The role of myo-inositol in preparing women for assisted reproductive technologies
Objective. To evaluate the effectiveness of administering the myo-inositol and folic acid complex on the quality of embryos and the pregnancy rate during preconception preparation for in vitro fertilization (IVF) in patients with infertility.Kvashnina E.V., Gvozdikova T.V., Druzhinina A.Yu., Masterova I.A., Murunova S.V., Plotavskaya T.B., Tutakov M.A., Pavlyuchenkova S.M., Shilova N.V., Dikke G.B.
Materials and methods. The study included 92 infertile patients planning to undergo IVF, among them 46 women (group I) received the complex of myo-inositol (MI) at a dose of 4000 mg and folic acid (FA) at a dose of 400 mcg (Fertina, Orion Pharma, Finland) per day for 12 weeks, and 46 women (group II) received only 400 mcg of folic acid per day. The research was performed in accordance with the Order of the Russian Ministry of Health No. 107n dated 30.08.2012. The criteria for evaluating the effectiveness of the complex were the facts of biochemical pregnancy based on the analysis of hCG and clinical pregnancy according to the ultrasound data.
Results. The women who took the MI/FA complex before the IVF protocol, in comparison with the women taking only FA, showed the following results: a shorter duration of ovarian stimulation, 9 (9–10) vs. 11 (10–11) days, p=0.04; a lower total dose of gonadotropins, 1470 (1425–1445) vs. 1990 (1775–2150) IU/ml, p=0.01; a larger number of oocytes at MII stage, 9 (4–17) vs. 6 (3–15) oocytes, p=0.03, respectively. The number of biochemical pregnancies was 19 (68%) vs. 10 (42%), p=0.05, and clinical pregnancies were noted in 15 (53%) vs. 8 (33%) cases, p=0.14, respectively.
Conclusion. Administration of the complex MI and FA for three months before IVF has a positive effect on the process of oocyte maturation and pregnancy rate.
Keywords
Due to the fact that the state significantly supports assisted reproduction programs, it is obvious that improving the effectiveness of in vitro fertilization (IVF) is an essential task [1].
The quality of oocytes and embryos is considered to be the main issue of IVF in assisted reproduction. Inositol plays an important role in FSH signaling, oocyte maturation, and embryo development [2]. The foreign researchers analyzed the results of treatment of 935 infertile patients using various ART protocols and found out that the inclusion of 2000–4000 mg of myo-inositol (MI) and 400 mcg of folic acid (FA) per day in the diet of women three months before the protocol resulted in a significant increase in the pregnancy rate and reduced the risk of spontaneous abortion [3]. However, this analysis did not show a significant difference in the number of retrieved oocytes at MII stage, the number of days of stimulation, and the doses of gonadotropins.
Among the few national studies on the effectiveness of taking MI and FA during preconception preparation, there is the work of E.V. Vartanyan et al., who studied the effect of inositol on women younger than 38 years preparing for IVF after ovarian surgery. According to the study, when the women receive inositol the number of mature oocytes and high-quality embryos increases and pregnancy occurs more frequently after embryo transfer [4].
The aim of the study is to evaluate the effectiveness of administering the myo-inositol and folic acid complex on the quality of embryos and the pregnancy rate during preconception preparation for IVF in patients with infertility.
Materials and Methods
This was a prospective, non-randomized, open-label, comparative clinical trial in parallel groups.
The study included 92 infertile patients planning to undergo IVF; they were examined and treated in the period from November 2018 to September 2020 at the network of clinics for reproductive technologies “ECO Center” (Moscow, Petrozavodsk, Lipetsk, Simferopol, Smolensk).
During the study, the patients were divided into two groups: the first group consisted of 46 patients who underwent preconception preparation, including the complex of MI and FA; the second group consisted of 46 patients selected according to the principle of “case – control” who underwent standard preparation (taking 400 mcg of FA) before starting the IVF protocol. On selecting pairs, the women met the criteria for age, body mass index (BMI), duration of infertility, number of previous IVF attempts, as well as the number of antral follicles at the beginning of the protocol and the starting dose of gonadotropin.
The research protocol was developed taking into account the theses of the Declaration of Helsinki created by the World Medical Association (revised in 2008), as well as the International Ethical Guidelines for Biomedical Research Involving Human Subjects. Informed consent to participate in the study was obtained from all patients.
The criteria for inclusion in the study were the age of patients 18–45 years, infertility, and compliance with the above-mentioned “case-control” principle. The exclusion criteria were severe somatic diseases, Rh-negative blood group, mental diseases, and cognitive disorders.
All the patients were examined in accordance with the Order of the Russian Ministry of Health No. 107n dated 30.08.2012 “On approval of the use of assisted reproductive technologies, contraindications and limitations to their use”.
Preconception preparation included administration of the complex of MI at a dose of 4000 mg and FA at a dose of 400 mcg (Fertina, Orion Pharma, Finland) for 12 weeks before IVF in group I, and administration of FA at the same dose also for 12 weeks in group II.
Protocols with gonadotropin-releasing hormone (GnRH) antagonists included the administration of gonadotropins 150–300 IU per day, depending on the number of antral follicles from the 2nd–5th day of the menstrual cycle. GnRH antagonists were started before the day of trigger injection if there was a follicle with a diameter of 14 mm. If there were more than 15 follicles with a diameter exceeding 11 mm, the trigger was replaced in protocols with GnRH antagonist with GnRH agonist. In order to increase the number of mature oocytes, a double trigger was used for a suboptimal response. Oocyte retrieval was performed 35–37 hours after the trigger was injected transvaginally with a Kitazato needle 19G 325 mm when more than three follicles were present.
The luteal phase was supported with progesterone 600 mg per day in the vagina or dydrogesterone 30 mg per day orally from the day of puncture to 10 weeks after embryo transfer. In the case of embryo transfer with GnRH agonist as a trigger, estrogen preparations were additionally prescribed (17β-estradiol, 0.1% gel for external use, from 2 to 4 mg or estradiol valerate, dragees from 2 to 6 mg orally) from the day of puncture and 5000 IU hCG intramuscularly on the 5th day.
The retrieved mature oocytes were fertilized in vitro 40±1 hours after the trigger injection. Fertilization was evaluated 17±1 hours later. The embryos were cultured for up to 3–5 days in a one-step medium of LifeGlobal Global Total in a humid CO2 atmosphere (6%). After evaluating the quality of the embryos according to the Gardner classification, there was a decision on transfer, biopsy, and cryopreservation. The transfer was performed using Kitazato #213325 catheter; in case of complicated transfers, CCD TDT Friedman catheter was used. The remaining embryos were frozen with vitrification on Kitazato Cryotop carriers using Kitazato Vitrification Medium VT601 solutions [5].
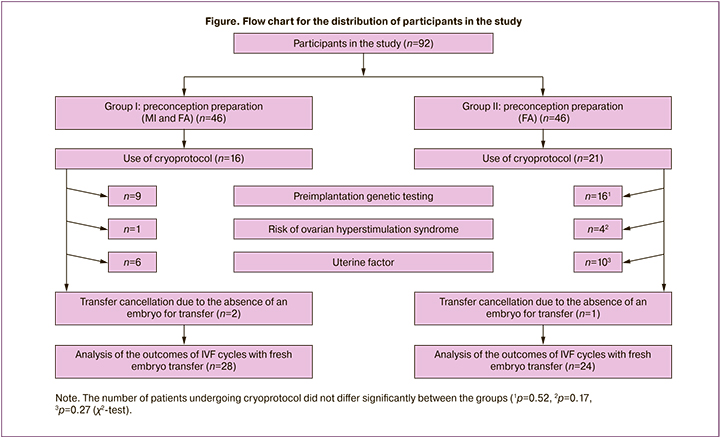
The distribution of the participants in the study is presented in the flow chart.
Treatment efficacy was evaluated using primary outcomes, which included such indicators as the total dose of gonadotropin, duration of stimulation, number of oocytes at MII stage, number of embryos at the time of transfer; it was also assessed on the basis of secondary outcomes including the rate of pregnancy confirmed biochemically and clinically.
Biochemical pregnancy was diagnosed based on the level of β-HCG in the blood 10–14 days after embryo transfer, the diagnosis of clinical pregnancy was confirmed by visualization of the gestational sac in the uterine cavity under ultrasound guidance 3–4 weeks after transfer, and the diagnosis of progressive pregnancy was verified 10 weeks after transfer.
Statistical analysis
The statistical analysis of the results was performed using Statistica 6.0 software package (Statsoft, USA). Median and interquartile range (Me [Q25–Q75%]) were calculated for quantitative variables, and absolute value (n) and percentage (%) of the total group volume were calculated for qualitative ones. The Mann– Whitney U test was used for intergroup comparison of indicators (their distribution was different from the normal one). The differences between qualitative variables were checked using the χ2-test and Fisher’s exact test (for small samples). If the achieved level of differences did not exceed 0.05, they were considered statistically significant [6]. Quantitative assessment of the relationship between the treatment factor and the outcome was evaluated using the value of the odds ratio (OR). In order to demonstrate the connection, a 95% confidence interval (95% CI) was calculated using the Woolf method.
Results
The analysis of medical and social indicators as well as the history of patients in both groups at the time of starting the IVF protocol showed no differences among them (Table 1).
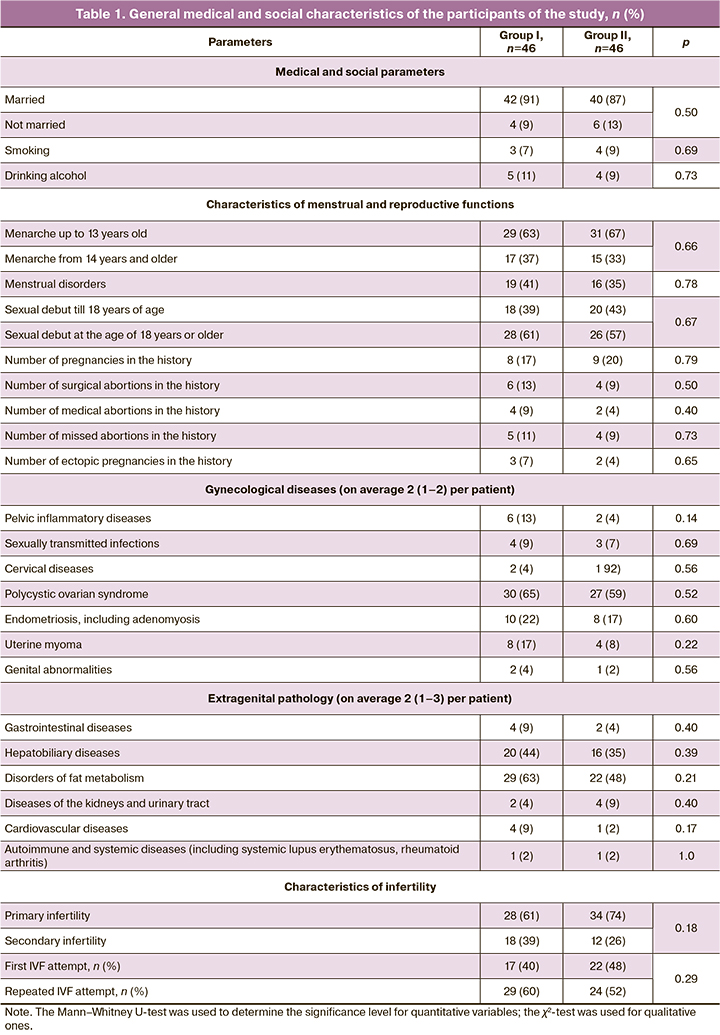
The average reproductive age of the patients was 33 (32–38) and 34 (32–39) years, respectively (p=0.71); they had obesity of the first degree (BMI 31 (22–34) and 32 (22–36) kg/m2, respectively, p=0.47); most of them had a history of unsuccessful ART attempts. The duration of infertility in both groups was 7 (5–9) years, p=0.75, and the number of repeated IVF attempts was the same, namely, 2 (2–3), p=0.68.
The patients who underwent preconception preparation for IVF using the complex of MI and FA received a statistically significantly lower total dose of gonadotropin, the period of stimulation was shorter, and there was a tendency to a higher frequency of fresh embryo transfer (Table 2).
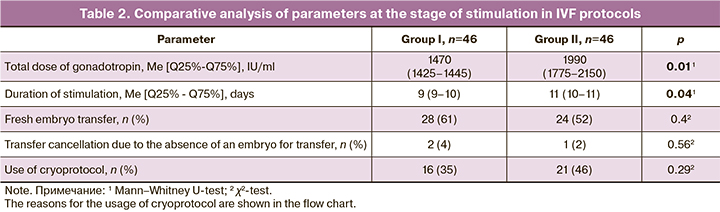
All other indicators of the IVF program of the patients did not show any significant differences. The reasons for cycle segmentation were also comparable in both groups. These reasons included preimplantation genetic testing of the embryo (20% of all cases of using cryoprotocol in group I and 15% in group II), uterine factor, which implied an unsatisfactory condition of the endometrium, and also the risk of ovarian hyperstimulation syndrome. Despite the absence of significant differences, the tendency to a higher incidence of this complication was observed in group II.
At the stage of embryo cultivation, it was found that the number of mature oocytes and the number of high-quality embryos on days 3 and 5 were statistically significantly higher in women of group I compared to these parameters in women of group II (Table 3).
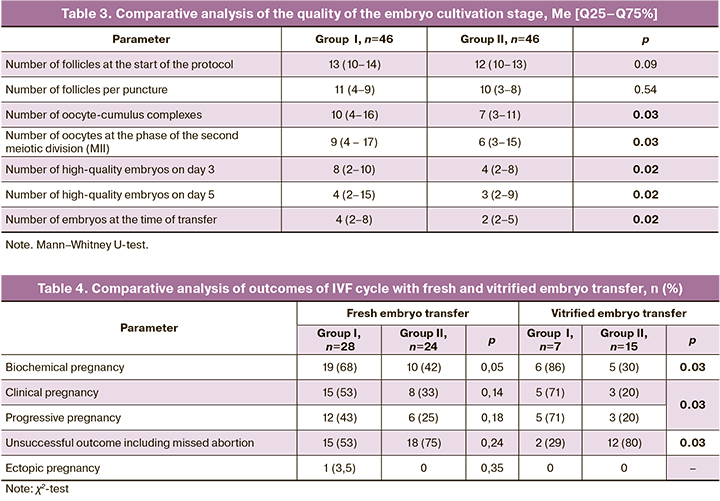
As cryoprotocol was used in some of the cycles, the results of IVF programs were analyzed after the transfer of the fresh and vitrified embryo (Table 4).
As for segmented IVF cycles, it should be noted that the median number of frozen embryos was higher when patients received MI: it was 8 (2–12) in group I, and it was 6 (1–10) in group II, p=0.04. By the end of the follow-up, not all segmented cycles were completed, primarily due to the difficult epidemiological situation in the spring of 2020 associated with the COVID-19 virus infection. In group I, there were 9 (56%) such cycles, and in group II there were 6 (28%), p=0.12.
The odds ratio (OR) of a positive outcome (biochemical, clinical, and progressive pregnancy) was calculated for the fresh embryo transfer and the use of the complex of MI and FA in comparison with FA in obese women, polycystic ovarian syndrome (PCOS), and repeated IVF attempts. It showed that OR in achieving biochemical pregnancy was 4.8 in obese patients (95% CI: 0.98–23.54, p=0.05). In achieving a clinical pregnancy that lasted until the end of the follow-up period (10 weeks), OR=5.2 (95% CI: 1.06– 25.97, p<0.001) in obese patients, OR=2.7 (95% CI: 0.43–16.94, p=0.01) in patients with PCOS, OR=1.5 (95% CI: 0.25–8.98, p=0.003) in patients without PCOS. The number of IVF attempts was not statistically significant.
Thus, the analysis of the outcomes of completed cycles allowed us to note that the inclusion of MI in preconception preparation has a positive effect on the rate of satisfactory outcomes of IVF programs including women with obesity and/or PCOS.
Discussion
Improving the effectiveness of ART programs is a relevant task all over the world. Given the high incidence of female obesity, which is an additional factor that complicates infertility and IVF programs [7], our data allow us to recommend women with a BMI of more than 30 kg/m2 to include a combination of MI and FA in the diet when preparing for IVF cycles. According to our research, the positive effect of these biologically active substances on the outcomes of the ART program may be caused by an improvement in the sensitivity of ovarian tissue to the gonadotropin stimulus. A similar conclusion was made by Caprio F. et al. [8], who noted a lower total dose of gonadotropin while taking MI. It should be noted that this study included women with poor response to stimulation, but with a normal BMI (from 23.3± 2.3 to 24.2±2.0 kg/m2), while we were able to observe a similar effect in obese women.
The researchers note a positive effect of MI on oocyte quality, but the main studies concern the cohort of women with PCOS. The reasons for the failure of IVF programs in such patients are likely to be low quality of oocytes and/or embryos, as well as the risk for ovarian hyperstimulation syndrome. When the assisted reproduction programs are preceded by the use of the complex of MI and FA three months before stimulation in the IVF cycle, the researchers note an improvement in the response of the ovarian tissue to the hormonal signal at a lower dose of follicle stimulating hormone, which is necessary for optimal development of the follicles. In addition, there is a decrease in estradiol levels on the day of ovulation trigger, which helps reduce the risk of ovarian hyperstimulation syndrome and reduces the number of canceled or incomplete IVF cycles due to this reason [9–11]. As for the risk of ovarian hyperstimulation syndrome, we also noted a tendency to its decrease in the group taking MI (one vs. four, p=0.17).
However, not all authors share the same opinion on the necessity of including MI in the diet before the IVF cycle. Gupta D. et al. stated that the improvement in the quality of oocytes and embryos in women with PCOS after taking MI before the IVF program is not statistically significant [12].
Our study included women with PCOS and without it. The decrease in the total dose of gonadotropin detected in the IVF program in our research is consistent with the decrease observed by Chiu T.T.Y. et al. They also included women with PCOS and without it in their study. The authors note that the amount of follicle stimulating hormone used to stimulate the ovaries during IVF cycles is reduced in women whose follicular fluid contains higher levels of MI. Therefore, it is reasonable to include MI in the diet of women who are planning ART [13].
We noted that taking MI before starting the IVF program is associated with the retrieval of mature oocytes, as well as obtaining good-quality embryos. Similar results were revealed by Alviggi S. et al., who showed that the intake of MI by women with PCOS before the IVF program resulted in an increase in the number of MII oocytes, namely 6.3±2.5 versus 4.5±2 in women who did not take MI (p=0.03) [14]. There is an opinion that PCOS is not necessarily associated with obesity, patients may experience latent obesity, this conclusion can be made after the assessment of the patients’ visceral fat [15]. In general, it is a rare situation when women undergoing ART are prescribed any additional measures such as preconception care only on the basis of BMI. Our study shows that MI administration to these women 12 weeks before starting stimulation in the IVF cycle can influence favorably both the course of the assisted reproduction program and its outcome.
Conclusion
Administration of the complex of myo-inositol at a dose of 4000 mg and folic acid at a dose of 400 mcg before the IVF program reduces the total dose of gonadotropin, reduces the duration of stimulation, and increases the number of high-quality oocytes at MII stage and embryos during the transfer.
Administration of the complex of myo-inositol and folic acid in IVF cycles with fresh embryo transfer increases the rate of biochemical pregnancy; in cycles with vitrified embryo, it increases the rate of both clinical and progressive pregnancy, moreover, it reduces the number of unsuccessful outcomes as well as the number of missed abortions.
Administration of the complex of myo-inositol and folic acid before IVF cycle increases the likelihood of achieving a clinical and progressive pregnancy compared to the administration of folic acid alone in women with obesity and/or PCOS in fresh embryo transfer cycles.
References
- Исупова О.Г. Вспомогательные репродуктивные технологии: новые возможности. Демографическое обозрение. 2017; 4(1): 35-64. [Isupova O.G. Assisted Reproductive Technologies: New Opportunities. Demographicheskoe obozrenie. 2017; 1: 33-63 (in Russian)].
- Simi G., Genazzani A.R., Obino M.E., Papini F., Pinelli S., Cela V., Artini P.G. Inositol and in vitro fertilization with embryo transfer. Int. J. Endocrinol. 2017; 2017: 5469409. https://dx.doi.org/10.1155/2017/5469409.
- Zheng X., Lin D., Zhang Y., Lin Y., Song J., Li S., Sun Y. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine (Baltimore). 2017; 96(49): e8842. https://dx.doi.org/10.1097/MD.0000000000008842.
- Вартанян Э.В., Цатурова К.А., Девятов Е.А., Михайлюкова А.С., Левин В.А., Сагамонова К.Ю., Громенко Д.С., Овсянникова Т.В., Эрлихман Н.М., Колосова Е.А., Сафронова Е.В., Фотина О.В., Красновская Е.В., Пожарищенская Т.Г., Аутлева С.Р., Гзгзян А.М., Нуриев И.Р., Воропаева Е.Е., Пестова Т.И., Здановский В.М., Ким Н.А., Котельников А.Н., Сафронов О.В., Назаренко Т.А., Ионова Р.М. Подготовка к лечению бесплодия методом экстракорпорального оплодотворения при сниженном овариальном резерве. Акушерство и гинекология. 2019; 8: 134-42. [Vartanian E.V.,Tsaturova K.A., Deviatov E.A., Mikhailiukova A.S., Levin V.A.,Sagamonova K.Yu., Gromenko D.S., Ovsiannikova T.V., Erlikhman N.M.,Kolosova E.A., Safronova E.V., Fotina O.V., Krasnovskaia E.V., Pozharishchenskaia T.G., Autleva S.R., Gzgzian A.M., Nuriev I.R., Voropaeva E.E., Pestova T.I., Zdanovskii V.M., Kim N.A., Kotelnikov A.N., Safronov O.V., Nazarenko T.A., Ionova R.M. Preparation for the treatment of infertility by in vitro fertilization with a reduced ovarian reserve. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 8: 134-42. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.134-142.
- Квашнина Е.В., Тутаков М.А., Томина Е.В., Берестецкая О.С., Немсцверидзе Э.Я., Шилова Н.В. Сегментация цикла вспомогательных репродуктивных технологий как инструмент повышения эффективности преодоления бесплодия. Уральский медицинский журнал. 2019; 5: 116-23. [Kvashnina E.V., Tutakov M.A., Tomina E.V. et al. Segmentation of the cycle of assisted reproductive technologies as a tool to improve the efficiency of overcoming infertility. Uralskiy meditsinskiy zhurnal/ Ural medical journal. 2019; 5(173): 116-23. (in Russian)].
- Harris M., Taylor G. Medical statistics made easy. London: Taylor and Francis; 2006. 114р.
- He Y., Tian J., Oddy W.H., Dwyer T., Venn A.J. Association of childhood obesity with female infertility in adulthood: a 25-year follow-up study. Fertil. Steril. 2018; 110(4): 596-604. e1. https://dx.doi.org/10.1016/j.fertnstert.2018.05.011.
- Caprio F., D’Eufemia M.D., Trotta C., Campitiello M.R., Ianniello R., Mele D., Colacurci N. Myo-inositol therapy for poor-responders during IVF: a prospective controlled observational trial. J. Ovarian Res. 2015; 8: 37. https://dx.doi.org/10.1186/s13048-015-0167-x.
- Papaleo E., Unfer V., Baillargeon J.-P., Fusi F., Occhi F., De Santis L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil. Steril. 2009; 91(5): 1750-4. https://dx.doi.org/10.1016/j.fertnstert.2008.01.088.
- Unfer V., Raffone E., Rizzo P., Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol. Endocrinol. 2011; 27(11): 857-61. https://dx.doi.org/10.3109/09513590.2011.564687.
- Rizzo P., Raffone E., Benedetto V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010; 14(6): 555-61.
- Gupta D., Khan S., Islam M., Malik B.H., Rutkofsky I.H. Myo-inositol’s role in assisted reproductive technology: evidence for improving the quality of oocytes and embryos in patients with polycystic ovary syndrome. Cureus. 2020; 12(5): e8079. https://dx.doi.org/10.7759/cureus.8079.
- Chiu T.T.Y., Rogers M.S., Law E.L.K., Briton-Jones C.M., Cheung L.P., Haines C.J. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte quality. Hum. Reprod. 2002; 17(6): 1591-6. https://dx.doi.org/10.1093/humrep/17.6.1591.
- Alviggi C., Cariati F., Conforti A., De Rosa P., Vallone R., Strina I. et al. The effect of FT500 Plus(®) on ovarian stimulation in PCOS women. Reprod. Toxicol. 2016; 59: 40‐4. https://dx.doi.org/10.1016/j.reprotox.2015.10.014.
- Чернуха Г.Е., Табеева Г.И., Удовиченко М.А. Неиспользованные возможности коррекции эндокринно-метаболических нарушений при синдроме поликистозных яичников. Акушерство и гинекология. 2019; 10: 140-7. https://dx.doi.org/10.18565/aig.2019.10.140-147. [Chernukha G.E., Tabeeva G.I., Udovichenko M.A. Unused opportunities for correction of endocrine and metabolic disorders in polycystic ovary syndrome. Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 10: 140-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.10.140-147.
Received 05.11.2020
Accepted 09.11.2020
About the Authors
Elena V. Kvashnina, PhD, leading reproductive specialist of the Network of Reproductive Health Clinics “Center of IVF”, the chief physician of the Rehabilitation Centerfor Reproductive Disorders “Partus”, Yekaterinburg. Tel.: +7(343)288-53-39. E-mail: doctor.kvashnina@gmail.com.
620026, Russia, Yekaterinburg, Mamina-Sibiryaka str., 171A.
Tatyana V. Gvozdikova, PhD, reproductologist, “Center of IVF”, Lipetsk, Tel.: +7(4742)56-30-64. Е-mail: slukina.t@gmail.com. 398007, Russia, Lipetsk, Ushinskogo str., 10.
Alena Yu. Druzhinina, gynecologist, “Medica-Mente”, Moscow. Tel.: +7(495)215-55-33. E-mail: dr.druzhinina@gmail.com. 129075, Russia, Moscow, Argunovskaya str., 3/1.
Irina A. Masterova, gynecologist, reproductologist, chief medical officer, “Center of IVF”, Petrozavodsk. Tel.: +7(900)459-89-01. E-mail: irramasterova@gmail.com.
185033, Russia, Petrozavodsk, Gogolya str., 6.
Svetlana V. Murunova, obstetrician-gynecologist, 295034, Russia, Simferopol, Polevaya str, 24/23, tel. +73652777245. E-mail:svetamurunova@mail.ru .
Tatiana B. Plotavskaya, obstetrician-gynecologist, reproductologist, “Center of IVF”, Smolensk. Tel: +7(4812)-30-31-41. E-mail: rakitskaya_t@mail.ru.
214031, Russia, Smolensk, Generala Paskevicha str., 19.
Maksim A. Tutakov, leading embryologist, Rehabilitation Center for Reproductive Disorders “Partus”, Yekaterinburg. Tel.: +7(343)288-53-39.
E-mail: tutakov@ivf-partus.ru. 620026, Russia, Yekaterinburg, Mamina-Sibiryaka str., 171A.
Svetlana M. Pavlyuchenkova, PhD, Director of embryology, “Center of IVF”, Moscow. Tel. +7(495)215-55-33. E-mail: emdir@sweetgroup.ru.
129075, Russia, Moscow, Argunovskaya str., 3/1.
Natalya V. Shilova, Сand. of Мed. Sci., Director, “Reprohelp”, Moscow. Tel.: +7(495)774-57-57. E-mail: nvshilova@gmail.com.
107078, Russia, Moscow, Kalanchevskaya str., 31/4.
Galina B. Dikke, Dr. Med. Sci., associate professor, Professor of the Department of obstetrics and gynecology with a course of reproductive medicine, F.I. Inozemtsev Academy of Medical Education, St. Petersburg. Tel.: +7(812)334-76-50. E-mail: galadikke@yandex.ru. ORCID: 0000-0001-9524-8962.
190013, Russia, St. Petersburg, Moscow Ave., 22M.
For citation: Kvashnina E.V., Gvozdikova T.V., Druzhinina A.Yu., Masterova I.A., Murunova S.V., Plotavskaya T.B., Tutakov M.A., Pavlyuchenkova S.M., N.V. Shilova, G.B. Dikke. The role of myo-inositol in preparing women for assisted reproductive technologies.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 11: 139-146 (in Russian).
https://dx.doi.org/10.18565/aig.2020.11.139-146



