A Russian model for evaluating the efficiency of the sFlt-1/PlGF test for preeclampsia
Objective. To study the Russian model for evaluating the efficiency of the sFlt-1 (soluble fms-like tyrosine kinase 1)/PlGF (placental growth factor) test versus standard practice for preeclampsia (PE) in the second half of pregnancy.Khodzhaeva Z.S., Kholin A.M., Shuvalova M.P., Ivanets T.Yu., Demura S.A., Galichkina I.V.
Material and methods. Based on the data of foreign studies on the nature of the flow of patients with PE, the national statistics of the Russian Federation, national clinical guidelines and protocols for a given algorithm of mathematical formulas (MS excel 2016), the outcomes were modeled taking into account the use of the sFlt-1/PlGF test (Elecsys/Cobas, Roche Diagnostics, Rotkreuz, Switzerland) for the diagnosis of PE.
Results. Analysis of priority publications, as well as mathematical modelling with the introduction of correction factors to the European models demonstrated the advantages of using the innovative sFlt-1/PlGF test in risk-group patients with symptomatic and asymptomatic pregnancy in the diagnosis of PE, reducing the risk of severe forms of the disease, which led to fetal growth restriction, premature birth, and perinatal mortality.
Conclusion. The mathematical modeling data on the use of the innovative sFlt-1/PlGF test in the second half of pregnancy confirm its efficiency in the diagnosis and prediction of PE.
Keywords
Over the past decade, hypertensive complications of pregnancy have remained a significant factor in maternal morbidity and mortality and are directly accountable for 6.9-17.4% of maternal death cases [1]. In the long-term, the women who experienced PE during pregnancy have a higher risk for obesity, chronic hypertension, diabetes, heart disease and stroke [2]. The rate of preterm delivery in this cohort is noted to be high. Premature birth often leads to physical and psychosomatic problems in preterm infants who may later develop full-blown metabolic, hormonal or cardiovascular disorders. [3].
Preeclampsia and eclampsia are associated with gestational hypertension and chronic hypertension and are considered to be major cardiovascular complications in pregnant women [4].
Every year, over 8.5 million women are diagnosed with PE. This condition is responsible for 15% of preterm births globally [4]. PE is a pregnancy-specific syndrome that usually develops after 20 weeks gestation and is characterized by hypertension (systolic BP levels > 140 mmHg and diastolic > 90 mmHg) and proteinuria (≥ 0.3 g/L in daily urine) [5].
Despite the fact that some pathophysiological changes (e.g., defective placentation) may occur in early pregnancy, as a rule, hypertension and proteinuria become apparent in the late second or third trimesters and are reported in 3-8% of all pregnancy cases.
According to the National Clinical Guidelines “Hypertensive Disorders During Pregnancy, Childbirth and Postpartum. Pre-eclampsia and Eclampsia.” designed by the Ministry of Health of Russia in 2016, currently there are no sensitive and specific assays available for detection and identification of PE [5].
The recent global research has produced the new promising diagnostic biomarkers, such as placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) [6]. Most often, these biomarkers are found in pregnant women at risk: those aged over 40 years, obese patients, or having chronic kidney or liver disease.
In the study conducted by Hadker et al. (2013), the gestational sFlt-1/PlGF ratio cut-off level was defined as 85:1 for 20 weeks, with reported test sensitivity of 82% and specificity of 95% [7]. The two innovative biomarkers (sFlt-1 and PlGF) could be assayed using automated analyzers available in medical centers and laboratories. The serum sFlt-1/PlGF ratio was shown to identify women at risk for PE in early pregnancy long before the clinical manifestations of the disease become evident.
The objective of the present study was to review the outcomes of mathematical modeling used for assessment of sFlt-1/PlGF ratio test in the second half of pregnancy and compare them with standard no-test diagnostic practice.
Materials and Methods
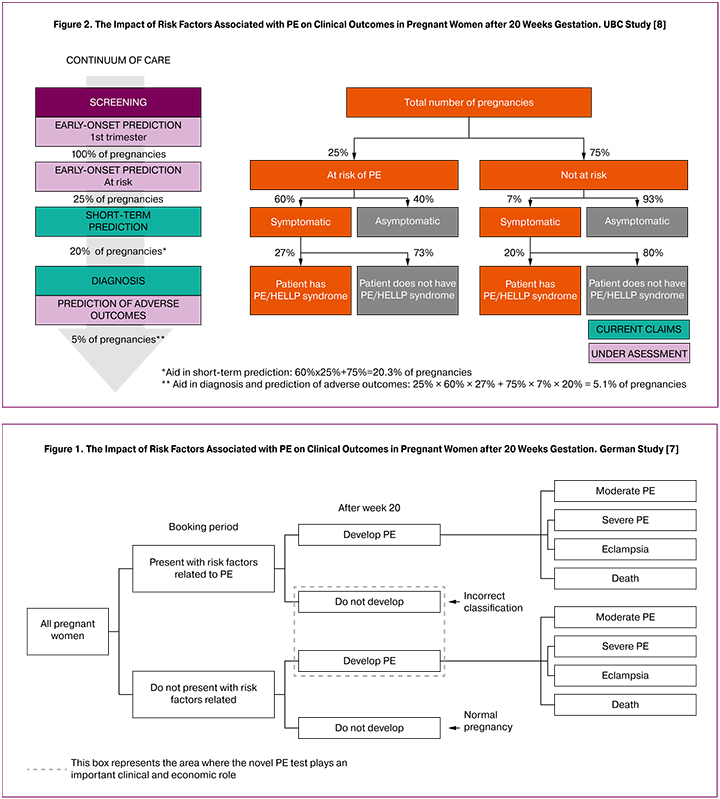
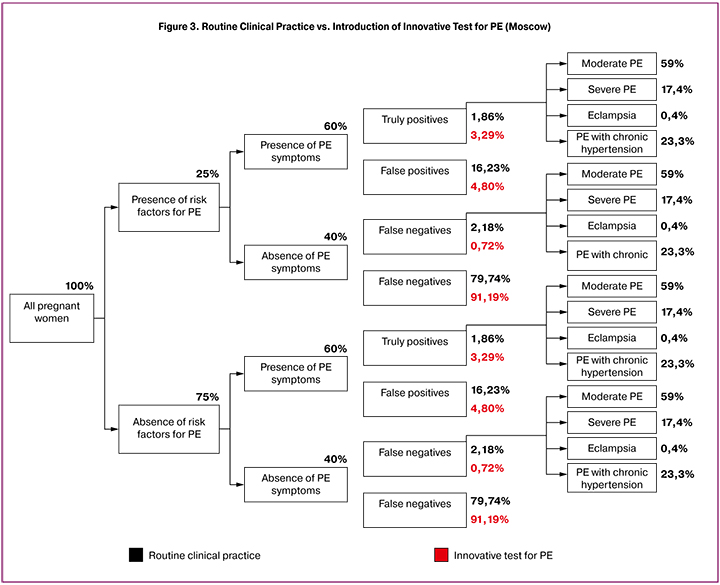
Two European research projects were used as reference to estimate the rate of PE patients in the relevant patient population. The rates of false positives and false negatives, truly positives and truly negatives, as well as the number of patients with different forms of PE in each scenario were calculated using data from the German study of Hadker et al. (2013) [7] (Fig. 1), the HECON UBC (2011) study [8] (Fig. 2), and the available Russian statistics [9, 10].
The data from the HECON model, which, according to local experts is the closest match to the routine clinical practice in Russia, was used to split patients into groups based on symptomatic/asymptomatic disease (60% and 40%, respectively) and the presence/absence of risks for developing PE (25% and 75%) [8]. The final distribution of patients based on the form of PE was made using local statistics [1, 10]. When creating an adapted evaluation model, the data from the German study was also used as the main source of reference, as it was viewed as the most relevant and meaningful for the local setting [7].
Two scenarios were selected for assessing the rates of PE development in the late second or third trimesters.
The first scenario (Standard Care or standard management of pregnant women in Russia) implied regular maternity clinic visits and the use of common diagnostic techniques during each antenatal visit (blood pressure measurement, biochemistry, urine test, ultrasound, Doppler, etc.) [9]. The second scenario (Innovative Test for PE) suggested supplementing routine practice with sFlt-1/PlGF ratio test after week 20.
In both scenarios the entire population of pregnant women in the Russian Federation is supposed to be screened for PE during the second half of pregnancy. Concurrently, 25% of the population under study were to be classified as patients at risk for developing PE. The risk factors for PE typically include the following: history of preeclampsia, absence of previous pregnancies, chronic hypertension, multifetal pregnancy, gestational diabetes [11]. Each group was categorized into smaller subgroups by presence (60%) or absence (40%) of PE symptoms. In addition, the patients were subdivided by clinical outcome into subjects with moderate PE, subjects with severe PE and subjects with both eclampsia and preeclamsia in presence of chronic hypertension.
When compared with data from international models, the local statistical information revealed certain discrepancies between the proportion of pregnant women with PE in Russia and the countries where the research was conducted [1, 7, 10]. For that reason, the decision was made to apply an adjustment factor to the Russian model.
Results
The Russian model was created to assess the impact of the risk factors associated with PE on clinical outcomes in pregnant women after 20 weeks’ gestation under two different scenarios.
It demonstrated substantial variation between the two scenarios of patient distribution depending on the clinical outcomes of PE. In the Standard Care scenario, the proportion of truly positives and truly negatives was significantly lower than in the Innovative Test scenario. At the same time, the rate of false negatives and false positives was higher under the Standard Care scenario. The distribution (%) of subjects in both scenarios is presented in Figure 3.
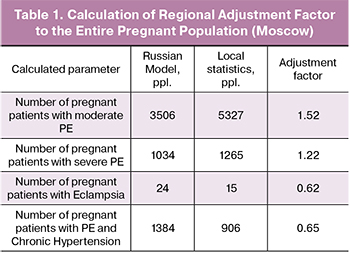
The final distribution of patients by form of PE was made on the basis of the Moscow statistics. According to most experts, it best reflects the real-world detection rates for PE in routine clinical practice.
In order to bring the calculated values in line with the current statistical consensus, a regional adjustment factor was introduced. The calculation of the adjustment factor was based on the Moscow medical statistics, according to which 147,150 pregnant women were registered in the city in 2017 (see Table 1).
The adjustment factor was initially calculated for the entire pregnant population (100%) and then applied to the group at risk for developing PE (25% of all pregnant women).
Calculation of the adjustment factor was performed separately for each region and each form of PE (moderate PE, severe PE, eclampsia and PE with chronic hypertension).
It was calculated as the ratio between the existing Russian statistics and the data obtained during simulation (mathematical modeling).
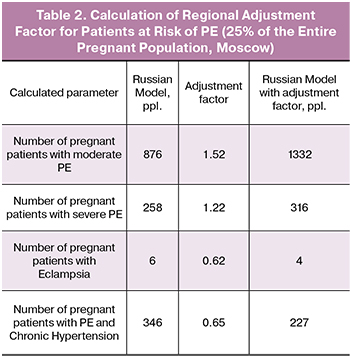 For instance, the adjustment factor for the patient group with moderate PE was calculated as =1.52.
For instance, the adjustment factor for the patient group with moderate PE was calculated as =1.52.
Subsequently, this adjustment factor was applied to the patient sample at risk for developing PE (25% of the total number of pregnant women (see Table 2):
For example, using adjustment factor, the number of patients with moderate PE in the group at risk for PE was calculated as follows: 876 * 1.52 = 1332.
The Russian model showed that the use of innovative sFlt-1/PlGF ratio test in routine practice can improve the diagnosis of PE in pregnant women by increasing the proportion of truly positive results by 24% (from 1.9% to 2.3%), while reducing the proportion of false positives (from 16.2% to 4.8%) and false negatives (from 2.2% to 0.7%) by 3 times, as well as increasing by 14% that of true negatives (from 79.74% to 91.2%).
Having analyzed the Russian statistical data, it was found that the country-adapted model, which implied the use of adjustment factor produced the following distribution of PE cases by severity: moderate PE - 59%; severe PE - 17.4%; eclampsia - 0.4%; PE with chronic hypertension - 23.3%.
Discussion
The mathematical modeling (simulation) was applied to assess the positive value of using innovative sFlt-1/PlGF ratio test in the second half of pregnancy in the pregnant population of Russia. Measurement of the sFlt-1/PlGF ratio provides new information that is likely to be valuable for the short-term prediction of PE.
The study of high-cited publications and the use of mathematical modeling validated on data from European models demonstrated the clear advantages of implementing innovative sFlt-1/PlGF ratio test in diagnosing symptomatic/asymptomatic PE in patients at risk and reducing the risks of severe complications which may result in retardation of fetal growth, premature birth and perinatal mortality.
During the research a number of suitable models were analyzed, which most accurately reflected the rates and distribution of patients with PE among the pregnant population. The European models were adapted to the local setting with introduction of adjustment factor to make them more relevant to the real-world situation in Russia.
This modeling analysis had several limitations. Firstly, the model assumes that the sensitivity and specificity of the new test for PE are equal to those reported during the clinical trials [7]. However, the actual clinical practice could deviate to a certain extent from what was described in the publications. Secondly, the model contains a number of assumptions with respect to sensitivity and specificity of standard PE testing practice. If the current test shows better performance, then the economic impact associated with it should be further examined. Thirdly, the information about the rates of antenatal visits in Russia was obtained from the local clinical guidelines for pregnancy management and the expert analysis concerning the group at risk for PE, while the latter information has never been published. Although the primary data collected from healthcare administrators and clinicians with sufficient experience in managing patients with PE provide relevant clinical and economic evidence for building a model, the use of this data has not been extensively validated. And, finally, when creating an evaluation model the emphasis was particularly made on the maternal health during pregnancy. Any long-term complications caused by PE detrimental to both the mother’s and infant’s health were not accounted for here [12]. It is obvious that the direct and indirect costs associated with these complications are quite substantial [13]. Also, taking into consideration that the clinical and economic effects of PE play a significant role during the neonatal stage, the cost savings expected from the use of innovative test for PE seem to be underestimated. However, these economic benefits were not factored in our model. There is no doubt that if this had been the case, the outcomes of the analysis would demonstrate an even greater cost saving potential for the Russian healthcare budget.
Therefore, the results of a literature search and the modeling analysis showed consistent advantages of the use of the innovative test for detection and identification of PE in the second half of pregnancy. This test significantly improves diagnosis and treatment of PE and prevents the onset of life-threatening complications. Also, the new diagnostic approach helps to streamline the allocated healthcare funds and resources through better diagnosis and management of patients with suspected PE.
The Russian model for assessing innovative sFlt-1/PlGF ratio test revealed a great potential for improving the diagnosis of PE. It is recommended to consider these findings when developing the clinical guidelines and recommendations for management of pregnant women, including those at risk for PE.
Conclusion
Measuring maternal serum concentrations of sFlt-1 and PlGF, as well as sFlt-1/PlGF ratio allows efficient profiling of pregnant women at risk for PE/eclampsia. Unlike the current standard care, the use of this innovative test may help to considerably reduce the rate of false-positives and false-negatives on PE testing (subclinical course of the disease) and improve the early identification of PE.
References
- Запорожец Э.Е., Шувалова М.П., Цымлякова Л.М., Фролова О.Г., Огрызко Е.В., Суханова Л.П. Основные показатели деятельности службы охраны здоровья матери и ребенка в Российской Федерации. Статистическая форма 32 за 2012 год. М.: Российское общество акушеров-гинекологов; 2013. [Zaporozhets E.E., Shuvalov MP, Tsymlyakova L.M., Frolova O.G., Ogryzko E.V., Sukhanova L.P. Main indicators of the activities of the maternal and child health services in the Russian Federation. Statistical form 32 for 2012. M .: Russian Society of Obstetricians and Gynecologists; 2013. (in Russian)]
- Andersgaard A.B., Acharya G., Mathiesen E.B., Johnsen S.H., Straume B., Øian P. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: a population-based study. Am. J. Obstet. Gynecol. 2012; 206(2): 143. e1-8.
- Young B., Hacker M.R., Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens. Pregnancy. 2012; 31(1): 50-8.
- WHO Recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva: World health Organization; 2011.
- Адамян Л.В., Артымук Н.В., Башмакова Н.В., Белокриницкая Т.Е., Беломестнов С.Р., Братищев И.В., Вученович Ю.Д., Краснопольский В.И., Куликов А.В., Левит А.Л., Никитина Н.А., Петрухин В.А., Пыроев А.В., Серов В.Н., Сидорова И.С., Филиппов О.С., Ходжаева З.С., Холин А.М., Шешко Е.Л., Шифман Е.М., Шмаков Р.Г. Гипертензивные расстройства во время беременности, в родах и послеродовом периоде. Преэклампсия. Эклампсия. Клинические рекомендации (протокол лечения). М.: Минздрав России; 2016. [Adamyan L.V., Artymuk N.V., Bashmakova N.V., Belokrinitskaya T.E., Belomestnov S.R., Bratishchev I.V., Vuchenovich Yu.D., Krasnopolsky V.I., Kulikov A. .V., Levit A.L., Nikitina N.A., Petrukhin V.A., Pyroev A.V., Serov V.N., Sidorova I.S., Filippov O.S., Khodjaeva Z.S. ., Kholin A.M., Sheshko E.L., Shifman E.M., Shmakov R.G. Hypertensive disorders during pregnancy, during childbirth and the postpartum period. Pre-eclampsia. Eclampsia. Clinical recommendations (treatment protocol). M .: Russian Ministry of Health; 2016. (in Russian)]
- Zeisler H., Llurba E., Chantraine F., Vatish M., Staff A.C., Sennström M. et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016; 374(1): 13-22.
- Hadker N., Garg S., Costanzo C., van der Helm W., Creeden J. Are there financial savings associated with supplementing current diagnostic practice for preeclampsia with a novel test? Learnings from a modeling analysis from a German payer perspective. Hypertens. Pregnancy. 2013; 32(2): 105-19.
- UBC. Health economic strategy. Market research carried out by United Biosource LLC. 2011.
- Приказ Министерства здравоохранения РФ от 1 ноября 2012 г. № 572н «Об утверждении Порядка оказания медицинской помощи по профилю «акушерство и гинекология (за исключением использования вспомогательных репродуктивных технологий)». [Order of the Ministry of Health of the Russian Federation of November 1, 2012 No. 572n „On approval of the procedure for providing medical care in the profile of” obstetrics and gynecology (except for the use of assisted reproductive technologies).(in Russian)]
- ЕМИСС. Государственная статистика. Число принятых родов с 22 недель беременности. 2018. [EMISS. State statistics. The number of births taken from 22 weeks of pregnancy. (in Russian)] 2018.Available at: https://fedstat.ru/indicator/41684
- DGGG. Diagnostik und Therapie hypertensiver Schwangerschaftserkrankungen AWMF-Leitlinien-Register Nr. 015/018. Deutsche Gesellschaft fur Gynakologie und Geburtshilfe; 2013.
- Cain M.A., Salemi J.L., Tanner J.P., Kirby R.S., Salihu H.M., Louis J.M. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am. J. Obstet. Gynecol. 2016; 215(4): 484. e1-484. e14.
- Chaiworapongsa T., Chaemsaithong P., Yeo L., Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014; 10(8): 466-80.
Received 14.02.2018
Accepted 21.09.2018
About the Authors
Khodzhaeva, Zulfia S., MD, professor, head of Department of Maternal-Fetal Medicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. Е-mail: zkhodjaeva@mail.ruKholin, Alexey M., research scientist, Department of Maternal-Fetal Medicine, head of Department of Telemedicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: a_kholin@oparina4.ru
Shuvalova, Marina P., PhD, assistant professor, deputy director – head of Department of Regional Cooperation and Integration, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia.
117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: m_shuvalova@oparina4.ru
Ivanets, Tatyana Yu., PhD, head of the Clinical Diagnostic Laboratory, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia. 117997, Russia, Moscow, Ac. Oparina str. 4. E-mail: t_ivanets@oparina4.ru
Demura, Sofia A., PhD, assistant professor, I.M. Sechenov First Moscow State Medical University (Sechenov University).
119146, Russia, Moscow, Bolshaya Pirogovskaya str. 19s1. E-mail: s.demura@aston-health.com
Galichkina, Irina V., master of Social and Economic Sciences, JSC «Aston Health». E-mail: i.galichkina@aston-health.com
For citations: Khodzhaeva Z.S., Kholin A.M., Shuvalova M.P., Ivanets T.Yu., Demura S.A., Galichkina I.V. A Russian model for evaluating the efficiency of the sFlt-1/PlGF test for preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (2): 52-8. (in Russian)
http://dx.doi.org/10.18565/aig.2019.2.52-58



