosed with infertility.
Authors' contributions: Spirina L.V., Romanova E.A., Samoilova Yu.G., Stakheeva M.N., Okkel Yu.V. – conception and design of the study; Sidorenkova K.A., Romanova E.A. – data collection and processing; Romanova E.A., Merkulov E.D. – statistical analysis; Spirina L.V., Romanova E.A., Kubykina M.I. – drafting of the manuscript; Spirina L.V., Petrov I.A., Samoilova Yu.G., Stakheeva M.N., Arkhipova Ya.I. – editing of the manuscript. All authors approved the final version of the article before submission, agreed to be accountable for all aspects of the study, implying appropriate investigation and resolution of questions related to the accuracy or integrity of any part of the study.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted with financial support from the Siberian State Medical University.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Siberian State Medical University (Ref. No: №8 of 18.01.2023).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Romanova E.A., Sidorenkova K.A., Petrov I.A., Spirina L.V., Samoilova Yu.G., Stakheeva M.N., Merkulov E.D., Okkel Yu.V., Kubykina M.I., Arkhipova Ya.I. Cytokine levels and lipid peroxidation status in
the blood, follicular fluid, and saliva of infertile women and their relationship with
the effectiveness of in vitro fertilization.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 124-132 (in Russian)
https://dx.doi.org/10.18565/aig.2025.94
Keywords
The prevalence of infertility is 17.8% in high-income countries and 16.5% in low- and middle-income countries [1], affecting approximately one in six adults worldwide. The etiology of infertility encompasses a wide range of factors that affect human reproductive function [2]. Currently, the role of immune disorders in the pathogenesis of acute and chronic infectious and inflammatory diseases of the female reproductive system has not been sufficiently studied [3, 4].
Cytokines secreted by immune cells can stimulate or inhibit cell growth, regulate cell proliferation, induce chemotaxis, and modulate the expression of other cytokines [5]. Immunocompetent cells, along with epithelial cells, secrete a significant number of cytokines into follicular fluid (FF), which regulates follicle maturation and ovulation through autocrine and paracrine mechanisms [6–8].
It is believed that a high concentration of interleukin (IL)-8 in patients with idiopathic infertility may negatively impact the outcomes of in vitro fertilization (IVF) [9]. The development of ovarian hyperstimulation syndrome is associated with increased levels of vascular endothelial growth factor (VEGF) in the FF. However, a study by Volchenok et al. (2019) noted an opposite relationship: VEGF is essential for the vascular support of follicles that have entered growth, as well as for the proper process of ovulation and the functioning of the corpus luteum [10].
Monocyte chemoattractant protein 1 (MCP-1), also known as CCL2, influences the progression of malignant gynecological diseases. However, new aspects of MCP-1 activity that contribute to embryo implantation have yet to be sufficiently studied [11]. MCP-1 may also play a role in suppressing cyclooxygenase-2, which is associated with oxidative stress, thereby increasing the likelihood of successful pregnancy [12].
Studies examining the ratios of IL-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α in the blood have shown that the balance between cytokines produced by Th1 and Th2 cells is crucial for successful pregnancy [13, 14].
The activation of mononuclear phagocytes leads to an increase in reactive oxygen species and intensifies lipid peroxidation processes [15]. Reactive oxygen species (ROS) released by the immune system and epithelial cells can act as mediators in various processes associated with the female reproductive system. ROS are involved in ovarian steroidogenesis, ovulation, oocyte maturation, and embryo development [16].
Consequently, numerous contradictory data have emerged regarding the role of cytokines and the characteristics of oxidative stress in the regulation of reproductive function. However, the question of creating effective laboratory approaches to predict the effectiveness of assisted reproductive technology (ART) remains unresolved.
This study aimed to determine the levels of TNF-α, interleukin-8, interleukin-10, VEGF, MCP-1, malondialdehyde, and the activity of catalase and superoxide dismutase (SOD) in the blood serum, follicular fluid, and saliva of infertile women undergoing IVF, and to assess their impact on treatment effectiveness.
Materials and methods
This retrospective study involved 32 women diagnosed with infertility who underwent IVF and intracytoplasmic sperm injection (ICSI) at the ART center (Head of Department: Petrov I.A.) of the Siberian State Medical University, Ministry of Health of Russia, in Tomsk, Russia.
The study design and group formation were conducted 6 months after ART procedures. The first group consisted of women with a positive ART outcome (n=10), and the second group included women who did not achieve pregnancy after the procedure (n=22). The groups were comparable in age (35.4 (4.4) years). Body mass index (BMI) for both groups was 24.3 (5.8) kg/m2. A successful outcome of the IVF program was defined as the presence of a progressive pregnancy, indicated by an embryo with a heartbeat in the uterine cavity.
The inclusion criteria were informed consent for this study; patients with infertility who met the indications for IVF as per order 803n, Appendix No. 2 [17]; patients with preserved ovarian reserve, indicated by an anti-Müllerian hormone concentration in blood serum of more than 1.2 ng/ml, and those obtaining three or more oocytes on the day of transvaginal follicle puncture.
The exclusion criteria included refusal to participate in the study at any stage, patients with infertility who had contraindications for IVF as per order 803n, Appendix No. 2 [17], patients with low ovarian reserve, indicated by an anti-Müllerian hormone concentration in blood serum of less than 1.2 ng/ml, and those with fewer than 3 oocytes on the day of transvaginal follicle puncture.
All patients underwent a comprehensive examination in accordance with order No. 107n of 30.08.2012 regarding the use of assisted reproductive technologies, including contraindications and restrictions [18]. Diagnosis was verified based on general clinical and laboratory studies, transvaginal echography at different phases of the menstrual cycle, laparoscopy, hysteroscopy, and morphological examination of the biopsies. Following the examination and pre-gravid preparation, all women participated in the IVF program. Superovulation was induced in both groups according to a protocol using gonadotropin-releasing hormone antagonists. Oocyte fertilization was achieved through IVF or ICSI, followed by selective transfer of one blastocyst on the 5th day of cultivation.
Samples of blood serum, non-blood-contaminated FF, and saliva were collected from all patients on the day of transvaginal follicle puncture. All studies adhered to the ethics committee's regulations and the Helsinki Declaration (protocol No. 8 of 18.01.2023). Biological materials were frozen at -20°C and stored until analysis.
The levels of TNF-α, IL-8, IL-10, VEGF, and MCP-1 were measured using enzyme-linked immunosorbent assay (No. RZN 2017/5961, No. RZN 2017/6005, No. RZN 2017/6011, No. RZN 2017/5974, RZN 2017/5969) in FF, blood serum, and saliva (Uniplan, Russia) using the Vector Best kit, following the manufacturer's recommendations, and expressed in ng/ml.
Determination of MDA Content. This method is based on the formation of a colored complex of MDA and thiobarbituric acid (TBA). For the assay, 0.1 ml of blood serum was mixed with 1.0 ml of 20% trichloroacetic acid (TCA) and 1.0 ml of 0.8% TBA solution. The samples were incubated for 10 min at 100°C and then centrifuged. The intensity of the color was measured at a wavelength of 540 nm in a cuvette with a 1 cm optical path length and expressed in μmol/ml.
Determination of Catalase Activity. To measure catalase activity, 2.0 ml of 0.03% hydrogen peroxide solution was added to 0.1 ml of blood serum. The samples were incubated for 10 min at 37°C and the reaction was stopped by adding 1.0 ml of 4% ammonium molybdate solution. The samples were then centrifuged for 10 min at 4000 rpm. The intensity of the developing color was measured using a spectrophotometer at a wavelength of 410 nm against the control. For the blank sample, 2.0 ml of distilled water was used instead of hydrogen peroxide. Catalase activity was expressed as μmol/min/l.
Determination of SOD Activity. The SOD activity was measured by quantifying the amount of adrenaline oxidation product, which absorbs light at 347 nm. To prepare the sample, 100 μl of a 0.1% adrenaline solution was added to 2 ml of 0.2 M carbonate buffer (pH=10.65), mixed quickly, and the optical density was measured at a wavelength of 347 nm every 30 s for 5 min. For the experimental sample, 10 μl of the sample and 100 μl of 0.1% adrenaline were added to a cuvette containing 2 ml of buffer, and the increase in optical density was measured as previously described. For comparison purposes, the control sample did not contain adrenaline.
Statistical analysis
The normality of the distribution of quantitative variables was assessed using the Kolmogorov–Smirnov test with Lilliefors correction. Statistically significant differences in continuous variables between the two independent groups were analyzed using the Mann–Whitney U test. For comparisons involving more than two independent groups, the Kruskal–Wallis test with the Bonferroni correction was applied. Statistical analysis was conducted using Statistica 10.0.
Results and discussion
This study included 32 women diagnosed with infertility caused by various factors (Table 1). Six women had tubal infertility, ten had female infertility associated with male factors, and 16 had other forms of infertility. Two groups were formed based on the ART outcomes: positive (n = 10) and negative (n=22). Patients were also divided into two groups according to BMI: <25 kg/m2 (n=17) and ≥25 kg/m2 (n=15). The clinical characteristics of the patients with infertility are presented in Table 1.

A special role is played by the interaction of immunocompetent and epithelial cells, which together secrete a significant amount of cytokines in FF [6]. During the study, it was found that the highest level of most of the studied cytokines, regardless of the effectiveness of ART, was noted in FF, whereas the levels of TNF-α in this environment was almost zero (Table 2). The concentrations of IL-8, VEGF, and MCP-1 were increased in FF by 3.53, 46.94 and 3.6 times, respectively, compared with those in blood serum. Simultaneously, a decrease was observed in some indicators of lipid peroxidation. The MDA level and catalase activity were reduced in FF by 2.26 and 2.09 times, respectively, compared to the indicators in blood serum.
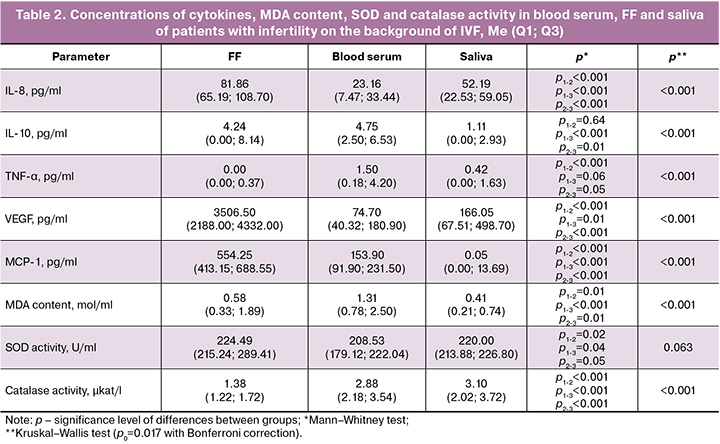
It is worth noting that the levels of IL-10 and TNF-α increased in the blood by 4.28 and 3.5 times, respectively, compared to saliva, which was accompanied by the accumulation of MDA in the blood (an increase of 3.2 times compared to saliva).
The decrease in the levels of IL-8, IL-10, VEGF, and MCP-1 was recorded in saliva by 1.57, 3.82, 21.12, 11000 times, respectively, compared to the indicators in FF against the background of high catalase activity, which was increased by 2.5 times, compared to that in FF.
During folliculogenesis, various cytokine mediators perform key regulatory functions, including pro-inflammatory interleukins, chemotactic factors, and colony-stimulating growth factors, which determine the ovarian potential and quality of the obtained oocytes [14]. The study found that in women whose IVF program ended successfully (group 1), the concentrations of IL-8 and MCP-1 in FF were increased by 1.27 and 1.42 times, respectively, compared to those in group 2 (Table 3). There were no significant differences in the blood serum and saliva.
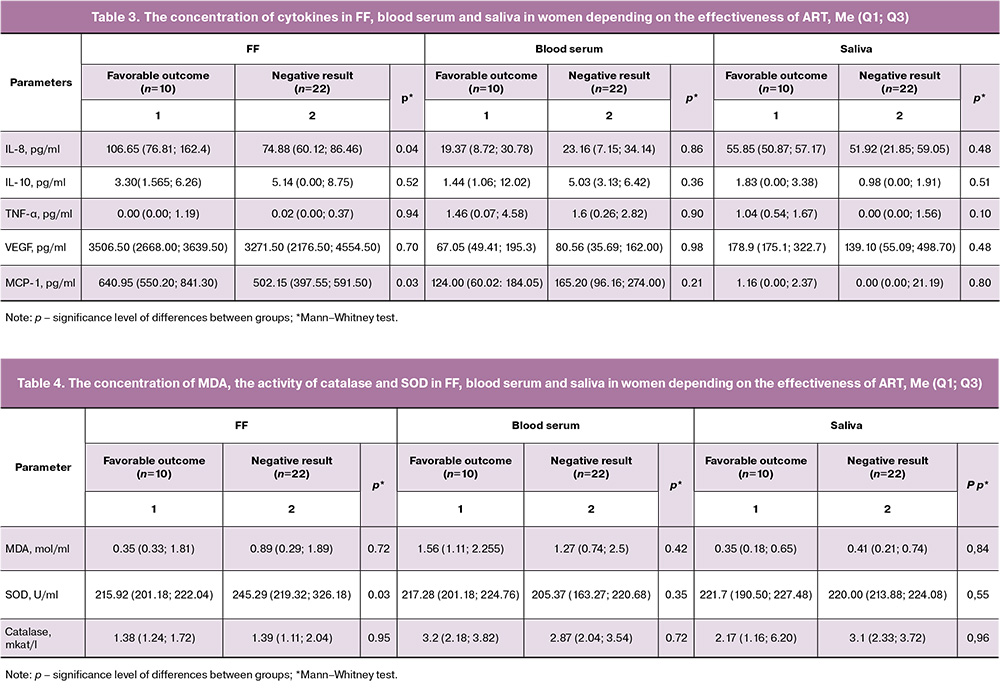
FF refers to biological fluids that reflect hormonal changes that occur in the oocyte microenvironment. Changes in lipid peroxidation are often associated with adverse ART outcomes. SOD activity in patients with a favorable ART outcome was 2.54 times higher compared to that in the group with a negative outcome (Table 4).
In a study by Artini et al. (2022), it was noted that the concentration of pro-inflammatory cytokines in blood serum was significantly lower than in FF obtained from unfertilized oocytes; at the same time, the level of the anti-inflammatory cytokine IL-10 was higher in FF obtained from fertilized oocytes than in unfertilized ones [8].
In another study, IL-8 was elevated in conjunction with MCP-1, which suppressed the activity of cyclooxygenase-2 and increased the effectiveness of IVF [12]. Additional evidence for this is the increase in SOD activity. In this case, even with a high level of proinflammatory cytokines, a favorable IVF outcome was noted.
IL-8 acts as a key regulator of local inflammation, characterized by increased production under conditions of oxidative stress and the realization of its biological activity by attracting immune cells to the site of inflammation [19]. At the same time, the role of MCP-1 in the maturation of reproductive structures is significant, but its effect on the effectiveness of IVF requires further study. However, it is known that in the tissues of the vaginal and cervical epithelium, the directed movement of macrophage and dendritic cell populations to the surface epithelial layer occurs, forming an immune barrier to protect reproductive organs [20].
The complex mechanism of action of IL-8 and MCP-1 in FF may be due to an increase in luteinizing hormone levels, which stimulates the expression of cytokine genes and causes an influx of macrophages into the preovulatory follicle, leading to its maturation. At this stage, the volume of the follicle increases, which allows the assessment of the concentration of its individual biochemical and immunological properties [21]. Thus, the results obtained indicate that an isolated increase in pro-inflammatory markers is not a prognostically unfavorable sign in the presence of a balanced immune response, which expands the understanding of the pathophysiological mechanisms underlying the successful conduct of IVF.
The results of this study demonstrate that the optimal ratio of cytokine-mediated regulatory mechanisms in the microenvironment of the follicle is a determining factor that influences the quantitative and qualitative characteristics of maturing oocytes, which in turn determines the success of the fertilization process of the obtained gametes. These results emphasize the importance of inflammatory processes in the follicular microenvironment for successful pregnancy outcomes. It is believed that pregnancy in mammals is characterized by an increase in basal oxygen consumption; with its development, oxidative stress increases [22], which was also confirmed in this study. It is worth noting that in the blood serum and saliva, it was not possible to identify an increase in the levels of cytokines, migration factors, and indicators of lipid peroxidation.
Correlations were found between cytokine levels in the blood serum, FF, and saliva. When the composition of one biological fluid changes, corresponding changes are observed in others, which can be used as a new approach in the diagnosis of infertility.
To identify the relationship between FF cytokines and each other, correlation analysis was performed. An inverse relationship between TNF-α activity and IL-8 concentration (rxy=-0.54, p=0.05) was observed. It was found that in saliva, the concentration of TNF-α was positively correlated with the concentration of IL-8 (rxy=0.56, p=0.05), whereas in blood serum, a direct relationship between SOD activity and TNF-α in FF was determined (rxy=0.49, p=0.05). A high level of SOD in the blood is usually considered a positive factor, indicating good antioxidant protection of the body, whereas an increase in the functional activity of SOD in this environment may indicate the presence of an inflammatory process in the ovaries [23].
An increase in the levels of TNF-α in FF, with an increase in the total activity of SOD and a decrease in the concentration of IL-8 in blood serum, may have a positive value. IL-8 attracts immune cells to the site of inflammation, acting as a chemokine and stimulating the migration of neutrophils to the focus of inflammation [24], while the low concentration of this cytokine may be due to the influence of SOD and TNF-α. An increase in SOD activity in the blood of patients with infertility indicates the stimulation of antioxidant protection and may be associated with the reduced consumption of these enzymes to neutralize reactive oxygen species [25]. At the same time, TNF-α, a soluble cytokine, can enhance or suppress the inflammatory process, affecting the expression of adhesion molecules and chemokines. At the same time, TNF-α causes inflammatory reactions and has a significant inhibitory effect on steroidogenesis and folliculogenesis [11].
Saliva is a clinically informative biological fluid because of its high levels of biomarkers [26]. Given the wide range of systemic factors affecting oxidative stress, the use of specific markers produced locally in saliva may be more accurate and sensitive in predicting ART outcomes. It should be noted that the practical application of this method requires additional studies to assess its diagnostic significance and standardize analytical procedures.
Indicators such as the levels of cytokines, MDA, SOD, and catalase in the blood serum, FF, and saliva demonstrated prognostic significance as potential markers for determining IVF and ICSI outcomes. Among all studied indicators, only IL-8 and MCP-1 in FF had good prognostic significance (AUC 0.844, 95% CI: 0.657–0.952, p<0.001) (figure). Sensitivity and specificity were 60% and 100%, respectively.
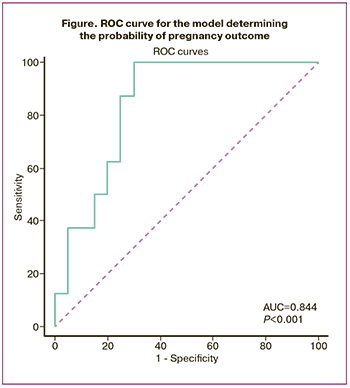
The analysis of the results obtained allowed us to consider IL-8 and MCP-1 as promising biomarkers for predicting the success of ART in patients with infertility.
Changes in the concentrations of IL-8 and MCP-1 in FF in patients contribute to the formation of an inflammatory response, which can potentially affect pregnancy outcomes. These changes can disrupt the microenvironment of the follicle, which negatively affects the quality of the oocytes and embryo implantation. Such disorders can lead to adverse pregnancy outcomes, including a decrease in the probability of successful implantation and an increased risk of early miscarriage.
Conclusion
This study examined the characteristics of cytokine levels and lipid peroxidation parameters in the biological fluids of patients. In FF, cytokine levels were found to be maximal, while TNF-α content was nearly absent. Notably, women who successfully became pregnant through ART exhibited significantly higher levels of IL-8 and monocyte MCP-1 in FF than those whose IVF attempts were unsuccessful. This finding underscores the critical role of inflammatory processes in the follicular microenvironment in successful pregnancy.
Consequently, this study highlights the significance of investigating inflammatory cytokines such as IL-8 and MCP-1 in FF as predictors of ART outcomes. These results can inform the development of targeted interventions to enhance treatment and improve the ART success rates in this patient group.
References
- Ban M., Jiao J., Zhou J., Cui L., Wang H., Chen Z.J. Association of age at menarche and different causes of infertility: a retrospective study of 7634 women undergoing assisted reproductive technology. J. Ovarian Res. 2025; 18(1): 40. https://dx.doi.org/10.1186/s13048-025-01629-y
- Loukopoulos T., Zikopoulos A., Kolibianakis E., Vatopoulou A., Gkrozou F., Sotiriou S. et al. High-Risk outcomes in in vitro fertilization pregnancies for women of a very advanced maternal age: insights from a multi-hospital study in Greece. J. Clin. Med. 2025; 14(4): 1323. https://dx.doi.org/10.3390/jcm14041323
- Moreira T., Leal C., Barreiro M., Tomé A., Vale-Fernandes E. Predictors of pregnancy after artificial insemination in women with polycystic ovary syndrome. JBRA Assist. Reprod. 2025; 29(2): 201-10. https://dx.doi.org/10.5935/1518-0557.20240095
- Basso C.G., Rocha B.A., Hauer I.R., Cruz J.C., Filho F.F., Barbosa F. et al. Associations between urinary and follicular fluid concentrations of phthalate metabolites and reproductive outcomes in Brazilian women undergoing fertility treatment. Reprod. Toxicol. 2025; 133: 108868. https://dx.doi.org/10.1016/j.reprotox.2025.108868
- Sarapik A., Velthut A., Haller-Kikkatalo K., Faure G.C., Béné M.C., de Carvalho Bittencourt M. et al. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin. Dev. Immunol. 2012; 2012: 606459. https://dx.doi.org/10.1155/2012/606459
- Базиева Т.А., Ордиянц И.М., Джабраилова Б.А., Тунгузбиева Р.У. Современные представления о нарушениях состояния эндометрия при привычном невынашивании. Медицинский вестник Юга России. 2022; 13(4): 53-7. [Bazieva T.A., Ordiyants I.M., Dzhabrailova B.A., Tunguzbieva R.U. Modern ideas about the violation of endometrial receptivity in recurrent miscarriage. Medical Herald of the South of Russia. 2022; 13(4): 53-7 (in Russian)]. https://dx.doi.org/10.21886/2219-8075-2022-13-4-53-57
- Хонина Н.А., Андреева Е.А., Тихонова М.А., Баторов Е.В., Останин А.А., Пасман Н.М., Черных Е.Р. Интерлейкин-6 как возможный регулятор интрафолликулярных Foxp3+-регуляторных T-клеток у женщин в цикле экстракорпорального оплодотворения. Иммунология. 2019; 40(2): 30-7. [Khonina N.A., Andreeva E.A., Tikhonova M.A., Batorov E.V., Ostanin A.A., Pasman N.M., Chernykh E.R. Interleukin-6 as a possible regulator of intrafollicular FoxP3+ T-cells in women undergoing IVF treatmen. Immunologiya. 2019; 40(2): 30-7 (in Russian)].https://dx.doi.org/10.24411/0206-4952-2019-12005
- Artini P.G., Scarfò G., Marzi I., Fusi J., Obino M.E., Franzoni F. et al. Oxidative stress-related signaling pathways predict oocytes' fertilization in vitro and embryo quality. Int. J. Mol. Sci. 2022; 23(21): 13442. https://dx.doi.org/10.3390/ijms232113442
- Rimon-Dahari N., Yerushalmi-Heinemann L., Alyagor L., Dekel N. Ovarian Folliculogenesis. Results Probl. Cell. Differ. 2016; 58: 167-90;https://dx.doi.org/10.1007/978-3-319-31973-5_7
- Волченок Д.А., Тихоновская О.А., Мустафина Л.Р., Логвинов С.В. Экспрессия факторов паракринной регуляции в яичниках крыс при коррекции экспериментальных функциональных кист яичников. Journal of Siberian Medical Sciences. 2019; 1: 67-77. [Volchenok D.A., Tikhonovskaya O.A., Mustafina L.R., Logvinov S.V. Expression of paracrine regulation factors in rat’s ovaries when correcting experimental functional ovarian cysts. Journal of Siberian Medical Sciences. 2019; 1: 67-77 (in Russian)]. https://dx.doi.org/10.31549/2542-1174-2019-1-67-77
- Hidalgo A.I., Ulloa-Leal C., Gajardo G., López G., Carretta D., Burgos R.A. et al. Ovulation induced by intrauterine seminal plasma increases total protein, PGE2, IL-8, and IL-1β in uterine fluid of llamas (Lama glama). Animals. 2023; 13(4): 554. https://dx.doi.org/10.3390/ani13040554
- Gonzalez M.B., Lane M., Knight E.J., Robker R.L. Inflammatory markers in human follicular fluid correlate with lipid levels and body mass index. J. Reprod. Immunol. 2018; 130: 25-9. https://dx.doi.org/10.1016/j.jri.2018.08.005
- Лихачева В.В., Зорина Р.М., Баженова Л.Г., Маркдорф А.Г., Архипова С.В., Филимонов С.Н. Содержание и прогностическое значение некоторых цитокинов в сыворотке крови и фолликулярной жидкости у женщин с синдромом поликистозных яичников, участвующих в программе ЭКО. Медицина в Кузбассе. 2017; 16(4): 34-8. [Likhacheva V.V., Zorina R.M., Bazhenova L.G., Markdorf A.G., Arkhipova S.V., Filimonov S.N. Content and prognostic value of some cytokines in blood serum and follicular fluid in women with polycystic ovary syndrome participating in the IVF program. Medicine in Kuzbass. 2017; 16(4): 34-8 (in Russian)].
- Mahdian S., Zarrabi M., Moini A., Shahhoseini M., Movahedi M. In silico evidence for prednisone and progesterone efficacy in recurrent implantation failure treatment. J. Mol. Model. 2022; 28(4): 105. https://dx.doi.org/10.1007/s00894-022-05093-z
- Awonuga A.O., Camp O.G., Abu-Soud H.M. A review of nitric oxide and oxidative stress in typical ovulatory women and in the pathogenesis of ovulatory dysfunction in PCOS. Reprod. Biol. Endocrinol. 2023; 21(1): 111. https://dx.doi.org/10.1186/s12958-023-01159-6
- Dai M., Hong L., Yin T., Liu S. Disturbed follicular microenvironment in polycystic ovary syndrome: relationship to oocyte quality and infertility. Endocrinology. 2024; 165(4): bqae023. https://dx.doi.org/10.1210/endocr/bqae023
- Приказ Минздрава России от 31.07.2020 № 803н «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению» (зарегистрировано в Минюсте России 19.10.2020 № 60457). Доступно по: https://www.consultant.ru/document/cons_doc_LAW_365474/ [Order of the Ministry of Health of the Russian Federation No. 803n dated July 31, 2020 "On the procedure for using assisted reproductive technologies, contraindications, and restrictions on their use" (registered with the Ministry of Justice of the Russian Federation on October 19, 2020, No. 60457). Available at: https://www.consultant.ru/document/cons_doc_LAW_365474/ (in Russian)].
- Приказ Минздрава России от 30.08.2012 № 107н (ред. от 01.02.2018) «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению» (зарегистрировано в Минюсте России 12.02.2013 № 27010). Доступно по: https://www.consultant.ru/document/cons_doc_LAW_142595/ [Order of the Ministry of Health of the Russian Federation No. 107n dated August 30, 2012 (as amended on February 1, 2018) "On the procedure for using assisted reproductive technologies, contraindications, and restrictions on their use" (registered with the Ministry of Justice of the Russian Federation on February 12, 2013, No. 27010). Available at: https://www.consultant.ru/document/cons_doc_LAW_142595/(in Russian)].
- Алегина Е.В., Тетруашвили Н.К., Агаджанова А.А., Трофимов Д.Ю., Донников А.Е. Полиморфизм гена интерлейкина-8 у женщин с привычными потерями беременности. Акушерство и гинекология. 2015; 9: 33-7. [Alegina E.V., Tetruashvili N.K., Agadzhanova A.A., Trofimov D.Yu., Donnikov A.E. Interleukin-8 gene polymorphism in women with recurrent miscarriages. Obstetrics and Gynecology. 2015; (9): 33-7 (in Russian)].
- Соснин Д.Ю., Галькович К.Р. Содержание моноцитарного хемотаксического фактора в нормальной сперме и в образцах эякулята с пониженной фертильностью. Пермский медицинский журнал. 2019; 36(3): 28-37. [Sosnin D.Yu., Galkovich K.R. Monocyte chemotactic factor content in normal sperm and in samples of ejaculate with diminished fertility. Perm Medical Journal. 2019; 36(3): 28-37 (in Russian)] https://dx.doi.org/10.17816/pmj36328-37
- Турдыбекова Я.Г. Фолликулогенез и фолликулярный запас яичника в норме и патологии: аспекты (этапы) клинико-морфологического изучения (литературный обзор). Вестник Казахского Национального медицинского университета. 2019; 1: 41-5. [Turdybekova Y.G. Folliculogenesis and follicular reserve of the ovary in health and disease: aspects of clinical and morphological study (review). Bulletin of the Kazakh National Medical University. 2019; (1): 41-5 (in Russian)].
- Граф А.В., Байжуманов А.А., Маслова М.В., Крушинская Я.В., Маклакова А.С., Соколова Н.А., Каменский А.А. Активность антиоксидантной системы при беременности в норме и при гипоксии. Вестник Московского университета. Серия 16. Биология. 2021; 76(3): 126-33. [Graf A.V., Baizhumanov A.A., Maslova M.V., Krushinskaya Y.V., Maklakova A.S., Sokolova N.A., Kamensky A.A. The antioxidant system activity during normal pregnancy and pregnancy following by hypoxic stress. Vestnik Moskovskogo universiteta. Seriya 16. Biologiya. 2021; 76(3):126-33 (in Russian)].
- Пушкина Т.А., Токаев Э.С., Попова Т.С., Бородина Е.Н. Супероксиддисмутаза в составе антиоксидантной терапии: состояние вопроса и перспективы (обзор литературы). Журнал им. Н.В. Склифосовского «Неотложная медицинская помощь». 2016; 4: 42-7. [Pushkina T.A., Tokayev E.S., Popova T.S., Borodina E.N. Superoxide dismutase as a component of antioxidant therapy: current state of the issue and prospects (a literature review). Sklifosovsky journal of emergency medical care. 2016; (4): 42-7 (in Russian)].
- Меняйло М.Е., Малащенко В.В., Шмаров В.А., Газатова Н.Д., Мелащенко О.Б., Гончаров А.Г., Селедцова Г.В., Селедцов В.И. Интерлейкин-8 способен поддерживать провоспалительную активность моноцитов (макрофагов) человека. Гены и клетки. 2018; 13(1): 65-9. [Meniailo M.E., Malashchenko V.V., Shmarov V.A., Gazatova N.D., Melashchenko O.B., Goncharov A.G., Seledsova G.V., Seledsov V.I. Interleukin-8 is able to promote pro-inflammatory activity of human monocytes (macrophages). Genes and Cells. 2018; 13(1): 65-9 (in Russian)]. https://dx.doi.org/10.23868/201805007
- Bulavenko O.V., Kelman V.V. Effect of stress on ovulatory function. Reports of Vinnytsia National Medical University. 2023; 27(3): 523-7. https://dx.doi.org/10.31393/reports-vnmedical-2023-27(3)-28
- Hoffmann J.A., Gründler K., Richter D.U. Stubert J. Prediction of spontaneous preterm birth using CCL2 and CXCL10 in maternal serum of symptomatic high-risk pregnant women: a prospective cohort study. BMC Pregnancy Childbirth. 2023; 23(1): 697. https://dx.doi.org/10.1186/s12884-023-06016-3
Received 07.04.2025
Accepted 10.07.2025
About the Authors
Ekaterina A. Romanova, student, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2, katyromanova24@mail.ru, https://orcid.org/0009-0006-1925-538XKira A. Sidorenkova, PhD student at the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2, +7(952)884-37-49, kirasidorenkova@mail.ru, https://orcid.org/ 0009-0007-6263-6716
Ilya A. Petrov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2, +7(952)899-83-66, obgynsib@gmail.com, https://orcid.org/0000-0002-0697-3896
Liudmila V. Spirina, Dr. Med. Sci., Professor, Head of the Department of Biochemistry and Molecular Biology with a course of clinical laboratory diagnostics, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2; Leading Researcher at the Laboratory of Tumor Biochemistry, Research Institute of Oncology of Tomsk Scientific Research Center, 634009, Russia, Tomsk, Cooperative lane, 5, spirinalvl@mail.ru, https://orcid,org/0000-0002-5269-736X
Yulia G. Samoilova, Dr. Med. Sci., Professor, Professor at the Department of Faculty Therapy with Clinical Pharmacology, Professor at the Department of Pediatrics with Endocrinology, Head of the Clinical Research Center, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2; Chief Specialist for Medical Prevention of the Department of Health of the Tomsk Region, 634050, Russia, Tomsk, Moscowski Trakt str., 4; Director of the Institute of Medicine and Medical Technologies, Novosibirsk National Research State University, 630090, Russia, Novosibirsk, Pirogova str., 1, +7(913)826-74-24, samoilova_y@inbox.ru,
https://orcid.org/0000-0002-2667-4842
Marina N. Stakheeva, Dr. Med. Sci., Professor at the Department of Biochemistry and Molecular Biology with a course of clinical laboratory diagnostics, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2; Leading Researcher at the Laboratory of Tumor Biochemistry, Research Institute of Oncology of Tomsk Scientific Research Center, 634009, Russia, Tomsk, Cooperative lane, 5, stakheyevam@oncology.tomsk.ru, https://orcid,org/0000-0003-0601-2240
Evgeny D. Merkulov, Laboratory Assistant, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2,
evmerc@mail.ru, https://orcid,org/0000-0002-7082-9389
Yulia V. Okkel, PhD, Teaching Assistant at the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2, okkel_uv@mail.ru, https://orcid.org/0000-0001-6386-1535
Marina I. Kubykina, Obstetrician-Gynecologist, Reproductologist at the Center of ART, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2, +7(983)235-54-21, marina.kubykina@mail.ru, https://orcid.org/0009-0002-4598-1079
Yana I. Arkhipova, Obstetrician-Gynecologist, Reproductologist at the Center of ART, Siberian State Medical University, Ministry of Health of Russia, 634050, Russia, Tomsk, Moscowski Trakt str., 2, +7(952)885-98-31, yana_98@icloud.com, https://orcid.org/0009-0002-1062-7344



