Reducing the incidence of early reproductive losses through sperm selection based on membrane zeta potential in assisted reproductive technology
Gamidova P.S., Makarova N.P., Smolnikova V.Yu., Lobanova N.N., Kulakova E.V.
Relevance: Preventing early reproductive losses in assisted reproductive technologies requires comprehensive examination and preparation of the couple, as well as the use of special methods to select high-quality germ cells for fertilization. A promising method for sperm selection is based on the zeta potential of the membrane. However, the indications for using this selection method have not yet been determined, and its effectiveness in Russian couples with infertility remains unknown.
Materials and methods: We conducted a prospective cohort study involving 120 married couples with a history of infertility and early reproductive loss. The study included 62 couples undergoing ICSI with sperm selected based on membrane zeta potential. The control group consisted of 58 couples who underwent ICSI with standard density-gradient sperm isolation. We compared the groups in terms of fertilization and blastulation rates, embryo quality, clinical pregnancy rates, and pregnancy development up to 12 weeks (early reproductive losses).
Results: We observed a statistically significant trend towards higher-quality embryos in the zeta-potential selection group compared to the control group (p<0.001). The pregnancy rate was 35.4% in the study group and 31.0% in the control group (p=0.69; RR=0.87; 95% CI 0.52–1.45). Reproductive loss rates, based on actual pregnancies, were 4.5% in the study group and 33.3% in the control group (p=0.03; RR 0.13; 95% CI 0.01–1.00).
Conclusion: Based on the results obtained, fertilization using ICSI with sperm selection based on membrane zeta potential is effective in reducing the incidence of early reproductive losses. It can be recommended for married couples with male factor infertility, provided that ejaculatory parameters allow selection.
Authors' contributions: Gamidova P.S. – collection and analysis of relevant literature, material processing and drafting of the manuscript; Makarova N.P. – collection and analysis of relevant literature, editing of the manuscript; Smolnikova V.Yu. – editing of the manuscript, critical analysis; Lobanova N.N. – analysis of literary sources, review of the manuscript; Kulakova E.V. – scientific editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gamidova P.S., Makarova N.P., Smolnikova V.Yu., Lobanova N.N., Kulakova E.V. Reducing the incidence
of early reproductive losses through sperm selection based on membrane zeta potential in assisted reproductive technology.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (7): 106-112 (in Russian)
https://dx.doi.org/10.18565/aig.2024.59
Keywords
Infertility is a significant medical and social issue that not only affects individual couples but also has implications for the overall demographics of a country. Infertility is defined as the inability of a couple to achieve pregnancy within 12 months before the age of 35 years (or within 6 months in the late reproductive age) despite regular sexual activity without contraception. However, achieving pregnancy does not guarantee the birth of a child. The European Society of Human Reproduction and Embryology (ESHRE) has introduced the concept of "early reproductive losses," which refers to the loss of pregnancy before reaching 12 weeks of gestational age [1].
The most comprehensive approach for addressing recurrent miscarriage (defined as the loss of 2 or more pregnancies before 22 weeks of gestation) involves a thorough examination of both the woman and man to identify any pathological conditions that may increase the risk of pregnancy loss. This is followed by treatment by a gynecologist and urologist-andrologist. However, there are situations that require a faster solution to the problem, such as couples of late reproductive age, individuals experiencing high levels of psychological stress, or when there is low compliance from one or both partners. In such cases, the use of assisted reproductive technology (ART) with a personalized approach to the embryological stage of treatment is justified.
However, ART alone does not completely eliminate the risk of miscarriage. The use of ART bypasses several natural gamete and embryo selection mechanisms. For example, intracytoplasmic sperm injection (ICSI) into oocytes allows the use of single and immobile sperm for fertilization.
When ART is used in couples with recurrent miscarriages, it is necessary to employ techniques that aim to eliminate or compensate for the factors that lead to reproductive losses during natural conception. Typically, these involve manipulations of gametes and embryos in vitro, which can only be performed with the assistance of ART. One approach to address low sperm quality is to select a subpopulation of the highest-quality male germ cells for subsequent ICSI.
An advanced method for selecting high-quality sperm is based on the presence of sialic acids on the surface of the mature male reproductive cells. These sialic acids give the cells a negative electrical charge, known as the zeta potential, which can reach up to -20 mV [2]. Sperms with such characteristics are believed to have higher chromatin integrity and are more suitable for fertilization [3, 4]. Therefore, using these spermatozoa for injection into the oocyte as part of ART may prove to be an effective method for overcoming reproductive losses.
This study aimed to evaluate the effectiveness of sperm selection based on membrane zeta potential in the treatment of infertility using ART in married couples with recurrent miscarriages.
Materials and methods
This prospective cohort study involved married couples referred to the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility in V.I. Kulakov NMRC for OG&P. All participants had no contraindications or restrictions for infertility treatment using ART and signed an informed consent to participate in the study.
Inclusion criteria were a woman's age from 18 to 40 years, history of two or more episodes of early reproductive pregnancy losses (up to 12 weeks of gestation), changes in basic sperm parameters (number, motility, proportion of morphologically normal sperm), normal ovarian reserve according to ultrasound and hormonal screening, regular menstrual cycle, normal karyotype of spouses, embryo transfer into the uterine cavity without preimplantation genetic testing, and absence of uterine factor infertility. Exclusion criteria were the use of donor gametes and surrogacy, structural anomalies of internal organs, endocrinopathies, conducting the ART program in the natural menstrual cycle, fertilization with sperm isolated from the epididymis or testicular tissue, external genital endometriosis stage III–IV spread, and lack of fertilization using the ICSI.
Before inclusion in the treatment program, all married couples were examined in accordance with Order of the Ministry of Health of Russia dated July 31, 2020 No. 803n “On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use.” Stimulation of ovarian function in all patients was performed according to a standard protocol using recombinant follicle-stimulating hormone (FSH) and gonadotropin-releasing hormone antagonists (GnRH antagonists). Human chorionic gonadotropin (hCG) at a dose of 10,000 IU or GnRH agonists 0.2 mg) was used for final follicular maturation. Follicular puncture was performed 36 h after the administration of the trigger under short intravenous anesthesia in a small operating room using atraumatic aspiration needles (VitroLife, Sweden). According to the inclusion and exclusion criteria, 120 married couples were included in this study. To evaluate the effect of the membrane zeta potential-based sperm selection method, patients were divided into two groups. In the study group (n=62), patients underwent ICSI using sperms selected based on the membrane zeta potential. In the control group (n=58), patients underwent ICSI using sperm isolated using the standard density gradient method.
Ejaculate was collected in sterile containers after 2-3 days of sexual abstinence. Spermatozoa from the study group were first processed using density gradient centrifugation (DGC) according to the manufacturer's protocol (Sage, ORIGIO, Denmark). The isolated fraction of male germ cells was then selected based on zeta potential according to a previously described method [5]. Briefly, after complete liquefaction of the ejaculate, the motility and morphology of the male germ cells were assessed according to the strict Kruger criteria. To 1 ml of native ejaculate, 3 ml of serum-free culture medium Multipurpose Handling Medium (Irvine Scientific, USA) was added and centrifuged at 300G for 10 min (Centrifuge CM-6M, ELMI, Latvia). The resulting precipitate was resuspended in 5 mL of serum-free MHM culture medium. The tube was then exposed to a positive surface charge and incubated at 37°C for 1 min. The supernatant was removed and male gametes with a negative membrane charge on the surface of the tube were diluted with 0.2 mL of Sperm Washing Medium (Irvine Scientific, USA) and used for ICSI.
In the control group, the ejaculate was processed using standard density gradient technology, according to the manufacturer's instructions (Sage, ORIGIO, Denmark). The primary endpoints of the study were the fertilization and blastocyst formation rates. Secondary endpoints included clinical pregnancy and pregnancy rates up to 12 weeks. Blastocyst quality was assessed according to the Gardner classification [6]. For statistical analysis, embryos were divided into several quality categories: excellent quality (AA), good quality (AB, VA), fair quality (VV), and poor quality (score C embryoblasts and/or trophoblasts).
Statistical analysis
Statistical analysis was performed using the SPSS software. The normality of the distribution was tested using the Shapiro–Wilk test. All variables, except FSH levels, were not normally distributed. The chi-squared test was used to compare categorical data (Fisher's exact test for binary variables). The statistical significance of between-group differences for continuous variables was tested using the Mann–Whitney U-test; normally distributed continuous variables were compared using the Student's t-test. Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Results
To assess the differences between the study groups that might affect the pregnancy rate, comparisons were made on a number of demographic, clinical, anamnestic, and laboratory parameters (Table 1). As shown in Table 1, the median patient age was 32-33 years. There was no significant trend toward being overweight or underweight among the patients included in the study. According to the median endocrine parameters (anti-Müllerian hormone (AMH), FSH), there was also no tendency towards a decrease in the ovarian reserve in these patients. The median duration of infertility was four years in both groups. Ovarian function was stimulated according to standard protocols in both groups, and no differences were found.
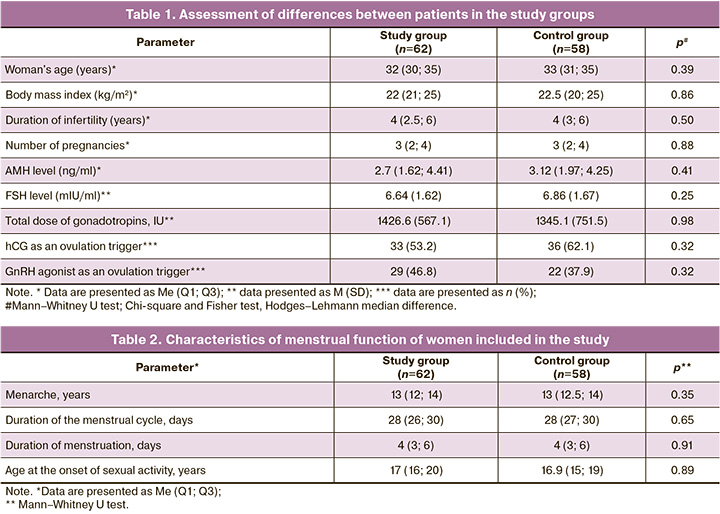
The menstrual function characteristics of the women included in this study are presented in Table 2. There were no significant differences between the patients.
Based on the data presented in Table 3, there is no reason to assume that the comparison results could be influenced by the differences in the severity of male factor infertility. The ages of the patients' partners did not differ significantly between the study and control groups. The groups did not differ in basic sperm parameters (sperm concentration, motility, and morphology).
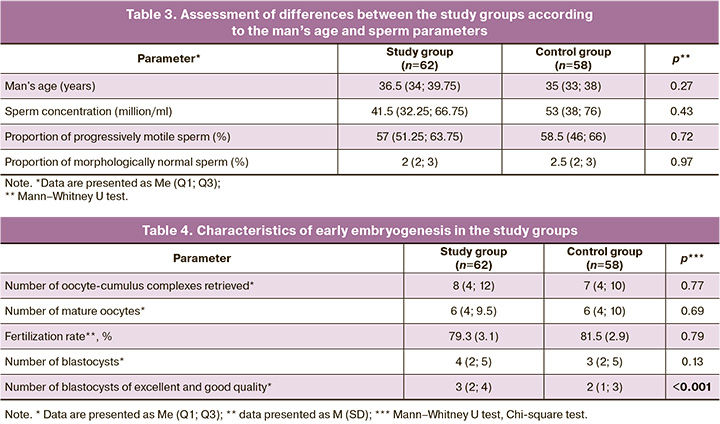
The characteristics of early embryogenesis in the two groups are presented in Table 4. The number of oocytes obtained during follicle puncture did not differ between the groups; therefore, this could not distort the results of their comparison in terms of ART effectiveness. In terms of the frequency of obtaining mature oocytes, fertilization, and blastocyst formation, both groups were comparable. There was a statistically significant trend towards the development of higher quality embryos in the study group (Table 4), indicating a greater potential for embryo development when sperm were selected by zeta potential in the couples in the study.
When assessing the effectiveness of sperm selection technology based on zeta potential, the following results were obtained: embryo transfer into the uterine cavity was performed in all patients. No cases of early embryonic arrest were observed. Embryos were transferred to all women on the 5th day of culture at the blastocyst stage. In the study group, pregnancy as a result of ART occurred in 22/62 couples; one patient had a miscarriage before 12 weeks of gestation. In the control group, pregnancy occurred in 18/58 cases, but 6/18 ended in termination before 12 weeks. Thus, the incidence of clinical pregnancy in the study and control groups was 35.4% and 31%, respectively (p=0.69; RR=0.87; 95% CI 0.52–1.45). The rates of early reproductive losses per ART cycle in the study and control groups were 1.6% and 10.4%, respectively. When calculating actual pregnancies, this figure was 4.5% and 33.3%, respectively, and this difference was statistically significant (p=0.03; RR=0.13; 95% CI 0.01–1.00).
Discussion
Oxidative stress and increased sperm DNA fragmentation are considered to be significant risk factors for reproductive loss. Therefore, men are typically prescribed antioxidant supplements [7]. Other male factors impact the effectiveness of assisted reproductive technology (ART), such as latent infections of the accessory gonads and changes in seminal plasma composition. These factors can lead to moderate sperm agglutination and ejaculate viscosity [8]. However, therapy can be lengthy and does not guarantee results, which is why an early transition to ART methods may be necessary in some cases.
Our study clearly demonstrated the benefits of using sperm zeta potential selection in ART for couples with a history of repeated reproductive loss. The study group did not significantly differ in fertilization frequency and blastulation frequency, which aligns with existing data that suggest minimal involvement of the paternal half of the genome in the earliest stages of embryo development [9]. However, the group that used spermatozoa selected by zeta potential for fertilization showed a statistically significant increase in the number of excellent- and good-quality blastocysts. It is important to note that this did not directly affect the clinical pregnancy rate, although it likely influenced the risk of miscarriage.
Determining the membrane zeta potential of sperm is a promising method for assessing its functional ability [10]. Vahidi S. et al. attempted to estimate the DNA fragmentation index in isolated sperm fractions using zeta potential selection and reaction with hyaluronic acid [11]. Both methods produced a subpopulation of sperm with lower DNA fragmentation than that of the native sample. Hyaluronic acid selection was more effective in this regard (chromatin dispersion test: 24.15% vs. 28.04%). However, clinical endpoints were not assessed in the study by Vahidi S. et al. When interpreting these results, it is important to remember that the sperm DNA fragmentation index is an important marker of sperm quality but not absolute.
Ghorbani-Sini R. et al. attempted to study an alternative marker of sperm quality - telomere length [12]. Spermatozoa selected by density gradient centrifugation and zeta potential analysis did not differ in telomere length from the native sample. However, the DNA fragmentation index measured by the TUNEL method was lower in sperm selected by the zeta potential. The clinical efficacy of ART using these cells has not been studied.Previously, in a study by Sefidgar Tehrani M. et al., the effectiveness of sperm selection through zeta potential was evaluated in men with oligo- and asthenozoospermia, specifically those with a DNA fragmentation index of over 30% [13]. Owing to differences in the inclusion criteria, a comprehensive comparison between the results of that study and our current study is not possible [13]. However, similar to our study, Sefidgar Tehrani M. et al. found no significant difference in fertilization rates when utilizing zeta potential selection but did observe a higher number of high-quality blastocysts. In contrast to our findings, a previous study reported a higher implantation rate using zeta potential selection compared with the control group (42% vs. 24%), which could be attributed to inherently poorer sperm quality. Interestingly, the incidence of miscarriages was not assessed in the study by Sefidgar Tehrani M. et al.
It should be noted that the main limitation of our study is the lack of randomization. This prospective study included a control group with data obtained from real-life clinical practice. One advantage of our study is that it evaluated both embryological and clinical endpoints, specifically the incidence of early reproductive loss until the 12th week of gestation.
Conclusion
Early reproductive losses after ART can greatly affect the success rate of in vitro fertilization. To reduce the risk of miscarriage, it is essential to conduct a thorough examination of both partners, engage in preconception preparation, and seek treatment from gynecologists and andrologists when needed. However, there are instances in which specialized technologies during the embryological stage are necessary to personalize infertility treatment. In this study focusing on teratozoospermia, our findings suggest that intracytoplasmic sperm injection (ICSI) with sperm selection based on membrane zeta potential is a potentially effective method in preventing early reproductive losses with low-quality male germ cells. Zeta potential selection is one of the few sperm selection techniques with evidence of clinical effectiveness, with cumulative evidence continuing to grow. Our study demonstrated the superiority of this technique over density gradient centrifugation for producing high-quality blastocysts. By selecting sperm based on zeta potential, we observed a reduction in the incidence of early reproductive losses following ICSI. Although this method appears to be both effective and safe, further research is needed to validate our results and to determine the appropriate indications for its use.
References
- ESHRE. https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Recurrent-pregnancy-loss
- Chan P.J., Jacobson J.D., Corselli J.U., Patton W.C. A simple zeta method for sperm selection based on membrane charge. Fertil. Steril. 2006; 85(2): 481-6. https://dx.doi.org/10.1016/j.fertnstert.2005.07.1302.
- Zarei-Kheirabadi M., Shayegan Nia E., Tavalaee M., Deemeh M.R., Arabi M., Forouzanfar M. et al. Evaluation of ubiquitin and annexin V in sperm population selected based on density gradient centrifugation and zeta potential (DGCZeta). J. Assist. Reprod. Genet. 2012; 29(4): 365-71. https://dx.doi.org/10.1007/s10815-011-9689-3.
- Nasr Esfahani M.H., Deemeh M.R., Tavalaee M., Sekhavati M.H., Gourabi H. Zeta sperm selection improves pregnancy rate and alters sex ratio in male factor infertility patients: A double-blind, randomized clinical trial. Int. J. Fertil. Steril. 2016; 10(2): 253-60. https://dx.doi.org/10.22074/ijfs.2016.4917.
- Гамидова П.С., Смольникова В.Ю., Макарова Н.П., Лобанова Н.Н. Успешный исход экстракорпорального оплодотворения при абсолютной тератозооспермии после отбора сперматозоида по дзета-потенциалу. Акушерство и гинекология. 2023; 6: 155-9. [Gamidova P.S., Makarova N.P., Smolnikova V.Yu., Lobanova N.N. A successful outcome of in vitro fertilization in absolute teratozoospermia after spermatozoon selection by the zeta potential. Obstetrics and Gynecology. 2023; (6): 155-9. (in Russian)].https://dx.doi.org/10.18565/aig.2023.29.
- Gardner D., Schoolcraft W. In vitro culture of human blastocysts. In: Jansen R., Mortimer, D., eds. Toward reproductive certainty: fertility and genetics beyond. Parthenon Press, Carnforth. 1999: 377-88.
- Гамидов С.И., Шатылко Т.В., Ли К.И., Гасанов Н.Г. Роль антиоксидантных молекул в терапии мужского бесплодия и подготовке мужчины к зачатию ребенка. Медицинский совет. 2020; 3: 122-9. [Gamidov S.I., Shatylko T.V., Li K.I., Gasanov N.G. Role of antioxidant molecules in the treatment of male infertility and preparation of a man for contraception. Medical Council. 2020; (3): 122-9. (in Russian)]. https://dxdoi.org/10.21518/2079-701X-2020-3-122-129.
- Наумов Н.П., Шатылко Т.В., Гамидов С.И., Попова А.Ю., Сафиуллин Р.И. Агглютинация сперматозоидов и время разжижения эякулята как негативный прогностический фактор при ICSI. Андрология и генитальная хирургия. 2022; 23(3): 61-71. [Naumov N.P., Shatylko T.V., Gamidov S.I., Popova A.Y., Safiullin R.I. Sperm agglutination and ejaculate liquefaction time as negative prognostic factor in ICSI. Andrology and Genital Surgery. 2022; 23(3): 61-71. (in Russian)]. https://dx.doi.org/10.17650/2070-9781-2022-23-3-61-71.
- Braude P., Bolton V., Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988; 332(6163): 459-61. https://dx.doi.org/10.1038/332459a0.
- Ionov M., Gontarek W., Bryszewska M. Zeta potential technique for analyzing semen quality. MethodsX. 2020; 7: 100895. https://dx.doi.org/10.1016/j.mex.2020.100895.
- Vahidi S., Narimani N., Ghanizadeh T., Yazdinejad F., Emami M., Mehravaran K. et al. The short abstinence may have paradoxical effects on sperms with different level of DNA integrity: a prospective study. Urol. J. 2021; 18(6): 682-7. https://dx.doi.org/10.22037/uj.v18i.6515.
- Ghorbani-Sini R., Izadi T., Tavalaee M., Azadi L., Hajian M., Rahimi Zamani M. et al. Comparison of sperm telomere length between two sperm selection procedures: density gradient centrifugation and zeta potential. Int. J. Fertil. Steril. 2020; 14(1): 51-6. https://dx.doi.org/10.22074/ijfs.2020.5981.
- Sefidgar Tehrani M., Amirian M., Jalali M., Attaranzadeh A., Fazel A., Ebrahimzadeh-Bideskan A. Role of the zeta method in intracytoplasmic sperm injection outcomes in high sperm DNA fragmentation inoligoasthenozoospermic men. Galen Med. J. 2018; 7: e1107. https://dx.doi.org/10.22086/gmj.v0i0.1107.
Received 18.03.2024
Accepted 02.07.2024
About the Authors
Gamidova Parvin Safail kizi, PhD student at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, gamidova.parvina@yandex.ru
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC
for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, np_makarova@oparina4.ru
Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC
for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, v_smolnikova@oparina4.ru
Natalia N. Lobanova, Junior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, n_lobanova@oparina4.ru
Elena V. Kulakova, Dr. Med. Sci., Senior Researcher at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, e_kulakova@oparina4.ru



