The role of interleukin-8 (IL-8) and polymorphism of IL-8 gene in formation of external genital endometriosis in patients of reproductive age
Aim. To study IL-8 production and IL-8 (251T>A) gene polymorphism in patients of reproductive age with external genital endometriosis (EGE).Avanesova T.G., Levkovich M.A., Ermolova N.V., Palieva N.V., Petrov Yu.A.
Materials and methods. The study included 71 patients with different stages of external genital endometriosis and 24 patients without endometriosis in the control group. The levels of IL-8 in serum and peritoneal fluid were determined by enzyme immunoassay using the kits produced by MedSystems (Austria). The distribution of alleles and genotypes of the 251T>A polymorphism of the gene IL-8 was carried out by the method of polymerase chain reaction followed by restriction analysis (GosNIIgenetika, Moscow).
Results. The increased IL-8 concentrations in the peritoneal fluid were found in patients with stages I-II EGE.
The distribution of IL-8 gene allelic variants among patients with various stages of ЕGE was characterized by a predominance of the T/T genotypes of the 251T> A polymorphism.
Conclusion. Increased IL-8 concentrations in the peritoneal fluid and the presence of the polymorphic marker 251T>A in IL-8 gene were associated with a risk of development of ЕGE in patients of reproductive age.
Keywords
External genital endometriosis (EGE) is one of the most important problems of gynecology. In the structure of gynecological pathology, EGE takes the third place and affects up to 50% of women with preserved menstrual function [1–5]. EGE is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain and infertility, often negatively affecting the psychoemotional state of women and overall quality of life [6, 7].
The pronounced negative impact of EGE on the quality of life of patients, their reproductive function, impaired adaptation of patients in society, and growing costs of treatment determine the social significance of the disease and the importance of studying it [8, 9].
Despite numerous theories describing the pathogenesis of endometriosis, there is a lack of knowledge about the development of the disease in a woman's body [10–14]. Considerable attention is currently being paid to the role of the immune system in the development of endometriosis. In patients with genital endometriosis, significant changes are observed in both local immunity and immunological components in circulating blood [15, 16]. There is few data on the role of interleukin (IL) -8 and its gene polymorphism in IGE [17].
In this regard, the aim of the research was to study the production of IL-8 and the polymorphism 251T> A of the IL-8 gene in patients of reproductive age with EGE.
Materials and methods
The study included 71 patients with EGE, who were divided into two groups: Group 1 – patients with stages I–II EGE (n=31); Group 2 – patients with stages III– IV EGE (n=40).
The control group consisted of 24 patients who underwent diagnostic laparoscopy with no evidence of EGE in the Department of Operative Gynecology of the Research Institute of Obstetrics and Pediatrics, Rostov State Medical University, Ministry of Health of Russia, who underwent the criteria for the inclusion of patients in the study: reproductive age 18–49 years, infertility and/or pelvic pain, body mass index 18.5–25 kg/m2, normal body temperature, laparo- and hysteroscopy with morphological verification of the diagnosis of EGE.
Exclusion criteria of the study: pubertal and perimenopausal age of patients, malignant neoplasms, severe decompensated extragenital pathology, acute infections forms.
Determination of IL-8 content in blood serum and peritoneal fluid was carried out by enzyme immunoassay using Bendermedsystems kits (Austria).
Allelic variants of IL-8 genes were determined by polymerase chain reaction followed by restriction analysis using a commercial test system for molecular genetic analysis developed by GosNIIgenetics (Russia).
Statistical analysis
To create a database and conduct a statistical study, we used the capabilities of an Excel 2006 spreadsheet processor and application packages (Megastat and Statisticapp 6.0). When determining the differential validity between the study groups, Mann – Whitney test was used with the Bonferroni correction, in which the traditional level of type 1 error is divided by the number of comparisons. The number of comparison pairs was calculated using the formula m=n(n–1)/2, where n is the number of groups; therefore, the critical value of the significance level (p) in our study was 0.02. Data are presented as median (Me) and quartiles Q1 and Q3. Determination of statistically significant differences between the groups of genotypes and studied genes is conducted using the criterion or χ2 according to the formula.
Results and Discussion
At laparoscopy, ovarian endometriomas was found in 66.2% of patients, providing evidence on the late diagnosis of the diseas. Superficial foci of endometriosis on the ovaries were found in 23.9% of cases. Pelvic peritoneal lesions were found: in the rectal-uterine pouch in 71.8% of cases; on the uterosacral ligaments in 57.7%; in the vesicouterine space – in 40.8% of cases.
During hysteroscopy, the endometrial hyperplasia was found with similar incidence (70%) in both clinical groups with EGE and was histologically confirmed. In the results of the morphological study, simple hyperplasia without atypia prevailed.
Histological examination of endometrioid lesions removed during surgery revealed that the presence of fibrosis and sclerosis, which were considered the outcomes of inflammatory process was statistically significantly higher in patients of the Group 2 (with III-IV stages of EGE) than in patients of the Group 1 (with I–II stages EGE) – 28/40 (70%) versus 4/31 (12.9%), p=0.01, which is apparently the. Hemosiderosis and angiomatosis were also more frequent in the advanced stages of the disease 31/40 (77.5%) and 15/40 (37.5%) versus 4/31 (12.9%) and 1/31 (3.2%), respectively (p=0.01), and were considered as the signs of the disease progression.
Proinflammatory cytokines are the main mediators and communicators of the immune system, initiate and enhance inflammatory responses to infection or injury, and are able to recruit immune cells to the sites of injury and stimulate them to synthesize additional cytokines.
Disregulation of cytokines can be considered as an important aspect of the pathogenesis of endometriosis. However, their role in the survival of endometriotic lesions remains poorly understood. This is in part due to the fact that these modulators are pleiotropic proteins that have a variety of functions.
Chemokines, in particular IL-8, are important factors involved in inflammation associated with endometriosis. IL-8 induces chemotaxis of neutrophils and other immune cells, and is also a potent angiogenic agent, involved in all processes of disease development: adhesion, invasion, implantation of ectopic tissue. In addition, IL-8 plays a role in the growth and maintenance of endometrial ectopic tissue, directly affecting the proliferation of endometrial cells. IL-8 can also protect ectopic cells from death as a result of apoptosis [18, 19].
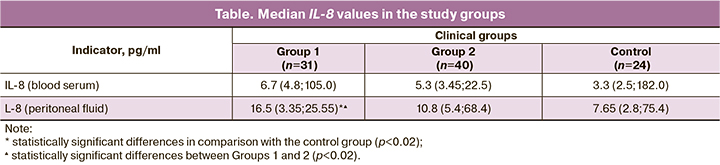
The results of studying the median values of IL-8 in the study groups are presented in the table.
In women in Group 1 (in the early stages of endometriosis), compared with the control and Group 2, there was a statistically significant increase in the IL-8 content in the peritoneal fluid, (p=0.01), moreover, the indicators in Group 1 exceeded the indicators in Group 2 1.5 times, (p=0.01) (Table 1). There were no statistically significant differences between the groups in IL-8blood serum levels.
According to modern concepts, the pathogenesis of endometriosis can be associated with the expression of inflammatory mediators, the induction of an immune response, and the level of their expression depends on the allelic variant of the gene in a genotype. Of particular importance is the study of genes encoding signaling molecules and causing the coordinated work of various systems and organs [20–22].
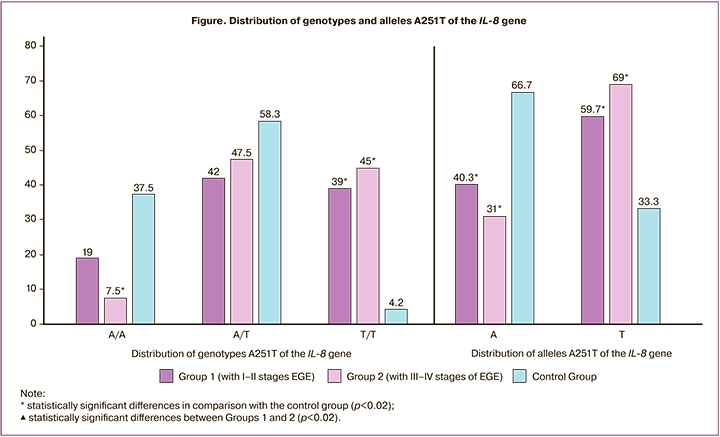
Since IL-8 promotes endometrial cell proliferation, adhesion and angiogenesis, an excess of this cytokine can lead to the growth of endometriotic foci and local neovascularization. In this regard, it was interesting to study the dependence of the risk of developing endometriosis in the presence of polymorphism in the IL-8 gene. The results of studying the frequency of polymorphism 251T> A of the IL-8 gene in patients are shown in the Figure.
When studying the distribution of the polymorphism 251T> A of the IL-8 gene, we did not find statistically significant differences between the patients of Groups 1 and 2.
The T/T genotype of the IL-8 gene was found 9.3 and 10.7 times more often in Groups 1 and 2, respectively, compared with the control group, χ2 =8.94 (p=0.01) and χ2 =8.94 (p=0.01).
In polymorphism 251T> A, the incidence of allele A in patients with EGE was lower, and the incidence of allele T was higher than in the control group (χ2 =7.52 at p=0.007, OR=2.9 (95% CI 1.35; 6.5) and χ2 =15.23 at p=0.01, OR=4.4 (95% CI 2.05; 9.45), respectively (Fig. 1).
EGE is one of the most common gynecological diseases affecting women of reproductive age, associated with an increase in the level of proinflammatory cytokines in the peritoneal cavity.
We found that the level of IL-8 was increased in the peritoneal fluid of women, mainly with stage I–II endometriosis. Our results indicate local IL-8 disregulation in the early stages of endometriosis. An increase in the level of IL-8 can lead to the development of proliferation of endothelial cells, and to inhibition of apoptosis processes [23]. IL-8 can act as an autocrine growth factor in the endometrium and promote a vicious cycle of endometrial cell attachment, which can lead to a transformation of acute inflammation to chronic. The data obtained indicate the decisive participation of IL-8 in the formation of endometrioid lesions. Since IL-8 promotes endometrial cells proliferation, adhesions development and angiogenesis, excess of these cytokines can promote growth and local neovascularization. We suggested that the IL-8 gene polymorphism associated with increased production of this cytokine may lead to the growth and maintenance of ectopic endometrial tissue, determining a more pronounced inflammatory response.
The data obtained show that the distribution of allelic variants of IL-8 genes among patients with different stages of EGE is characterized by a predominance of T/T genotypes of polymorphism 251T> A. Thus, the detection of 251T> A polymorphism of the IL-8 gene may serve as a marker of an increased risk of endometriosis in patients of reproductive age.
Conclusion
An increase in the level of IL-8 in the peritoneal fluid, the presence of the polymorphic marker 251T> A of the IL-8 gene is associated with the risk of EGE development in patients of reproductive age.
References
- Адамян Л.В., Фархат К.Н., Макиян З.Н., Савилова А.М. Молекулярно-биологическая характеристика эутопического эндометрия (обзор литературы). Проблемы репродукции. 2015; 21(5): 8-16. [Adamyan L.V., Farkhat K.N., Makiyan Z.N., Savilova A.M. The external genital endometriosis: theories and molecular investigations (a review). Problemy reproduktsii/ Problems of Reproduction. 2015; 21(5): 8-16 (in Russian)]. https://dx.doi.org/10.17116/repro20152158-16.
- Коган Е.А., Акопова Е.О., Унанян А.Л. Бесплодие при эндометриозе: краткий очерк современных представлений. Пространство и время. 2017; 1: 251-9. [Kogan E.A., Akopova E.O., Unanyan A.L. Infertility in Endometriosis: A Brief Sketch of Modern Concepts. Prostranstvo i vremya/Space and Time. 2017; 1: 251-9. Available at: 2226-7271provr_st1-27.2017.103 Accessed September 29, 2018. (in Russian)].
- Ермолова Н.В., Друккер Н.А., Погорелова Т.Н., Слесарева К.В., Томай Л.Р., Маркарьян И.В., Трушина С.А. Молекулярно-биологические технологии в диагностике и дифференцированном подходе к лечению эндометриоза. Журнал акушерства и женских болезней. 2016; 65 (Приложение): 8-10. [Ermolova N.V., Drukker N.A., Pogorelova T.N., Slesareva K.V., Tomay L.R., Markar'yan I.V. et al. Molecular biological technologies in the diagnosis and differentiated approach to the treatment of endometriosis. Zhurnal akusherstva i zhenskikh bolezney/Journal of Obstetrics and Womans Diseases. 2016; 65(Suppl.): 8-10. Available at: https://elibrary.ru/item.asp?id=28968529 Accessed November 19, 2018. (in Russian)].
- Wimberger P., Grübling N., Riehn A., Furch M., Klengel J., Goeckenjan M. Endometriosis – a chameleon: patients’ perception of clinical symptoms, treatment strategies and their impact on symptoms. Geburtshilfe Frauenheilkd. 2014; 74(10): 940-6. https://dx.doi.org/10.1055/s-0034-1383168.
- Rogers P.A.W., Adamson G.D., Al-Jefout M., Becker C.M., D'Hooghe T.M., Dunselman G.A.J. et al. Research priorities for endometriosis. Reprod. Sci. 2017; 24(2): 202-26. https://dx.doi.org/10.1177/1933719116654991.
- Ярмолинская М.И., Зайцев Д.В., Тхазаплижева С.Ш. Мелатонин и генитальный эндометриоз – новые возможности терапии. Журнал акушерства и женских болезней. 2015; 64(1): 67-75. [Yarmolinskaya M. I., ZaytsevD. V., Tkhazaplizheva S. Sh. Melatonin and genital endometriosis – new possibilities for therapy. Zhurnal akusherstva i zhenskikh boleznei/Journal of Obstetrics and Womans Diseases. 2015; 64(1): 67-75. (in Russian)]. https://dx.doi.org/10.17816/JOWD64167-75.
- Yeo S.G., Won Y.S., Lee H.Y., Kim Y.I., Lee J.W., Park D.C. Increased expression of pattern recognition receptors and nitric oxide synthase in patients with endometriosis. Int. J. Med. Sci. 2013; 10(9): 1199-208. https://dx.doi.org/10.7150/ijms.5169.
- Brosens I., Puttemans P., Benagiano G. Endometriosis: a life cycle approach? Am. J. Obstet. Gynecol. 2013; 209(4): 307-16. https://dx.doi.org/10.1016/j.ajog.2013.03.009.
- Acién P., Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet. Gynecol. 2013; 2013: 242149. https://dx.doi.org/10.1155/2013/242149.
- Сонова М.М., Адамян Л.В., Жорданиа К.И., Паяниди Ю.Г. Эндометриоз и рак яичников. Есть ли взаимосвязь? Oбщие патогенетические черты рака яичников и эндометриоза. Онкогинекология. 2013; 4: 30-40. [Sonova M.M., Adamyan L.V., Zhordania K.I., Payanidi Yu.G. Endometriosis and ovarian cancer. Is there a relationship? Common pathogenetic features of ovarian cancer and endometriosis. Onkoginekologija/Oncogynecology. 2013; 4: 30-40. (in Russian)].
- Левкович М.А., Ермолова Н.В., Аванесова Т.Г. Иммунопатогенез генитального эндометриоза. Российский аллергологический журнал. 2017; 14 (Приложение 1): 76-8. [Levkovich M.A., Ermolova N.V., Avanesova T.G. Immunopathogenesis of genital endometriosis. Rossiiskii allergologicheskii zhurnal/ Russian Journal of Allergology. 2017; 14 (Suppl. 1): 76-8. (in Russian)].
- Паяниди Ю.Г., Жорданиа К.И., Логинов В.И., Чемерис Г.Ю., Сивакова Н.Г. Эндометриоз и эндометриоидный рак яичников. Акушерство и гинекология: новости, мнения, обучение. 2017; 1: 44-8. [Payanidi Yu.G., Zhordania K.I., Loginov V.I., Chemeris G.Yu., Sivakova N.G. Endometriosis and endometrioid ovarian cancer. Akusherstvo i ginekologiya: novosti, mneniya, obuchenie/Obstetrics and gynecology: news, opinions, training. 2017; 1: 44-8. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2017-00016.
- Melin A., Lundholm C., Malki N., Swahn M.L., Sparen P., Bergqvist A. Endometriosis as a prognostic factor for cancer survival. Int. J. Cancer. 2011; 129(4): 948-55. https://dx.doi.org/10.1002/ijc.25718.
- Nassif J., Mattar S., Abu Musa A., Eid A. Endometriosis and cancer: what do we know? Minerva Ginecol. 2013; 65(2): 167-79.
- Асташкин E.И., Кречетова Л.В., Ванько Л.В. Роль нейтрофильных гранулоцитов в развитии эндометриоза. Акушерство и гинекология. 2020; 6: 5-12. [Astashkin E.I., Vanko L.V., Krechetova L.V. The role of neutrophilic granulocytes in the development of endometriosis. Obstetrics and Gynecology. 2020; 6: 5-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.6.5-12.
- Сельков С.А., Ярмолинская М.И. Эндометриоз как патология регуляторных механизмов. Журнал акушерства и женских болезней. 2017; 66(2): 9-13. [Selkov S.A., Yarmolinskaya M.I. Endometriosis as a pathology of regulatory mechanisms. Zhurnal akusherstva i zhenskikh boleznei/Journal of Obstetrics and Womans Diseases. 2017; 66(2): 9-13. (in Russian)]. https://dx.doi.org/10.17816/jowd6629-13.
- Рзаева А.Ш. Распространение полиморфизма интерлейкина-8 – 251 ТА среди женщин Азербайджана больными эндометриозом. Успехи современного естествознания. 2011; 3: 32-4. [Rzayeva A.Sh. The role of interleukin IL-8 – 251TA polymorphism in pathogenesis of endometriosis among women in Azerbeidzjan. Uspekhi sovremennogo estestvoznaniya/ Achievements of modern natural science. 2011; 3: 32-4. Available at: http://www.natural-sciences.ru/ru/article/view?id=15955 Accessed October 17, 2018. (in Russian)].
- Sikora J., Smycz-Kubańska M., Mielczarek-Palacz A., Kondera-Anasz Z. Abnormal peritoneal regulation of chemokine activation - the role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017; 77(4): e12622. https://dx.doi.org/10.1111/aji.12622.
- Красильникова А.К., Малышкина А.И., Сотникова Н.Ю., Анциферова Ю.С. Особенности функционального состояния фагоцитов крови у пациенток с эндометриозом I–II стадии и бесплодием. Российский вестник акушера-гинеколога. 2017; 17(3): 9-14. [Krasilnikova A.K., Malyshkina A.I., Sotnikova N.Yu., Antsiferova Yu.S. The functional state of phagocytes in patients with stage I–II endometriosis and infertility. Rossiiskii vestnik akushera-ginekologa/Russian Bulletin of Obstetrician-Gynecologist. 2017; 17(3): 9-14. (in Russian)]. https://dx.doi.org/10.17116/rosakush20171739-14.
- Питиримова Л.Н., Загороднева Е.А., Гумилевский Б.Ю. Особенности аллельного полиморфизма генов интерлейкинов и цитокиновый баланс женщин с невынашиванием беременности. Акушерство и гинекология. 2014; 3: 33-8. [Pitirimova L.N., Zagorodneva E.A., Gumilevskiy B.Yu. Specific features of allelic polymorphism of the interleukin genes and the cytokine balance in women with miscarriage. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2014; 3: 33-8. (in Russian)].
- Левкович М.А., Ермолова Н.В., Аванесова Т.Г., Маркарьян И.В. Современные взгляды на патогенез генитального эндометриоза: роль гормональных, иммунологических, генетических факторов. Таврический медико-биологический вестник. 2017; 20(2-2): 185-9. [Levkovich M.A., Ermolova N.V., Avanesova T.G., Markaryan I.V. Modern viewpoints on the pathogenesis of genital endometriosis: the role of hormonal, immunological, and genetic factors. Tavricheskii mediko-biologicheskii vestnik/Tauride Medical and Biological Bulletin. 2017; 20(2-2): 185-9. (in Russian)].
- Ярмолинская М.И., Айламазян Э.К., Сельков С.А. Иммунологические аспекты генитального эндометриоза. В кн.: Репьева Н.Н., ред. Генитальный эндометриоз. Различные грани проблемы. СПб.: ЭКО-Вектор; 2017: 126-73. [Yarmolinskaya M.I., Ailamazyan E.K., Sel'kov S.A. Immunological aspects of genital endometriosis. In: Yarmolinskaya M.I., Ailamazyan E.K. Genital endometriosis. Different facets of the problem. SPb.: EKO Vektor; 2017:126-73. (in Russian)].
- Laganà A.S., Sturlese E., Retto G., Sofo V., Triolo O. Interplay between misplaced Müllerian-derived stem cells and peritoneal immune dysregulation in the pathogenesis of endometriosis. Obstet. Gynecol. Int. 2013; 2013: 527041. https://dx.doi.org/10.1155/2013/527041.
Received 01.10.2020
Accepted 25.11.2020
About the Authors
Tatevik G. Avanesova, Ph.D., obstetrician-gynecologist, City Hospital No 8, Rostov-on-Don, Russia. Tel.: +7(905)453-59-58.E-mail: tata_avanesova@mail.ru. ORCID: 0000-0002-7225-7206. 344010, Russia, Rostov-on-Don, Krasnoarmeyskaya str., 19.
Marina A. Levkovich, Dr. Med. Sci., Associate Professor, Leading Researcher at the Department of Medical and Biological Problems in Obstetrics, Gynecology and Pediatrics, Rostov State Medical University, Ministry of Health of Russia, Research Institute of Obstetrics and Pediatrics. Tel.: +7(918)570-64-36.
E-mail: xlma@mail.ru. ORCID: 0000-0001-8047-7148. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Natalia V. Ermolova, Dr. Med. Sci., Associate Professor, Head of the Department of Obstetrics and Gynecology, Rostov State Medical University, Ministry of Health of Russia, Research Institute of Obstetrics and Pediatrics. Tel.: +7(929)813-32-54. E-mail: rniiap.ermolova@gmail.com.
ORCID: 0000-0002-6537-3436. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Natalia V. Palieva, Dr. Med. Sci., Professor at the Department of Obstet-rics and Gynecology No2, Leading Researcher at the Department of Obstetrics and Gynecology, Rostov State Medical University, Ministry of Health of Russia, Research Institute of Obstetrics and Pediatrics. Tel.: +7(928) 296-46-96.
E-mail: nat-palieva@yandex.ru. ORCID: 0000-0003-2278-5198. 344012, Russia, Rostov-on-Don, Mechnikova str., 43.
Yuriy A. Petrov, Dr. Med., Sci., professor, Head of the Department of Obstetrics and Gynecology No2, Rostov State Medical University, Ministry of Health of Russia.
Tel.: +7(863)250-42-00. E-mail: mr.doktorpetrov@mail.ru. ORCID: 0000-0002-2348-8809. 344022, Russia, Rostov-on-Don, Nakhichevanskiy str., 29.
For citation: Avanesova T.G., Levkovich M.A., Ermolova N.V., Palieva N.V., Petrov Yu.A. The role of interleukin-8 (IL-8) and polymorphism of IL-8 gene in formation of external genital endometriosis in patients of reproductive age.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 124-129 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.124-129



