Естественные киллеры (NK-клетки, natural killer cells) представляют собой гетерогенную популяцию лимфоцитов I группы врожденного иммунитета, обеспечивающих первую линию иммунной защиты против ряда патогенов. Клетки обладают естественной цитотоксической активностью, способны продуцировать цитокины и хемокины, осуществлять координацию взаимодействия врожденного и адаптивного звеньев иммунной системы; участвуют в противоинфекционном и противоопухолевом контроле, поддержании клеточного гомеостаза лимфоидной системы [1].
NK-клетки распространены в различных органах и системах организма, условно подразделяясь на NK-клетки периферической крови, где их численность составляет 5–20% лимфоцитов, и тканерезидентные NK-клетки (селезенка, печень, почки, костный мозг, тимус, лимфатические узлы, кожа и слизистые оболочки, слюнные железы, эндометрий) [2].
NK-клетки периферической крови проходят последовательные этапы дифференцировки из гематопоэтических стволовых клеток-предшественниц (ГСК) преимущественно в костном мозге, что координируется транскрипционными факторами (T-bet, Eomes и другие), стромальными элементами и цитокинами. По мере дифференцировки клетки приобретают зрелый фенотип и функциональную компетентность – способность к продукции литических молекул и цитокинов, после чего выходят из костного мозга в периферическую кровь [3].
Важнейшей функцией NK-клеток является их цитотоксическая активность по отношению к клеткам-мишеням. NK-клетки могут распознавать и атаковать опухолевые, инфицированные вирусами, бактериями, а также поврежденные в результате окислительного стресса клетки без предварительной сенсибилизации [4]. NK-клетки способны лизировать чужеродные или свои собственные измененные клетки в отсутствие молекул главного комплекса гистосовместимости I класса на мембране, независимо от антител и комплемента [4]. Уничтожение клеток-мишеней реализуется посредством нескольких механизмов. Контактный цитолиз происходит при высвобождении содержимого цитотоксических гранул NK-клеток, содержащих перфорин, гранзимы, сериновые протеазы, в область иммунологического синапса с клеткой-мишенью. Перфорин способствует проникновению в клетку цитолитических медиаторов, что запускает процессы апоптоза. Цитотоксический эффект натуральных киллеров также реализуется при связывании рецептора TRAIL- и Fas-активированных NK-клеток с их лигандами (TRAIL-R, Fas-R) на поверхности клеток-мишеней. При их взаимодействии в клетке-мишени происходит индукция каскада сигнальных реакций, активирующих каспазу-8 и каспазу-10, инициирующих ее апоптоз [5].
NK-клетки обладают иммунорегуляторной функцией, являясь одними из основных источников цитокинов и хемокинов, таких как интерферон гамма (IFNγ), фактор некроза опухоли альфа (TNFα), гранулоцитарный макрофагальный колониестимулирующий фактор роста, хемокин CCL5, регулируя активность макрофагов, нейтрофилов, дендритных клеток и Т-лимфоцитов, способствуя формированию врожденного и адаптивного иммунных ответов [6].
NK-клетки периферической крови
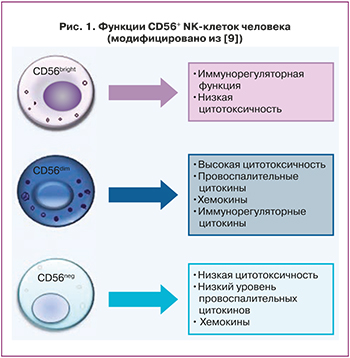 NK-клетки периферической крови (peripheral blood NK-сells, pbNK-клетки) подразделяют на две основные субпопуляции согласно экспрессии поверхностных антигенов кластерной дифференцировки лейкоцитов – CD56 и CD16 [7]. Около 90% pbNK-клеток слабо экспрессируют СD56 (CD56dim) и интенсивно CD16 (CD16bright); представлены фенотипом CD3-CD56dimCD16bright; для них характерно большое количество лизосомальных гранул и высокая цитотоксическая активность. Другая субпопуляция NK-клеток интенсивно экспрессирует CD56 (CD56bright) и средне/низко CD16 (CD16dim/neg) и имеет фенотип CD3CD56brightCD16dim/neg, составляя менее 10% от общего количества натуральных киллеров крови. Данные клетки обладают регуляторными свойствами, продуцируют цитокины, хемокины, факторы роста [7]. CD56brightNK-клетки более распространены в периферических тканях организма, что указывает на их тканеспецифическую функцию [8]. Помимо CD56bright и CD56dim, выделяют также субпопуляцию NK-клеток CD56neg, отличающихся пониженной экспрессией цитотоксических рецепторов, низким уровнем перфорина и, как следствие, общей гипореактивностью (рис. 1).
NK-клетки периферической крови (peripheral blood NK-сells, pbNK-клетки) подразделяют на две основные субпопуляции согласно экспрессии поверхностных антигенов кластерной дифференцировки лейкоцитов – CD56 и CD16 [7]. Около 90% pbNK-клеток слабо экспрессируют СD56 (CD56dim) и интенсивно CD16 (CD16bright); представлены фенотипом CD3-CD56dimCD16bright; для них характерно большое количество лизосомальных гранул и высокая цитотоксическая активность. Другая субпопуляция NK-клеток интенсивно экспрессирует CD56 (CD56bright) и средне/низко CD16 (CD16dim/neg) и имеет фенотип CD3CD56brightCD16dim/neg, составляя менее 10% от общего количества натуральных киллеров крови. Данные клетки обладают регуляторными свойствами, продуцируют цитокины, хемокины, факторы роста [7]. CD56brightNK-клетки более распространены в периферических тканях организма, что указывает на их тканеспецифическую функцию [8]. Помимо CD56bright и CD56dim, выделяют также субпопуляцию NK-клеток CD56neg, отличающихся пониженной экспрессией цитотоксических рецепторов, низким уровнем перфорина и, как следствие, общей гипореактивностью (рис. 1).
Особенностью NK-клеток является контроль их функциональной активности за счет экспрессии широкого репертуара активирующих и ингибирующих рецепторов на поверхности. Основные группы рецепторов представлены лектиноподобными рецепторами NKG (natural killer group), семейством иммуноглобулиноподобных рецепторов (killer immunoglobulinlike receptor, KIR), цитотоксическими рецепторами NKp30, NKp44, NKp46 и другими [10]. Активность NK-клеток зависит от плотности распределения групп рецепторов, их взаимодействия с клетками-мишенями, клеточным микроокружением, растворимыми факторами.
Некоторыми исследованиями продемонстрировано изменение показателей количества и функциональной активности pbNK-клеток в течение менструального цикла и при наступлении беременности. В работе Lee S. et al. (2010) обнаружено выраженное увеличение субпопуляции CD3-CD56dimNK-клеток крови в лютеиновую фазу цикла при отсутствии изменений количества CD3-CD56brighNK-клеток [11]. Также отмечено снижение цитотоксической активности pbNK-клеток в лютеиновой фазе цикла и при физиологической беременности [12, 13], что, впрочем, не подтверждается другими авторами [14, 15].
Таким образом, субпопуляции pbNK-клеток различаются по экспрессии цитокиновых и хемокиновых рецепторов, регуляторной и цитотоксической функции.
NK-клетки эндометрия и децидуальной оболочки
Количество иммунокомпетентных клеток эндометрия может достигать 40% от общего числа стромальных клеток, при этом NK-клетки являются самой многочисленной популяцией лимфоцитов эндометрия. Эндометриальные NK-клетки (uterine NK cells, uNK-клетки) составляют минимум 30% от общего количества эндометриальных лимфоцитов, при этом в секреторную фазу менструального цикла их количество прогрессивно увеличивается и в случае наступления беременности на ранних сроках достигает 75% [16].
На сегодняшний день не существует единого мнения относительно происхождения uNK-клеток. Возможность их локальной дифференцировки подтверждается рядом исследований, обнаруживающих способность ГСК эндометрия к приобретению фенотипического маркера CD56+NK-клеток [17]. Вторая теория предполагает образование uNK-клеток преимущественно путем трансэндотелиальной миграции pbNK-клеток за счет хемотаксиса с последующим приобретением эндометриального или децидуального фенотипа. Эндометрий человека продуцирует факторы, влияющие на дифференцировку NK-клеток, включая интерлейкины IL-15, IL-25, IL-7, хемокины CXCL10 и CXCL11, трансформирующий фактор роста β1 (TGFβ1), секреция которых увеличивается в лютеиновой фазе менструального цикла и начале беременности [18]. Миграция pbNK-клеток в эндометрий также может быть реализована в ответ на секрецию хемокинов клетками трофобласта [19] (рис. 2).
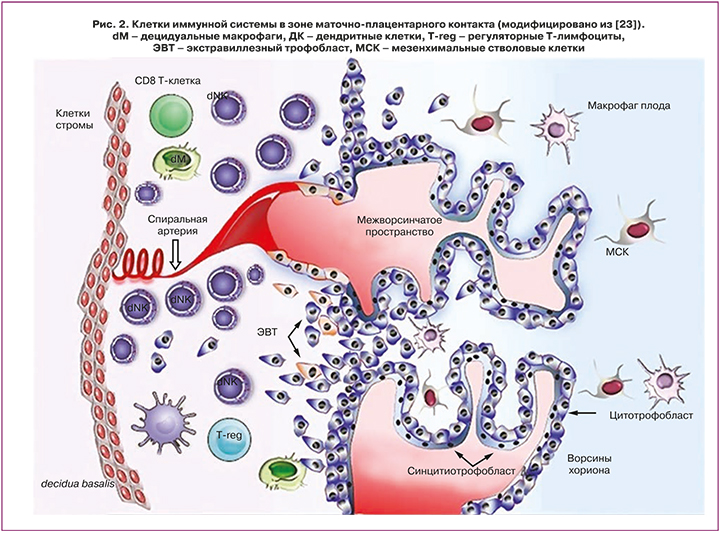
Установлено, что децидуальные NK-клетки располагаются вдоль спиральных артерий матки и являются источниками цитокинов, проангиогенных факторов, участвуя в ремоделировании сосудов матки и децидуальном ангиогенезе при беременности. uNK-клетки экспрессируют ряд рецепторов, включая KIR и лейкоцитарные иммуноглобулиноподобные рецепторы (LIRs), которые распознают HLA-E, HLA-G и HLA-C, экспрессируемые вневорсинчатым трофобластом [20].
За счет связывания рецепторов KIR2DL4 NK-клеток с молекулами локуса HLA-G, экспрессируемыми клетками трофобласта, происходит подавление цитотоксической активности NK-клеток. Связывание KIR2DL4 и HLA-G стимулирует секрецию NK-клетками IFNγ, индуцирующего экспрессию генов различных хемокинов, цитокинов и транскрипционных факторов клетками децидуальной ткани, что способствует построению сосудистой сети плаценты [21]. IFNγ также способствует подавлению иммунной реакции материнского организма в отношении полуаллогенного плода, стимулирует экспрессию и секрецию неклассических молекул главного комплекса гистосовместимости HLA-G и HLA-E эндотелиальными клетками и клетками трофобласта [22], что способствует поддержанию состояния иммунологической толерантности в системе мать–плацента–плод.
Предложена двухволновая гипотеза накопления uNK-клеток в матке при беременности. Первая волна происходит в процессе децидуализации эндометрия и обусловлена локальной пролиферацией тканерезидентных uNK-клеток, при этом циркулирующие pbNK-клетки имеют минимальный вклад в растущий пул uNK-клеток на ранних сроках беременности. Во второй волне происходит рекрутирование pbNK-клеток в матку в ответ на инвазию трофобласта, процессы плацентации [24]. uNK-клетки продуцируют проангиогенные факторы, в том числе фактор роста эндотелия сосудов и плацентарный фактор роста, которые способствуют развитию и росту плаценты в decidua basalis. Исследования человеческих uNK-клеток показывают, что эти процессы могут быть обусловлены связыванием рецепторов NKp30 и NKp44 на uNK-клетках с лигандами вневорсинчатого трофобласта и материнских стромальных клеток.
Установлено воздействие половых стероидных гормонов, эстрогена и прогестерона, на иммунорегуляторную и ангиогенную функцию uNK-клеток [25]. In vitro под влиянием эстрогенов происходит усиление экспрессии галектина-1 uNK-клетками. Эстрогены увеличивают секрецию CCL2 uNK-клетками, что влияет на пролиферацию кровеносных сосудов эндометрия; в то время как прогестерон индуцирует экспрессию IFNγ. За счет опосредованного прогестероном воздействия на клетки микроокружения эндометрия (Т-лимфоциты, стромальные клетки эндометрия) происходит продукция транскрипционного фактора Hoxa-10, прогестерон-индуцированного блокирующего фактора и цитокинов Th2 типа (IL-18), что в совокупности влияет на снижение цитотоксичности uNK-клеток.
Таким образом, uNK-клетки участвуют в регуляции инвазии бластоцисты и клеток трофобласта в стенку матки, контролируют ремоделирование спиральных артерий матки, поддержание плодово-материнской иммунологической толерантности.
Изменения количественно-качественных показателей NK-клеток обеих популяций ассоциированы с репродуктивно значимыми заболеваниями, в том числе с повторными неудачами имплантации (ПНИ) и привычным невынашиванием беременности (ПНБ).
NK-клетки при повторных неудачах имплантации
На сегодняшний день термин ПНИ (Recurrent implantation failure, RIF) не имеет унифицированного определения и наиболее часто описывает клиническую ситуацию отсутствия наступления беременности после проведения 2 и более циклов ЭКО при переносе эмбрионов (не менее 4 для эмбрионов на стадии дробления; не менее 2 для бластоцист) хорошего качества женщинам моложе 40 лет [26].
Активно изучаются NK-клетки обеих субпопуляций при повторных неудачах имплантации. Так, Marron K. et al. (2019) в образцах эндометрия середины лютеиновой фазы цикла обнаружили повышение количества uNK-клеток в группе пациентов с ПНИ по сравнению с ПНБ, а также в группах пациенток с бесплодием без проведенных протоколов ЭКО. При этом продемонстрировано увеличение количества NK-клеток цитотоксического фенотипа CD16+CD56dim и их функциональной активности, снижение количества Т-регуляторных лимфоцитов (Treg) [27]. Похожие данные сообщены Tuckerman E. et al. (2010), обнаружившими изменение пропорций CD56brightCD16- (уменьшение) и CD56dimCD16+ (увеличение) uNK-клеток при ПНИ [28].
Предприняты попытки оценки диапазонов нормальных значений количества uNK-клеток и отклонений от них. Так, Chen X. et al. (2017) относят количество uNK-клеток в «окно имплантации» от 1,2 до 4,5% от общего количества стромальных клеток к нормальному, более 4,5% – к высокому, менее 1,2% – к низкому. В большинстве случаев при ПНИ авторы отмечали значительно более высокий процент uNK-клеток, однако в исследовании продемонстрировано и снижение процента uNK-клеток в группах пациентов с ПНБ и ПНИ [29].
Результаты проведенных исследований pbNK-клеток при неудачах имплантации также неоднозначны. В ряде работ продемонстрировано значительное повышение количества pbNK-клеток, в других приводятся противоположные данные или не обнаруживается различий показателей.
Так, Sacks G. et al. (2012) обнаружили, что пациентки с ПНИ имеют значительное увеличение количества pbNK-клеток по сравнению с фертильными женщинами [30]. Подобные результаты получены в работе Santillan I. et al. (2015), продемонстрировавших, что количество и pbNK-клеток, и uNK-клеток повышается при повторных неудачах ЭКО. Уровни pbNK-клеток составили 13,4% у пациентов с ПНИ и 8,4% в группе фертильных пациенток [31].
Напротив, в работе Ho Y.K. et al. (2019) количество pbNK-клеток у пациентов с ПНИ, равное 10,6%, установлено как пограничное для прогнозирования исходов беременности в цикле ЭКО. Количество pbNK-клеток менее 10,6% ассоциировано со снижением показателей наступления имплантации и беременности по сравнению с количеством pbNK-клеток >10,6% [32].
Zhang H. et al. (2019) не обнаружили различий в количестве, фенотипе, цитотоксической активности и рецепторном профиле pbNK-клеток при ПНИ [33]. Аналогично, Kolanska K. et al. (2019) провели исследование в группах пациентов с ПНБ, ПНИ и группой фертильных пациенток с оценкой pbNK-клеток без учета фазы менструального цикла. Не обнаружено различий в относительном содержании и субпопуляциях клеток между исследуемыми группами и контролем, а также в исходах беременности (выкидыш/роды) [34].
Проведенные по данной тематике метаанализы Seshadri S., Sunkara S.K. (2014) и VonWoon E. et al. (2020) подтверждают повышение количества обеих субпопуляций NK-клеток, однако заключают, что необходимы дальнейшие исследования pbNK-клеток и uNK-клеток, прежде чем рекомендовать их в качестве критериев диагностики эффективности протоколов ЭКО, исходов беременности и назначения терапии [35, 36].
Трудности в сравнении исследований возникают в результате использования различных методов количественной и функциональной оценки uNK-клеток (проточная цитофлуометрия/иммуногистохимия), методов оценки цитотоксичности, отсутствия единых референсных интервалов и трактовки термина ПНИ в циклах ЭКО (2 и более, 3 и более неудачных циклов ЭКО; различное количество и качество перенесенных эмбрионов, день переноса), стандартизации групп.
Дальнейшие исследования могут позволить подробнее изучить роль обеих популяций NK-клеток в патогенезе неудач имплантаций, определить референсные показатели, оценить прогностическую роль данных клеток в эффективности циклов ЭКО, а также расширить возможные методы терапии.
NK-клетки при привычном невынашивании беременности
ПНБ неустановленной этиологии составляет до 50% случаев повторных выкидышей [26]. Большое количество исследований подтверждает роль иммунологического фактора, в частности, изменения в показателях периферических и эндометриальных NK-клеток, в генезе невынашивания беременности.
Изменение количества и цитотоксической активности NK-клеток может приводить к чрезмерному цитокиновому ответу Th1-типа, в частности, увеличению синтеза TNFα и IFNγ и снижению цитокинов Th-2-профиля, IL-4 и IL-10, обуславливая иммунологический дисбаланс, что создает неблагоприятную среду для имплантации и пролонгирования беременности [37]. Однако на сегодняшний день парадигма Th1/Th2-иммунного ответа, отражающая важность определенного соотношения провоспалительных и противовоспалительных факторов для индукции поддержания толерантности при беременности, сменяется на парадигму, включающую Т-регуляторные клетки (Th1/Th2/Th17/Тreg). Т-регуляторным лимфоцитам (Treg) отводится важная роль в сохранении иммунотолерантности за счет супрессирующего действия на генерацию провоспалительных регуляторных клеточных типов, таких как Th1 и Th17 [38]. Treg также подавляют пролиферацию и активность NK-клеток, в том числе влияют на экспрессию активирующего рецептора NKG2D in vitro. В основе супрессорного действия Treg лежит способность к продукции TGFβ и IL-10 [39].
Баланс активирующих и ингибирующих сигналов от клеток трофобласта также может оказывать регуляторное воздействие на функциональную активность NK-клеток. Клетки трофобласта продуцируют TGFβ, что может являться одним из факторов, снижающих цитотоксичность NK-клеток и увеличивающих количество pbNK-клеток с регуляторным фенотипом [40]. Потенциальным механизмом нарушения имплантации также может являться нарушение взаимодействия HLA-G инвазирующего трофобласта и материнских иммуноглобулиноподобных рецепторов uNK-клеток, что приводит к повышению их цитотоксической активности. Важную роль играют обусловленный uNK-клетками ангиогенез и ремоделирование спиральных артерий эндометрия. При этом увеличение количества uNK-клеток может вызывать увеличение периимплантационного кровотока и раннее формирование плодово-материнского кровообращения, приводя к чрезмерному окислительному стрессу, повышая вероятность самопроизвольного выкидыша [41].
По данным ряда работ, при ПНБ увеличивается количество pbNK-клеток с цитотоксическим фенотипом с параллельным снижением иммунорегуляторного фенотипа CD56brightpbNK-клеток, что приводит к цитокиновому дисбалансу. Так, в работе Azargoon A. et al. (2019) показано значительное увеличение цитотоксичности pbNK-клеток и содержания в них перфорина в середине лютеиновой фазы в группах ПНБ по сравнению с контрольной группой. При этом количественная разница pbNK-клеток не отмечена [42]. Аналогичные результаты получены Ghafourian M. et al. [43], которые сообщили, что процент активированных pbNK-клеток значительно повышается у пациентов с ПНБ и при ПНИ. Однако Baczkowski T. и Kurzawa R. не обнаружили подобных различий в данных подгруппах [44].
Повышенная цитотоксичность pbNK-клеток может сохраняться в течение нескольких месяцев после самопроизвольного выкидыша, что подтверждается данными обследования пациентов через 3–12 месяцев [45]. Также необходимо отметить разницу в pbNK- и uNK-клетках у пациентов с ПНБ в зависимости от наличия родов в анамнезе. Пациенты с ПНБ и отсутствием родов в анамнезе демонстрируют значительно более высокое количество NK-клеток по сравнению с пациентами с ПНБ, имевшими хотя бы 1 роды в анамнезе [46].
Дискуссионным является вопрос корреляции pbNK-клеток с uNK-клетками эндометрия при ПНБ и ПНИ. Некоторые исследователи продемонстрировали, что pbNK-клетки могут отражать изменения в локальной эндометриальной среде [47], другие данные результаты не подтвердили [48].
Все больше исследований подтверждают роль uNK-клеток в патогенезе ПНБ. Продемонстрированы более высокие концентрации uNK-клеток в сравнении с фертильным контролем [49]. Также отмечено значительное уменьшение субпопуляции CD16-CD56bright uNK-клеток у пациенток с ПНБ по сравнению с фертильными женщинами. Продемонстрировано увеличение функциональной активности NK-клеток обеих популяций, увеличение количества цитотоксических uNK-клеток [49] и uNK-клеток с повышенной экспрессией рецепторов естественной цитотоксичности NKp46, NKp44 и NKp30 у женщин с идиопатическим ПНБ [50].
Согласно проведенному метаанализу, при ПНБ отмечается более высокое относительное и абсолютное содержание количество pbNK-клеток по сравнению с фертильными женщинами. Однако не выявлено существенных различий количества uNK-клеток в группе ПНБ [35].
На сегодняшний день несколько исследований предлагают критерии оценки количественных изменений NK-клеток. В работе Kuon R.J. et al. (2017) был проведен анализ концентрации uNK-клеток в группе пациентов с ПНБ и фертильными женщинами. Количество uNK-клеток: 0–40 uNK-клеток/мм2 оценено как низкое, 40–300 – нормальное, более 300 – высокое, ассоциированное с высоким риском потери беременности [51]. В работе Tang et al. (2013) количество uNK-клеток более 5% от общего количества стромальных клеток определено как высокое [52].
Ebina Y. et al. (2017) изучали активность pbNK-клеток в середине лютеиновой фазы в группе пациенток с идиопатическим ПНБ и ПНБ с установленной этиологией, их влияние на наступление и исход беременности. Авторами продемонстрировано значительное повышение активности pbNK-клеток в группе с идиопатическим ПНБ. Уровень pbNK-клеток более 33% до беременности ассоциирован с повышением риска самопроизвольного выкидыша или наступления биохимической беременности в 3,4 раза [53]. Более ранние исследования предлагали пороговые значения активности NK-клеток, связанные с повышенным риском прерывания беременности: более 34,3% в работе Lee S.K. et al. [54] и более 18% в исследовании Beer A.E. et al. [55].
Таким образом, современная доказательная база имеет достаточно сведений для подтверждения роли NK-клеток при ПНБ, однако данные носят противоречивый характер. В основном это связано с гетерогенностью изучаемых популяций, различиями в самом определении патологии (согласно одним авторам, ПНБ – последовательная потеря 3 и более беременностей до 24 недель, другим – 2 потери до 20 недель), методах исследований, интерпретации результатов.
Заключение
Баланс звеньев локального и системного иммунного ответа имеет фундаментальное значение для наступления и успешной пролонгации беременности. Исследования последних лет подтверждают роль как периферических, так и эндометриальных NK-клеток в репродуктивном здоровье женщины.
NK-клетки крови обладают высокой цитотоксической активностью, а также выполняют иммунорегуляторную функцию, поддерживая иммунологический гомеостаз. Согласно некоторым исследованиям, изменяясь в количестве, фенотипе и функциональной активности в течение менструального цикла, NK-клетки крови могут пополнять пул NK-клеток матки путем трансэндотелиальной миграции с последующим приобретением эндометриального и децидуального фенотипа. В некоторых работах продемонстрирована корреляция между показателями pbNK-клеток и uNK-клеток, в связи с чем существует предположение, что pbNK-клетки могут отражать изменения в локальной эндометриальной среде.
Эндометриальные NK-клетки участвуют в процессах децидуализации, регуляции инвазии бластоцисты и клеток трофобласта в стенку матки, контроле ремоделирования спиральных артерий матки, поддержании плодово-материнской иммунологической толерантности, выполняя иммунорегуляторную и ангиогенную функции.
Продемонстрированное многочисленными исследованиями изменение количественно-качественных показателей pbNK-клеток и uNK-клеток у пациенток с ПНИ и ПНБ подтверждает гипотезу о причинно-следственной связи данных заболеваний и функции NK-клеток. Однако полученные данные противоречивы. С одной стороны, обнаруженное увеличение количества и/или функциональной активности pbNK-клеток и uNK-клеток, а также изменение пропорций их субпопуляций при репродуктивных неудачах может быть связано с патологией инвазии трофобласта, цитокиновым дисбалансом, увеличением периимплантационного кровотока, ранним формированием плодово-материнского кровообращения, окислительным стрессом. С другой стороны, имеются данные о снижении количества и цитотоксической активности как pbNK-клеток, так и uNK-клеток при ПНБ и неудачах ЭКО, что также может быть связано с дефектами плацентации, приводящими к прерыванию беременности или развитию ее осложнений.
Таким образом, NK-клетки могут рассматриваться как важный иммунологический параметр при ПНБ и ПНИ, однако необходимо дальнейшие изучение роли и механизмов их действия. Необходимы дальнейшие исследования для подтверждения или опровержения прогностической ценности NK-клеток крови в качестве маркеров наступления беременности в циклах ЭКО и при ПНБ, ее пролонгирования, исходов, а также критериев назначения и эффективности иммунотропной терапии.



