The role of neurokinin B in the pathophysiological mechanisms of vasomotor symptoms and sleep disturbances in postmenopausal women
Objective. To carry out a systematic analysis of the serum level of neurokinin B (NKB) in women of different age periods and in those with and without vasomotor symptoms and sleep disturbances. To assess the time course of changes in the level of NKB during menopausal hormone therapy (MHT).Ebzieva Z.Kh., Yureneva S.V., Ivanets T.Yu., Averkova V.G.
Subjects and methods. The investigation enrolled 50 postmenopausal women and 30 reproductive-aged women having a regular menstrual cycle. The patients were divided into 3 groups: 1) 25 postmenopausal patients with vasomotor symptoms and sleep disturbances; 2) 25 postmenopausal patients without vasomotor symptoms and sleep disturbances. A control group (Group 3) included 30 reproductive-aged women having a regular menstrual cycle. Group 1 received MHT. The dynamics of menopausal symptoms (Greene Climacteric Scale), a subjective sleep rating scale, and NKB levels were assessed after 12 weeks of therapy.
Results. There was a significant increase in serum NKB levels in postmenopausal women compared with the controls. In postmenopausal women with sleep disturbances and vasomotor symptoms, NKB levels were statistically significantly higher than in women without the above symptoms. A strong relationship was established between the level of NKB and the severity of menopausal syndrome. There was a significant decrease in the severity of menopausal symptoms after 12 weeks of MHT, which was accompanied by a 3.2-fold decrease in NKB levels.
Conclusion. The findings suggest that the activation of KNDy neurons and the increased secretion of NKB play a possible role in the pathogenesis of sleep disturbances and vasomotor symptoms in postmenopausal women.
Keywords
Menopausal vasomotor symptoms (hot flashes and/or night sweats) and their accompanying symptoms of sleep disturbances are significant social, economic and medical problems occuring in almost 80% of perimenopausal women and 65% of postmenopausal women [1, 2]. The definition of these symptoms as menopausal ones implies their transient nature until spontaneous resolution. However, some experts question whether this term underestimates the biological essence of this phenomenon [3]. To date, it is necessary to conduct a deeper study of pathophysiology of vasomotor symptoms in menopause, as well as the underlying processes of neuronal dysregulation in order to create adequate therapeutic agents, improve the quality of life of women and eliminate the potential cause of age-associated diseases and their complications in the future [4, 5].
Despite the impressive amount of accumulated scientific data on the genesis of menopausal symptoms, the obvious role of estrogens, neurotransmitters norepinephrine (NA) and serotonin, various vasoactive substances, there is still neither clearly defined sequence of events accompanying it nor the description of the specific role of individual biological agents. Considerable attention is currently being paid to relatively new data on the key role of increased activity of specific estrogen-sensitive KNDy neurons of the infundibular nucleus of the hypothalamus in the genesis of hot flashes. KNDy neurons and secreted peptides there (kisspeptin / neurokinin B / dynorphin) play a central role in the functioning of the hypothalamus. Neurokinin B (NKB) is a neuropeptide that binds to neurokinin receptors in the hypothalamus and along with kisspeptin regulates the participation of GnRH in the reproduction. Thanks to the projection in close proximity to the structures involved in the control of thermoregulation, KNDy neurons and their neuropeptides are likely capable of influencing thermoregulatory processes in the brain and act as triggers of vasomotor symptoms [6].
In works performed by Rance on autopsy materials of the brain, hypertrophic changes in neurons of infundibular nucleus were detected in women after ovariectomy or in postmenopausal women in comparison with premenopausal women. Increased expression of NKB genes and synthesis of its m-RNA in these neurons were detected later [6-8]. This fact was further supported by results of a randomized, double-blind, placebo-controlled trial in which young healthy women were given intravenous injection of NKB that caused hot flashes similar to those in the presence of climacteric syndrome in women during menopause [6, 9]. Despite published in the literature research data on the effect of NKB receptor antagonists on the severity and frequency of vasomotor symptoms, no publications have been found on the study of NKB levels in different age periods of women’s lives at the time of writing this article. In this regard, it is relevant to study the level of NKB in the blood of postmenopausal women to determine its role in the occurrence of vasomotor symptoms and sleep disturbances, as well as the dynamics of its level during menopausal hormone therapy (MHT) [6].
Materials and Methods
The study included 50 women aged 45 to 60 years in stages (+1b and +1c) according to STRAW + 10 criteria and 30 women of reproductive age with a regular menstrual cycle. The patients were stratified into three groups: group 1 included 25 women with menopausal symptoms, including sleep disturbances and vasomotor symptoms, according to STRAW+10 (+1b and +1c). The comparison group (group 2) included 25 women by STRAW+10 (+1b and +1c) criteria without vasomotor symptoms and sleep disturbances. There were 30 women of reproductive age with regular menstruation in the control group (group 3).
Comprehensive examination of women included the collection of clinical data, history taking, determination of gynecological status, study of hormonal profile with determination of FSH level. The level of FSH was determined in women of reproductive age on the 2nd-4th day of the menstrual cycle. Also, all women had cytological examination with assessment of smears from the cervical canal by the Bethesda system (2004), mammography according to BI-RADS, ultrasound examination of pelvic organs on the device 2000 Toshiba SSA-240 (Japan) using transvaginal convection sensor with frequency of 7.5 MHz. Initially and at the end of treatment, all patients had venous blood tests. Determination of FSH concentration in blood serum was performed with electrochemiluminescent method on automatic immunochemical analyzer Cobas E411 (Roche Diagnostics GmbH, Germany.) Determination of serum levels of NKB in postmenopausal and reproductive-aged women was performed using enzyme immunoassay solid-phase method (“Neurokinin B” cat. No. S-1271 Peninsula Laboratories International, Inc., USA.)
All patients signed a voluntary informed consent to participate in the study.
Patients from group 1 received 17-β estradiol in the form of transdermal gel 0.75 mg once per day daily in combination with micronized progesterone in the form of capsules in a continuous mode of 100 mg daily vaginally as therapy of menopausal symptoms. According to the clinical recommendations of the Ministry of Health of the Russian Federation, women in menopause are prescribed the lowest effective dose of MHT [13].
As criteria for evaluating the effectiveness of treatment of menopausal symptoms, including hot flashes and sleep disturbances, we used a questionnaire by the Greene Climacteric Scale and a score of subjective sleep characteristics.
The data are presented as the mean±standard error of the mean (M±m). Statistical analysis was carried out in the program Excel (Microsoft) using Statistica 8 (Statsoft, Inc.). The nonparametric Mann-Whitney U–test, designed to compare two independent samples, was used to assess the validity of the differences between the groups. The frequency (%), risk ratio (RR) and its confidence interval (CI) were determined for binary data. The criterion χ2 was used to compare groups by qualitative characteristics with only two values. Pearson’s correlation coefficient was applied in the analysis. Evaluation of diagnostic significance of the studied indicators was performed by the results of ROC-analysis. Methods of regression analysis (binary logistic regression, multiple logistic regression) were used to estimate probabilities. Binary logistic regression calculated the probability of an event dependence on the values of the independent variables. The probability of the event was determined by the formula:
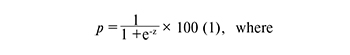
p - probability of belonging to one of the analyzed groups,
z - a regression function that has the form: z = b1*X1 +
b2*x2+ ...+ bn*Xn+ a (2).
The difference between the groups was considered statistically significant at a significance level of p < 0.05.
Results and Discussion
Analysis of clinical and anamnestic data showed that the patients of the first two groups were comparable in age, anthropometric and hormonal parameters (Table 1). It was revealed that in patients with vasomotor symptoms and sleep disturbances, bad habits were more common in comparison with women who did not have these symptoms (p = 0.0001). The relative risk (RR) of sleep disturbances in smokers was 2.5 (95% CI 1.3; 4.5). Similar data were obtained by Smith R. L. et al. [10]: according to the study, women who quit smoking were less likely to suffer from hot flashes compared to smokers (RR = 0.55, 0.80, 0.76), but more likely to experience more severe hot flashes than those who never smoked (RR = 2.55; CI 1.68, 1.46). The findings of our and other studies, however, point to an increased risk and severity of menopausal syndrome, as well as the potential benefits of quitting smoking for both prevention and relief of existing menopausal symptoms.
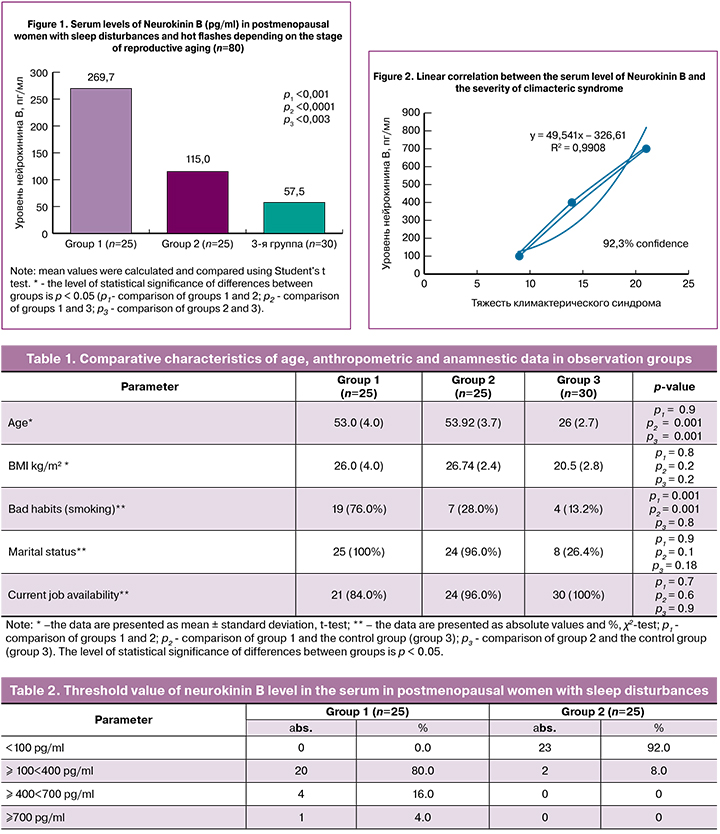
When assessing the level of NKB in the groups of studied patients, it was found that the values of NKB were 4.6 times higher (p1 < 0.001) in women in group 1, and in group 2 they were 2.0 times higher than those obtained in group 3. At the same time, NKB indicators in groups 1 and 2 were statistically significantly higher in comparison with the control group (p2 < 0.0001, p3 = 0.003) (Fig. 1). A significant increase in serum levels of NKB in postmenopausal women compared to women of reproductive age may indicate the relationship of these conditions with changes in the activity of the main regulatory neuropeptide systems of the brain (KNDy neurons), as well as it confirms the increase in their activity in response to a decrease in estrogen levels.
In the course of a further study, threshold values of NKB and RR of vasomotor symptoms and sleep disturbances in postmenopausal women were calculated (Table 2). The threshold value of the NKB level exceeding 100 pg/ml was associated with an increase in the RR of hot flashes by 6.6 times (95% CI 2.2; 19.6), the development of sleep disturbances by 3.3 times (95% CI 1.6; 6.8).
On the basis of the obtained data, a linear correlation analysis was carried out between the level of neurokinin B and the severity of climacteric syndrome (Greene Climacteric Scale) in group 1 (Fig. 2).
Pearson correlation coefficient (r) was 0.99, which corresponds to a very high degree of relationship between serum levels of NKB and the severity of menopausal syndrome.
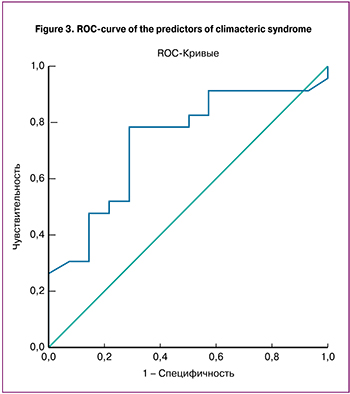 To identify markers associated with the severity of climacteric syndrome according to the Greene Climacteric Scale, a model was constructed using logistic regression (Fig. 3). The outcome of the model was a variable characterizing the severity of climacteric syndrome, its predictor was the level of neurokinin B. Neurokinin B can be used as an independent marker to assess the severity of climacteric syndrome in postmenopausal women. The sensitivity of this method was 75.7%, specificity was 71.4%. The value of the area under the ROC curve was AUC = 0.733±0.085 (p = 0.019).
To identify markers associated with the severity of climacteric syndrome according to the Greene Climacteric Scale, a model was constructed using logistic regression (Fig. 3). The outcome of the model was a variable characterizing the severity of climacteric syndrome, its predictor was the level of neurokinin B. Neurokinin B can be used as an independent marker to assess the severity of climacteric syndrome in postmenopausal women. The sensitivity of this method was 75.7%, specificity was 71.4%. The value of the area under the ROC curve was AUC = 0.733±0.085 (p = 0.019).
It was interesting and important from a practical point of view to analyze clinical and laboratory data after the treatment of menopausal symptoms with MHT drugs during the course of our study. A 3-month course of treatment was completed by 25 patients out of 25 (100%).
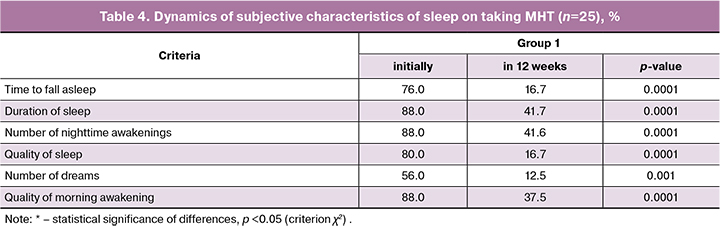
When analyzing the severity of menopausal symptoms by the Greene Climacteric Scale during the therapy, a statistically significant decrease in their severity was found in group 1 (Table 3). Complete disappearance of hot flashes was noted by 10 patients (40.0%), statistically significant relief was detected in 15 patients (60%). Night sweating significantly decreased in 9 patients (36.0%), complete disappearance was noted by 16 women (64.0%). In 16 patients (64.0%) there was a decrease or complete disappearance of sleep disturbances (p = 0.0001), the intensity of symptoms of sleep disturbances decreased in 36.0% of the studied women (p = 0.0001).
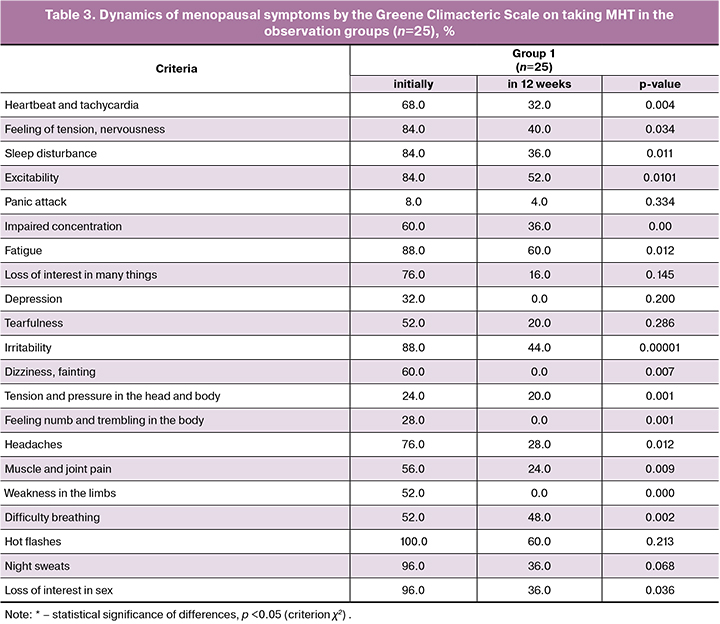
After 12 weeks of treatment, the severity of climacteric syndrome had the following degrees: mild - in 12 patients (48%), medium – in 12 patients (48%) and severe – in 1 patient (4%). Statistically significant reduction of vasomotor symptoms and sleep disturbances on taking MHT once again proves the important role of estrogens in the pathogenesis of menopausal disorders (Tables 3 and 4).
Relief of vasomotor symptoms during treatment may have been associated with improved quality of sleep. This result is not unexpected, as the relief of vasomotor symptoms is often considered as a clinical parameter to control the treatment of sleep disturbances. In the subgroup of women who reported on moderate to severe vasomotor symptoms at the beginning of our study, their relief was correlated with increased sleep duration.
It is important to note that during the 12-week course of treatment, there was an almost 3-fold decrease in the level of NKB in group 1: from 269.72±40.79 pg/ml to 83.56±26.07 pg/ml (p = 0.0003), MHT had a significant effect on the level of neurokinin B, indicating a direct central effect of sex hormone preparations on KND neurons.
Conclusion
The increase in serum levels of NKB in postmenopausal women compared to women of reproductive age may be due to the activation of hypothalamic KNDy neurons. The increase in NKB level in patients with menopausal symptoms compared to one in women without symptoms, as well as its decrease while taking MHT, confirms the hypothesis of an important role of NKB in the pathogenesis of vasomotor symptoms and sleep disturbances in postmenopausal women.
References
- Sturdee D.W., Hunter M.S., Maki P.M., Gupta P., Sassarini J., Stevenson J.C., Lumsden M.A. The menopausal hot flush: a review. Climacteric. 2017; 20(4): 296-305. doi: 10.1080/13697137.2017.1306507.
- Gartoulla P., Islam M.R., Bell R. Gartoulla P., Islam M.R., Bell R.J., Davis S.R. Prevalence of menopausal symptoms in Australian women at midlife: a systematic review. Climacteric. 2014; 17(5): 529-39. doi: 10.3109/13697137.2013.865721.
- Miller V.M., Kling J.M., Files J.A., Joyner M.J., Kapoor E., Moyer A.M. et al. What’s in a name: are menopausal ‘‘hot flashes’’ a symptom of menopause or a manifestation of neurovascular dysregulation? Menopause. 2018; 25(6): 700-3. doi: 10.1097/GME.0000000000001065.
- Franco O.H., Muka T., Colpani V., Kunutsor S., Chowdhury S., Chowdhury R., Kavousi M. Vasomotor symptoms in women and cardiovascular risk markers: systematic review and meta-analysis. Maturitas. 2015; 81(3): 353-61.
- Muka T., Oliver-Williams C., Colpani V., Kunutsor S., Chowdhury S., Chowdhury R., et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta-analysis. PLoS One. 2016; 11(6): e0157417. https://doi.org/10.1371/journal.pone.0157417
- Юренева С.В., Эбзиева З.Х. Роль гипоталамических (триггеров) нейропептидов в генезе приливов жара. Перспективы новых терапевтических подходов к лечению вазомоторных климактерических симптомов. Акушерство и гинекология. 2017; 8: 115-20.[Yureneva S.V., Ebzieva Z.Kh.Role of hypothalamic (trigger) neuropeptides in the genesis of hot flushes. Prospects for new therapeutic approaches to treating vasomotor menopausal symptoms. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (8): 115-20. (in Russ.)]. http://dx.doi.org/10.18565/aig.2017.8.115-20
- Moore A.M., Coolen L.M., Porter D.T., Goodman R.L., Lehman M.N. KNDy Cells Revisited. Endocrinology. 2018;159(9):3219-3234. doi: 10.1210/en.2018-00389.
- Uenoyama Y., Inoue N., Maeda K.I., Tsukamura H. The roles of kisspeptin in the mechanism underlying reproductive functions in mammals. J Reprod Dev. 2018; 64(6): 469–476. doi: 10.1262/jrd.2018-110. Epub 2018 Oct 8.
- Jayasena C.N., Comninos A.N., Stefanopoulou E., Buckley A., Narayanaswamy S., Izzi-Engbeaya C., et al. Neurokinin B Administration induces hot flushes in women. Sci. Rep. 2015; 5: 8466. doi: 10.1038/srep08466.
- Smith R.L., Flaws J.A., Gallicchio L. Maturitas. Does quitting smoking decrease the risk of midlife hot flashes? A longitudinal analysis. 2015; 82(1):123-7. doi: 10.1016/j.maturitas.2015.06.029
- Anderson R.A., Skorupskaite K., Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2019; 22(1): 51-54. doi: 10.1080/13697137.2018.1540564. Epub 2018 Dec 21.
- Prague J.K., Roberts R.E., Comninos A.N., et al. Neurokinin 3 receptor antagonism rapidly improves vasomotor symptoms with sustained duration of action. Menopause. 2018; 25: 862–9. doi:10.1097/GME.0000000000001090.
- Клинические рекомендации. Менопауза и климактерическое состояние у женщин. М.: Российское общество акушеров-гинекологов, 2016.[Klinicheskie rekomendacii. Menopauza i klimaktericheskoe sostojanie u zhenshhin. M.: Rossijskoe obshhestvo akusherov-ginekologov, 2016.(in Russ.)].
- Oakley A.E., Steiner R.A., Chavkin C. Kappa agonists as a novel therapy for menopausal hot flash Menopause. 2015; 22(12): 1328-34. doi: 10.1097/GME.0000000000000476.
- Prague J.K., et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017; 389(10081): 1809–20. doi: 10.1016/S0140-6736(17)30823-1
Received 27.05.2019
Accepted 21.06.2019
About the Authors
Zukhra Kh. Ebzieva, postgraduate of gynecological endocrinology department, Federal State Budget Institution “Research Center for Obstetrics, Gynecology and Perinatology” Ministry of Healthcare and Social Development of the Russian Federation. Phone: +7252580319; e-mail: zu87@list.ru4, Acad. Oparin street, Moscow, Russian Federation 117997.
Svetlana V. Yureneva, MD, Federal State Budget Institution “Research Center for Obstetrics, Gynecology and Perinatology” Ministry of Healthcare and Social Development
of the Russian Federation. Phone: +79161797400; e-mail: syureneva@gmail.com
4, Acad. Oparin street, Moscow, Russian Federation 117997.
Tatiana Yu. Ivanets, Clinical Diagnostic Laboratory, Federal State Budget Institution “Research Center for Obstetrics, Gynecology and Perinatology”
Ministry of Healthcare and Social Development of the Russian Federation. Phone: +79104042669; e-mail: t_ivanets@oparina4.ru
4, Acad. Oparin street, Moscow, Russian Federation 117997.
Viktoriya G. Averkova, postgraduate of gynecological endocrinology department, Federal State Budget Institution “Research Center for Obstetrics,
Gynecology and Perinatology” Ministry of Healthcare and Social Development of the Russian Federation. Phone: +79152590810; e-mail: buch1202@mail.ru
4, Acad. Oparin street, Moscow, Russian Federation 117997.
For citation: Ebzieva Z.Kh., Yureneva S.V., Ivanets T.Yu., Averkova V.G. The role of neurokinin B in the pathophysiological mechanisms of vasomotor symptoms and sleep disturbances in postmenopausal women.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2019; (11): 160-166.(in Russian).
http://dx.doi.org/10.18565/aig.2019.11.160-166



