Качество жизни пациенток, больных раком молочной железы (РМЖ), остается нерешенной клинической задачей. И перед врачами во всем мире становится эндокринная дилемма: необходимо одновременно блокировать эффекты эстрогенов для снижения риска рецидивов РМЖ и купировать проявления дефицита эстрогенов (климактерического синдрома) для поддержания качества жизни больных [1].
За последние 40 лет наблюдается улучшение в частоте выживаемости после постановки диагноза РМЖ. Однако большинство женщин имеют низкое качество жизни, связанное с тяжелыми приливами жара, бессонницей, сухостью во влагалище, диспареунией, остеопорозом и другими проявлениями климактерического синдрома [2].
Число женщин, перенесших РМЖ в анамнезе, постоянно повышается, поэтому климактерические расстройства в указанной группе становятся крайне важной проблемой с коротко- и долгосрочными последствиями для здоровья и качества жизни (табл. 1) [3].
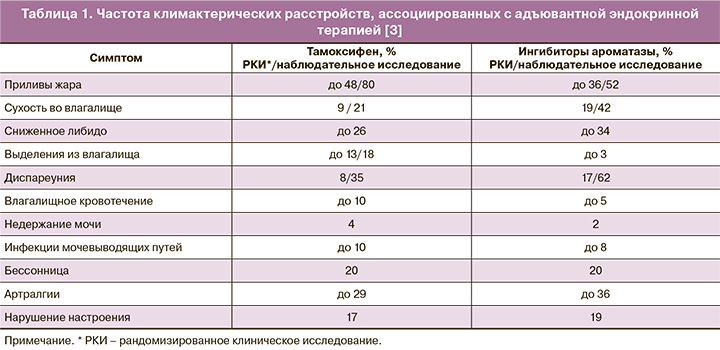
Около 80% женщин, получающих терапию тамоксифеном, имеют вазомоторные симптомы (ВМС), при дополнительной овариальной супрессии приливы развиваются у 93% женщин [4].
Полагают, что интенсивность приливов жара у пациенток, больных РМЖ, выше, чем у женщин с естественной менопаузой. Приливы жара на фоне терапии тамоксифеном обычно более тяжелые по сравнению с терапией ингибиторами ароматазы (ИА) [3]. У молодых пациенток с индуцированной менопаузой климактерические симптомы более тяжелые, чем у женщин в постменопаузе.
Климактерические расстройства у женщин, больных РМЖ, могут быть связаны: с естественной менопаузой, возникшей параллельно с диагнозом РМЖ; возобновлением симптомов после отмены менопаузальной гормонотерапии (МГТ) в случае постановки диагноза РМЖ; риск-редуцирующей билатеральной аднексэктомией; химиотерапией или супрессией функции яичников агонистами гонадотропин-рилизинг-гормона (аГнРГ) в пременопаузе; адъювантной терапией тамоксифеном или ИА [5].
Выявлено, что тяжелые приливы жара могут приводить к отказу от лечения антиэстрогенами [6]. Как следствие, по данным зарубежных авторов, до 28% женщин, больных РМЖ, не привержены адъювантной терапии, 34% отмечают, что врачами не задавался вопрос о приливах жара и ночной потливости, и только 25% женщин было рекомендовано лечение [7]. Полученные данные указывают на возможное повышение риска рецидивов РМЖ за счет низкого качества жизни на фоне терапии заболевания и, как следствие, отказа от нее.
Коррекция вазомоторных симптомов у больных раком молочной железы
Известно, что МГТ может купировать до 90% приливов, однако вызывают обеспокоенность системное воздействие эстрогенов и возможное повышение частоты рецидивов у женщин, перенесших РМЖ, что крайне ограничивает ее использование [8].
За последние 20 лет проведено множество исследований, направленных на поиск эффективных не-эстрогенсодержащих методов лечения для женщин, перенесших РМЖ [2].
Фитоэстрогены похожи на 17β-эстрадиол по химической структуре [9] и могут обладать эстрогенным и антиэстрогенным эффектом. Результаты исследований влияния их на прогноз у больных РМЖ неоднозначны. Большинство данных поддерживают представления о безопасности их использования, некоторые даже указывают на снижение риска рецидивов и смертности на фоне их приема [3].
Согласно данным исследования на клеточной линии РМЖ, экстракт цимицифуги блокировал рост, особенно при повышенной экспрессии HER2 [9, 10]. Согласно данным систематического обзора 26 статей, не выявлено взаимосвязи между приемом цимицифуги и повышением риска РМЖ [11].
Однако, согласно данным метаанализа 16 исследований, не выявлено различий между цимицифугой и плацебо в частоте и тяжести приливов [12]. Эффективность фитоэстрогенов при РМЖ не была доказана в рандомизированных клинических исследованиях [3]. В целом эффект плацебо может достигать 50% у данной группы женщин [13].
Таким образом, не выявлено значительной эффективности влияния цимицифуги на приливы жара у больных РМЖ, а также негативного влияния на риск РМЖ [11].
Альтернативные методы
Норадреналин и серотонин являются важными нейротрансмиттерами, влияющими на терморегуляцию, и участвуют в патогенезе приливов жара. Эстрогены влияют на их высвобождение и на количество их рецепторов.
Ингибиторы обратного захвата серотонина (и норадреналина) одинаково эффективны у женщин, перенесших РМЖ, независимо от приема тамоксифена или ИА (несмотря на то, что на фоне приема тамоксифена приливов обычно больше) [14].
Однако некоторые из них (пароксетин, флуоксетин, сертралин) способны ингибировать цитохром Р450 2D (CYP2D6), метаболизирующий тамоксифен в его активную форму – эндоксифен [4]. Оптимальным вариантом является выявление показаний и подбор препарата совместно с неврологом/психиатром. Ниже представлены основные препараты, благоприятно воздействующие на ВМС у женщин (табл. 2).
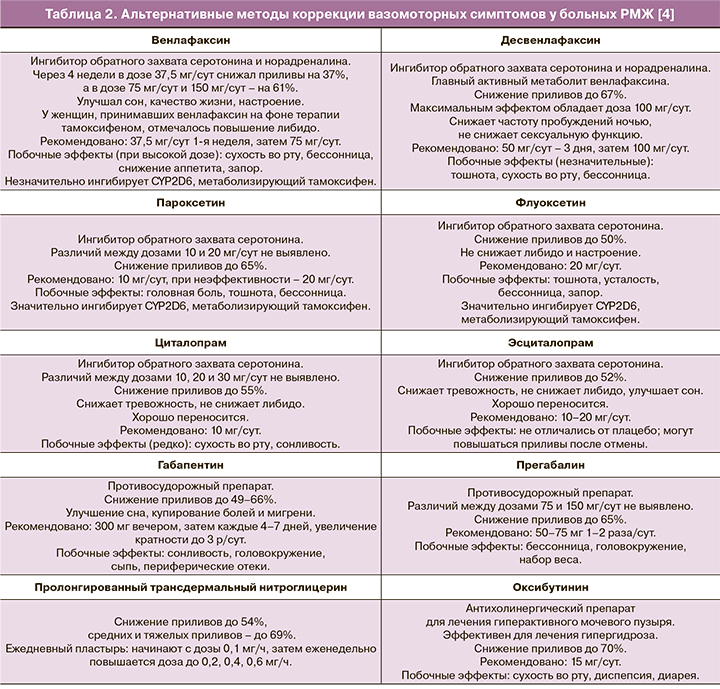
Иглоукалывание, когнитивная поведенческая терапия и некоторые другие альтернативные методы, по всей видимости, обладают меньшей эффективностью в отношении ВМС [15, 16].
Системная гормональная терапия
МГТ – наиболее эффективное лечение ВМС, однако она не является оптимальной опцией для женщин, перенесших РМЖ, в особенности рецептор-позитивный [4, 17, 18].
При этом ряд наблюдательных исследований [19–21], большое исследование случай-контроль [22] и систематический обзор [23] указывают на отсутствие повышения риска рецидивов РМЖ на фоне приема МГТ.
Несмотря на это, 2 из 3 рандомизированных клинических исследования выявили повышение риска рецидива РМЖ.
Исследование HABITS (Hormonal replacement therapy after breast cancer – it is safe? - Заместительная гормональная терапия после рака молочной железы – это безопасно?) – рандомизированное контролируемое исследование, в которое вошли 442 женщины, наблюдение в течение 4 лет: рецидивы РМЖ выявлены у 39/221 пациенток, принимавших МГТ, и у 17 из группы контроля (ОР 2,4; 95% ДИ 1,3–4,2) [24].
Стокгольмское исследование: рандомизированное открытое клиническое исследование 378 женщин, 10 лет наблюдения, остановленное в 2003 г. в связи с результатами исследования HABITS: не выявлено различий в рецидивах РМЖ между группами (ОР 0,82; 95% ДИ 0,35–1,9). Однако доза гестагена в Стокгольмском исследовании была ниже [25].
Согласно данным обоих исследований, среди женщин, принимавших МГТ, не выявлено повышения смертности от РМЖ или от любого другого онкологического заболевания [1].
LIBERATE (Livial Intervention following Breast cancer: Efficacy, Recurrence, And Tolerability Endpoints – Применение Ливиала после рака молочной железы – конечные точки: эффективность, рецидивы, переносимость) – рандомизированное плацебо-контролируемое исследование 3148 женщин, больных РМЖ, со значительными приливами жара с назначением тиболона 2,5 мг/сут. Через 3,1 года наблюдения выявлен повышенный риск рецидива РМЖ (ОР 1,40; 95% ДИ 1,14–1,70); исследование было закончено досрочно. В группе тиболона были отмечены значительное снижение приливов жара, улучшение сна, сексуальной функции, купирование сухости во влагалище и улучшение настроения, а также повышение минеральной плотности кости по сравнению с контрольной группой [26].
На сегодняшний день клинические рекомендации в основном рассматривают РМЖ как противопоказание к МГТ. Однако у некоторых женщин облегчение симптомов может перевешивать потенциальные риски МГТ [1, 18].
Согласно рекомендациям Североамериканского общества по менопаузе от 2017 г., у женщин с РМЖ в анамнезе, особенно эстроген-рецептор-позитивным, решение о назначении системной МГТ должно производиться по веским причинам после попытки терапии негормональными препаратами, после подробной консультации, с принятием решения совместно с онкологом (Уровень III) [27].
Современная альтернатива МГТ, используемая в США и некоторых странах Европы, – тканеселективный эстрогеновый комплекс, куда входят конъюгированные эквинэстрогены в сочетании с базедоксифеном, блокирующим эстрогеновые рецепторы в молочной железе и матке, – на сегодняшний день не рекомендован для больных РМЖ из-за недостаточных данных по безопасности [28].
К запланированным исследованиям гормональных методов коррекции климактерического синдрома у больных РМЖ можно отнести изучение эффективности эстрогена эстетрола, который синтезируется в печени плода человека и блокирует эстрогеновые рецепторы в молочной железе, в качестве терапии для снижения побочных симптомов дефицита эстрогенов при лечении РМЖ [29, 30].
Коррекция генитоуринарного менопаузального синдрома у больных раком молочной железы
По данным исследований, 50–75% женщин с РМЖ в анамнезе имеют один или более симптомов генитоуринарного менопаузального синдрома (ГУМПС) [31].
Сексуальная дисфункция включает в себя снижение желания/либидо, возбуждения, оргазма, а также диспареунию и вагинизм, что приводит к снижению самооценки, настроения и нарушению межличностных отношений, что снижает качество жизни пациентки и ее партнера. Более тяжелые симптомы наблюдаются в постменопаузе по сравнению с пременопаузой, а также на фоне химиотерапии или ИА [32].
Полагают, что проявления ВМС и ГУМПС могут снижаться после окончания адъювантной эндокринной терапии, за исключением снижения либидо, которое персистирует у 30% женщин как минимум еще на протяжении 2 лет после окончания лечения [33].
В исследовании у 763 женщин, которым было проведено лечение по поводу РМЖ, в течение 6 лет безрецидивного течения отмечались снижение ВМС, но увеличение сухости во влагалище, недержание мочи и снижение сексуальной активности [34].
Негормональные методы коррекции
На сегодняшний день рекомендуется оценивать признаки и симптомы сексуальной дисфункции и генитоуринарные симптомы у больных РМЖ и выявлять обратимые факторы сексуальной дисфункции и воздействовать на них. Необходимо начинать с негормональных лубрикантов и увлажняющих средств на водной основе при наличии сухости во влагалище и назначать консультации психолога [35].
Онкологи сдержаны в назначении местных эстрогенов из-за возможной системной абсорбции и повышения риска рецидивов РМЖ (особенно на фоне терапии ИА).
Многие пациенты хорошо отвечают на простые меры: использование средств гигиены без мыльной основы, увлажняющие средства на водной основе, лубриканты [2].
Первая линия терапии – негормональные лубриканты и увлажняющие средства на водной основе [36]. Данные рандомизированного двойного-слепого плацебо-контролируемого исследования указывают на большую эффективность лубрикантов на силиконовой основе по сравнению с водной для снижения дискомфорта при половой жизни у женщин в постменопаузе, больных РМЖ [37].
Двойное-слепое рандомизированное плацебо-контролируемое исследование вагинального геля, содержащего молочную кислоту, показало снижение сухости во влагалище и диспареунии, снижение pH и увеличение индекса созревания влагалищного эпителия [38].
Местное нанесение 4% раствора лидокаина на преддверие влагалища на протяжении 4 недель снижало проявления сексуальной дисфункции у женщин (46) в постменопаузе, больных РМЖ, с тяжелой диспареунией [39].
Неконтролируемое проспективное исследование использования фракционного СО2-лазера у 77 женщин в постменопаузе выявило значительное снижение симптомов через 12 недель [40].
Гормональные методы коррекции
Согласно рекомендациям Североамериканского общества по менопаузе 2017 г., при упорном ГУМПС возможно рассмотреть применение низкодозированной эстроген-терапии (рекомендации касаются применяемых в США вагинальных форм эстрадиола и конъюгированных эквинэстрогенов). Низкодозированная вагинальная эстроген-терапия, используемая при ГУМПС, имеет минимальную системную абсорбцию (уровень в крови в постменопаузальном диапазоне) и, исходя из некоторых наблюдений, не имеет риска рецидива рака эндометрия или РМЖ (Уровень II). Использование низкодозированных вагинальных эстрогенов (при неудаче негормональной терапии) у перенесших РМЖ, вероятно, безопасно и значительно улучшает качество жизни. Для женщин, перенесших РМЖ, решение о назначении низкодозированной вагинальной эстроген-терапии должно приниматься совместно с онкологом, особенно для женщин, принимающих ИА, при которых снижается уровень эстрадиола (Уровень III) [27].
Согласно клиническим рекомендациям «Менопауза и климактерическое состояние у женщины» Российского общества акушеров-гинекологов и Российской ассоциации по менопаузе от 2016 г., при РМЖ в анамнезе назначение низкодозированных локальных эстрогенов зависит от желания женщины, ее информированности о потенциальных рисках, после консультации с врачом-онкологом [14].
По данным небольших исследований женщин, получающих ИА по поводу РМЖ, эстриол не может трансформироваться в эстрадиол или эстрон и не повышает уровень эстрадиола в сыворотке крови [41, 42]. Таким образом, местный эстриол теоретически должен быть безопасным, однако необходимо больше исследований по безопасности и эффективности.
Вагинальный дегидроэпиандростерон (ДГЭА) повышает индекс созревания влагалищного эпителия, снижает pH влагалища без повышения уровня эстрогенов в сыворотке крови [43]. Результаты 2 двойных слепых рандомизированных плацебо-контролируемых исследований (фаза III) показали положительные эффекты на все 4 аспекта сексуальной функции, включая желание, возбуждение, оргазм и болевые ощущения при половых контактах [44].
FDA недавно одобрило препарат ДГЭА для вагинального применения у здоровых женщин в постменопаузе для лечения диспареунии средней и тяжелой степени. Однако на сегодняшний день нет данных по безопасности данного препарата у пациенток с РМЖ в анамнезе.
Оспемифен – единственный селективный модулятор эстрогеновых рецепторов, одобренный для лечения диспареунии средней и тяжелой степени, связанной с вульвовагинальной атрофией. Оспемифен 60 мг/сут улучшал индекс созревания влагалищного эпителия, рН влагалища, сухость. Данные in vitro на животных указывают на антиэстрогенный эффект на молочную железу при раке [45], однако эффективность и безопасность у женщин не доказаны.
Использование тканеселективного эстрогенового комплекса может также дать положительный эффект на ГУМПС. Данные больших клинических исследований выявили значительное снижение проявлений вульвовагинальной атрофии при безопасности для эндометрия, а также значимое улучшение индекса созревания влагалищного эпителия, рН влагалища, сухости, снижение наиболее беспокоящих вагинальных симптомов [46]. Однако на сегодняшний день нет данных по безопасности данного препарата у больных РМЖ [47].
Коррекция снижения минеральной плотности кости у больных раком молочной железы
Снижение минеральной плотности кости и переломы у больных РМЖ являются следствием целого ряда причин: прямое или системное влияние опухоли, эстрогенная депривация вследствие терапии РМЖ (химиотерапия, овариальная супрессия, ИА), естественная менопауза, прием глюкокортикоидов.
Наиболее высокая степень снижения минеральной плотности (7–8% в год в поясничном отделе позвоночника) выявлена у женщин в пременопаузе на фоне химиотерапии или аГнРГ с ИА.
У женщин в пременопаузе использование тамоксифена ассоциировано со снижением минеральной плотности кости, в отличие от женщин в постменопаузе [5].
Терапия ИА ассоциирована с большей потерей МПК, чем при естественной менопаузе. Ежегодная частота переломов, ассоциированная с приемом ИА (анастрозол), составила 2,2 по сравнению с 1,3% у здоровых женщин в постменопаузе и остеопенией [48].
Также к факторам риска остеопороза и переломов у больных РМЖ относится высокая распространенность дефицита витамина D (до 76%) [49], сниженная физическая активность, потенциальное повышение риска падений, связанное с индуцированной химиотерапией нейропатией.
Бисфосфонаты и деносумаб, согласно данным исследований, повышают общую выживаемость и выживаемость без прогрессирования болезни [5], а также входят в рекомендации по профилактике и лечению патологии костной ткани (включая остеопороз) при злокачественных новообразованиях [50].
Заключение
Таким образом, на сегодняшний день очевидно, что коррекция климактерических расстройств у больных РМЖ является не просто методом поддержания качества жизни, но и продолжительности жизни, в том числе за счет снижения частоты отказа от противорецидивной терапии.
Безусловно, самым эффективным методом коррекции климактерических расстройств остается менопаузальная гормонотерапия, которая противопоказана, согласно данным большинства рекомендаций (включая клинические рекомендации «Менопауза и климактерическое состояние у женщины» Российского общества акушеров-гинекологов и Российской ассоциации по менопаузе от 2016 г.), женщинам, перенесшим РМЖ, независимо от длительности безрецидивного течения. При этом признание того факта, что в ряде случаев вопросы качества жизни у больных РМЖ могут превалировать над возможным повышением риска рецидивов, позволило включить, в частности, в рекомендации Североамериканского общества по менопаузе в 2017 г. пункт о возможности назначения системной МГТ по веским причинам после попытки терапии негормональными препаратами, после подробной консультации, с принятием решения совместно с онкологом.
Фитоэстрогены, несмотря на схожесть по структуре с 17β-эстрадиолом, очевидно, являются хоть и менее эффективной, но более безопасной опцией для коррекции ВМС, однако необходимы дальнейшие исследования.
При выборе ингибитора обратного захвата серотонина (и норадреналина) предпочтение следует отдавать препаратам с максимально возможной эффективностью в отношении жалоб конкретной пациентки, с учетом данных о возможном влиянии ряда препаратов на метаболизм тамоксифена.
К перспективным препаратам, которые, возможно, будут доступны в будущем, можно отнести тканеселективный эстрогеновый комплекс и эстетрол.
Различные проявления ГУМПС, включая снижение либидо, которое персистирует у 30% женщин как минимум еще на протяжении 2 лет после окончания лечения РМЖ, также значительно снижают качество жизни пациенток.
На сегодняшний день рекомендуется оценивать признаки и симптомы ГУМПС и сексуальной дисфункции у больных РМЖ, выявлять и корригировать обратимые факторы сексуальной дисфункции. Необходимо начинать с негормональных лубрикантов и увлажняющих средств на водной основе, а также возможно рекомендовать интимную гигиену без мыльной основы, вагинальный гель, содержащий молочную кислоту, СО2-лазер, при необходимости назначать консультацию психолога.
Главный недостаток негормональных видов лечения – частичный или временный локальный эффект, меньший, чем на фоне вагинальных эстрогенов. Согласно данным зарубежных и российских рекомендаций, при отсутствии эффекта от негормональных методов можно совместно с онкологом рассмотреть вопрос о непродолжительной терапии низкодозированными вагинальными эстрогенами. Особую осторожность следует проявлять в случае применения эстрадиола или конъюгированных эквинэстрогенов на фоне ИА. В отличие от этого, есть данные, что эстриол при вагинальном применении не может трансформироваться в эстрадиол или эстрон и не повышает уровень эстрадиола в сыворотке крови. Однако необходимы дальнейшие исследования. К перспективным направлениям относится использование пероральных препаратов на основе селективных модуляторов эстрогеновых рецепторов – оспемифена и тканеселективного эстрогенового комплекса, а также препарата ДГЭА для вагинального использования.
Снижение минеральной плотности кости и переломы у больных РМЖ являются следствием целого ряда причин. К наиболее эффективным и безопасным относят бисфосфонаты и деносумаб, которые, согласно ряду данных, также играют позитивную роль в патогенезе РМЖ и метастазировании.



