The role of maternal gut microbiota in spontaneous preterm birth
Objective. To study the potential correlation between maternal gut microbiota and preterm labor.Gorina K.A., Khodzhaeva Z.S., Muravieva V.V., Muminova К.Т., Donnikov A.E., Priputnevich T.V.
Materials and methods. A prospective case control study included 40 puerperas. The patients were divided into two groups: group I consisted of women who had spontaneous preterm birth; group II included healthy women who had full-term births. The study of the gut microbiota was carried out using a culture method.
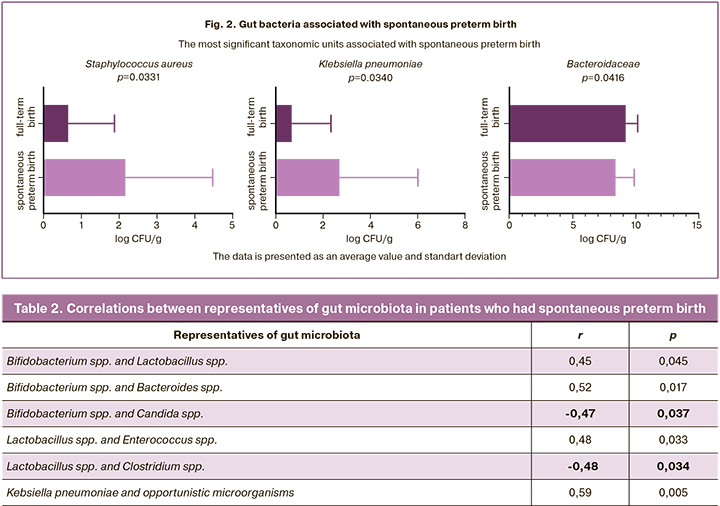
Results. The gut microbiota of patients in both groups was presented mainly by the microorganisms of Bifidobacterium spp., Lactobacillus spp., Bacteroides spp., Enterococcus and Escherichia spp. In group I, patients who gave birth prematurely had more frequently opportunistic pathogens of facultative anaerobic origin, namely Staphylococcus aureus (p=0.0365) and/or Klebsiella pneumoniae (p=0.0217); they had a higher logarithmic value of colony-forming units (CFU) compared to the group of patients who had full-term births; patients of group I also showed a poorer growth of obligate anaerobes – Bacteroides spp. (p=0.0416); the results were statistically significant.
Conclusion. The gut microbiota is likely to play a role in spontaneous preterm birth.
Keywords
Preterm birth is the leading cause of neonatal morbidity and mortality worldwide [1, 2], however, the underlying etiopathogenetic factors continue to be under active research nowadays. Thus, the issues related to the development of two clinical phenotypes of preterm birth, namely, intact fetal membranes and premature rupture of membranes, require further studies and details [3, 4]. Inflammation resulting from an ascending infection from the lower genital tract and/or an imbalance of the vaginal microflora (an increase in the population of Gardnerella vaginalis and/or Ureaplasma spp. accompanied by a sharp decrease in the content of normoflora – Lactobacillus spp.) is a well-researched factor of preterm birth among other identified causes [5, 6]. However, spontaneous preterm birth can be associated with an infectious and inflammatory process localized outside the vagina, for example, in the oral cavity, urinary tract, intestines, and with the microbiota of various organs and systems [7, 8]. The results of recent research confirm the significance of a comprehensive study of the mother’s gut microbiota. This microbiota cannot only initiate spontaneous preterm birth, but it may also affect the neurological status of a premature newborn.
More than one hundred trillion microorganisms live in the human intestinal tract and united in a single continuum, gut microbiota [9]. It is known that the intestine is a multifunctional organ, and gut microbiota in the context of spontaneous preterm birth has an immunomodulatory potential [10]. The disbalance of gut microbiota contributes to the development of mural inflammation with the formation of increased intestinal permeability and subsequent active migration of microorganisms (the so-called «leaky gut») [9]. High permeability facilitates the dissemination of microorganisms and their metabolites, such as lipopolysaccharides (LPS), that can be found in the cell wall of gram-negative bacteria. LPS contribute to systemic inflammation called metabolic endotoxinemia (a two- or three-fold increase in the level of circulating endotoxins in the blood) [11, 12]. Activation of the proinflammatory pathway from a physiological to an excessive level can also make a significant contribution to the development of other major obstetric syndromes, in particular, fetal growth retardation and preeclampsia [13, 14]. Being typical representatives of gut microbiota, microorganisms were found in the amniotic fluid of pregnant women with premature rupture of membranes; the presence of microorganisms proves their role, maybe etiological, in the development of intrauterine infection [15]. Currently, two theories of such microbial translocation have been proposed: the circulation of LPS with their subsequent stimulating effect on the synthesis of inflammatory mediators and prostaglandins, and hematogenic dissemination of pathogens from the intestine («leaky gut») to the placenta or uterus [16].
The aim of the research is to study the potential correlation between maternal gut microbiota and preterm birth.
Materials and Methods
A prospective case control study was carried out in the obstetric departments of the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow. According to the aim of the study, 40 women were examined. The patients were divided into two groups: group I (main) consisted of puerperas who had spontaneous preterm birth (the average gestational age at delivery was 34.3 ± 1.6 weeks); group II (control) included healthy women who had full-term birth (39.5 ± 1.1 weeks).
Anamnestic, clinical and laboratory data of all pregnant women included in the study were analyzed in detail: somatic, obstetric and gynecological history, the course of pregnancy and childbirth, the postpartum period, indicators of peripheral blood and the discharge of the genital tract. Modes of delivery and neonatal outcomes were analyzed. There was a survey that included questions about the diet and bowel habits during and before pregnancy, as well as the presence or absence of bad habits.
Patients with singleton pregnancy, spontaneous preterm birth till 366 weeks (the onset of labor and/or premature rupture of membranes) were eligible for inclusion in group I; patients with singleton pregnancy that ended in a full-term delivery were eligible for inclusion in group II. In order to minimize the impact of confounders, patients with severe obstetric pathology (except for the previous history of preterm birth, late and recurrent miscarriages) and extragenital diseases, as well as carriers of Streptococcus agalactiae (group B Streptococcus) were not included in both groups in the study. Exclusion criteria were multiple pregnancies, placenta previa/ increta, a long interval between membrane rupture and delivery (12 hours or more), taking antibacterial therapy just before the delivery (7 days or less), and diagnosed chronic inflammatory intestinal diseases. Before the research the patients signed an informed consent to participate in the study; the work was approved by the Local Ethics Committee for Biomedical Research of the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology.
The study of the gut microbiota was carried out using a culture method with selective and non-selective culture media and incubation under aerobic, microaerophilic and anaerobic conditions. Fecal samples collected in a sterile plastic container were delivered to the laboratory within two hours, 10-fold dilutions were prepared in saline solution, and followed by the inoculation of the media (100 ml each). For isolating facultative anaerobic and aerobic microorganisms, we used Columbia blood agar (Oxoid, Great Britain), chromogenic media Brilliance (Oxoid, Great Britain), mannitol salt agar (Himedia, India), Enterococcus agar (SRCAMB, Obolensk, Russia), Endo-agar GRM (SRCAMB, Obolensk, Russia), Salmonella-Shigella agar (Oxoid, Great Britain), dextrose agar Saburo (Oxoid, Great Britain). Lactobacilli were grown using the Lactobacagar medium (SRCAMB, Obolensk, Russia). Strict anaerobes were cultured with agar for bifidobacteria (Himedia, India), Schedler agar (Oxoid, Great Britain) with the necessary additives, basic agar for anaerobes (Oxoid, Great Britain), perfringens agar (Oxoid, Great Britain), iron-sulfite agar (Oxoid, Great Britain). Species identification of microorganisms was performed using the MALDI-TOF-MS method with the time-of-flight AutoflexIII mass spectrometer and the MaldiBioTyper software (Bruker Daltoniks, Germany).
Statistical analysis
Statistical analysis of the obtained data was performed using the methods of descriptive and variational mathematical statistics with GraphPad Prism 8.3 and IBM SPSS Statistics 22 programs following the general recommendations for medical and biological studies. Differences were considered statistically significant at the level of p<0.05. The data were processed using the following methods: test χ2 with the calculation of correction for continuity for contingency tables 2x2 and Fisher’s criterion, odds ratio (OR) with a confidence interval of 95% (95% CI) for comparison of binary data. Comparative analysis of variables was performed using Student’s t-test. In the absence of a normal distribution of data, the methods of nonparametric statistics (Mann–Whitney U-test) were used. For studying the dependencies between the parameters, Pearson correlation analysis was applied.
Results
The study included 40 patients who were divided into two groups depending on the gestational age at delivery (preterm and full-term birth). These groups were compared in age, weight and height, and body mass index (BMI). The demographic characteristics of the patients are presented in Table 1.
The analysis of the course of pregnancy did not show any statistically significant differences in the frequency of threatened preterm birth (p=0.75), gestational toxicosis (p=0.89), respiratory diseases (p=0.06), anemia of pregnant women in the third trimester (p=0.15). However, patients of group I in comparison with group II had a statistically significant increase in preterm birth in history (p=0.04), cervical incompetence (40% versus 10%, respectively, p=0.029) with performed cerclage (75%) or placement of a cervical pessary (25%). Antibacterial therapy was prescribed more frequently in the third trimester in the group of patients with spontaneous preterm birth than in the control group, namely in 7 out of 20 pregnant women (35%) versus 1 out of 20 patients (5%), p=0.02. However, when analyzing this indicator in the first and second trimesters, there were no significant differences (p=0.9 and p=0.43, respectively).
The comparative analysis of the characteristic features of delivery, regardless of the gestational age, showed that the predominant mode in both groups was vaginal delivery (85% and 90%, respectively). The indication for abdominal delivery in group I was the onset of labor/premature rupture of membranes in patients with a scar on the uterus after a previous cesarean section. The frequency of premature rupture of membranes (p=0.46), interval between membrane rupture and delivery (p=0.32), and the duration of labor (p=0.13) did not differ significantly in groups I and II.
In order to study the characteristic features of gut microbiota in spontaneous preterm birth, a detailed analysis of its composition was carried out in the study groups. Gut microbiota composition in patients of the study groups is presented in Figure 1. It should be noted that despite the same taxonomic diversity, there is a depletion of normal gut microbiota in patients with spontaneous preterm birth. The proportion of microorganisms is represented as a percentage.
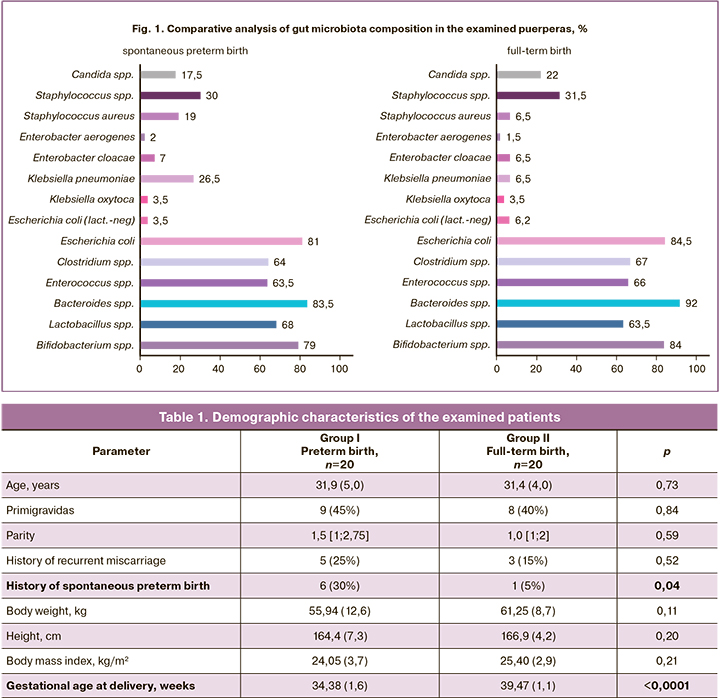
The statistically significant differences between the groups were found in the quantity of Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (Kl. pneumoniae) and bacteria in the family Bacteroidaceae (Fig. 2). Thus, patients who had spontaneous preterm birth had such pathogens as S. aureus (p=0.0331) and/ or Kl. pneumoniae (p=0.0340) more frequently and these patients had higher logarithmic values of colony-forming units (CFU), compared to the patients who had full-term births; at the same time, there was a depletion of the microbiota due to obligate-anaerobic bacteria of the family Bacteroidaceae (p=0.0416), which increase the dysbiotic processes, contributing to the activation of the proinflammatory pathway [17].
In order to assess the relationships of microorganisms in the patients of the main group and to understand the interaction of microorganisms with each other, a multiple correlation analysis was performed using linear r-Pearson correlation coefficient; its results are presented in Table 2.
The obtained data confirm the potential (direct dependence) influence of representatives of normal microflora on each other: the growth of some microorganisms contributes to the growth of others (r=0.45; p=0.045); the growth of opportunistic microorganisms creates a favorable environment for the pathogen growth (r=0.59; p=0.005); as well as a negative influence (inverse relationship) between normal symbiotes and opportunistic microorganisms, in particular, Candida spp. (-0.47; p=0.037) [18].
For a more detailed assessment of other risk factors affecting gut microbiota, we conducted a questionnaire of patients in both groups. No significant differences were found (p=0.74) in the diet; there were 5 options to choose from: a variety of food, including junk food (fast food, crisps, etc.), a variety of healthy food, excluding carbohydrates, proteins, and fats; 70% of respondents in both groups chose a variety of healthy food. Abnormal bowel pattern, namely constipation, occurred more frequently in the patients who had spontaneous preterm birth, but the differences between the groups were not significant (p=0.75). Groups I and II did not differ significantly in either frequency of bad habits (smoking) or the presence of pets (p=0.75 and p=0.31, respectively).
The postpartum period was not complicated in any of the groups; on average, the patients were discharged on the fourth day. The level of white blood cells in patients of group I was 11.38 ± 2.96 × 109/L, and it did not differ from that in the control group, 10.60 ± 3.3 × 109/L (p=0.42). Infectious complications characteristic of the postpartum period were not observed. However, infectious complications (congenital pneumonia, urinary tract infection, etc.) developed in 7 newborns (35%) from the group of spontaneous preterm birth, while there were no such complications in the control group (p=0.0004). The percentage of peripheral blood lymphocytes on the first or second day of life was significantly higher in group with spontaneous preterm birth (39.5 ± 13.2%) than in the control group (27.50 ± 7.78%, p=0.001).
In order to visualize the entire data array, a heat map was constructed on the Displayr platform (Fig. 3) and the total number of microorganisms was estimated. The obtained data and the full spectrum of microorganisms revealed in puerperas who had spontaneous preterm birth and full-term birth are presented in the graphic image. Each thin horizontal line represents the gut microbiota composition of the puerperas (n=40). Each cell in Figure 3 displays the degree of microbial contamination (labels are at the top) in log CFU/g.
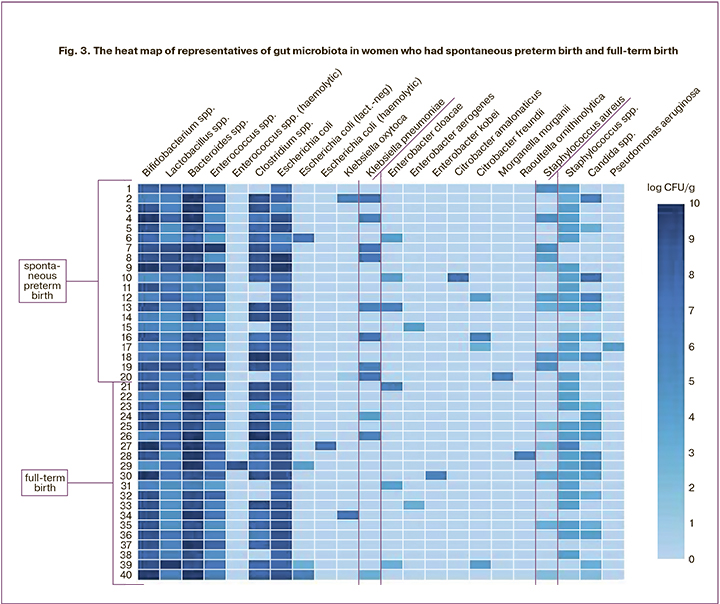
According to the data obtained, an increase in opportunistic microorganisms such as S. aureus can be associated with spontaneous preterm birth as this microorganism was observed in 50% (10/20) of patients who had spontaneous preterm birth and 25% (5/20) of patients having full-time birth; these differences were not statistically significant (p=0.19). However, the considerable growth of S. aureus (more than 1×104 CFU) was observed only in 5% (1/19) of women with full– term birth, while this indicator was 40% (8/20) in patients who gave birth prematurely; the result was statistically significant (p=0.02, OR 12.67, 95% CI: 1.8-146.6). The analysis of Kl. pneumoniae occurrence did not show significant differences either (p=0.16): women who had preterm birth showed an increase of 40% (8/10) and women with full-term birth had a 15% (3/20) increase. Considerable growth of Kl. pneumoniae was observed in 40% (8/20) of patients, that is, in all patients of group I, and the value was not less than 1×106 CFU, and it was found in 5% (1/19) of the patients of group II. Similar to S. aureus, these differences were statistically significant (p=0.02, OR 12.67, 95% CI: 1,8–146.6).
Discussion
In this case-control study, the indicators of gut microbiota of 40 puerperas who had spontaneous preterm birth and full-term birth were analyzed. More than 50 types of microorganisms are known to exist in the large intestine, but four main types are dominant, namely Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria [19]. According to the results obtained, the leading representatives of gut microbiota in both groups were microorganisms of the genera Bifidobacterium (type Actinobacteria), Bacteroides (type Bacteroidetes), Lactobacillus, Enterococcus, Staphylococcus, Clostridium (type Firmicutes) and Escherichia, Klebsiella (type Proteobacteria). According to the obtained results, all patients were divided into three groups, depending on the degree of dysbiotic disorders: normocenosis, moderate dysbiotic changes (severe anaerobes predominate over facultative anaerobes, growth of opportunistic microorganisms is less than 104 CFU/g) and severe dysbiosis (facultative anaerobes predominate over obligate anaerobes, growth of opportunistic microorganisms is more than 104 CFU/g, with increased generation of S. aureus, Klebsiella spp. and Clostridium spp. Dysbiotic disorders from moderate to severe prevailed in 90% (18/20) of patients in the main group, which is significantly higher than ones in the control group (2/20 (10%), p 0.001); at the same time, the frequency of severe dysbiosis was also higher (45 and 10%, respectively), which is statistically significant (p=0.004).
According to the obtained data, an increase in the number of facultative-anaerobic opportunistic microorganisms such as S. aureus and/or Kl. pneumoniae, as well as a poor amount of obligate-anaerobic component of gut microbiota, that is bacteroids, can be associated with spontaneous preterm birth. A decrease in the titers of bacteroids and other obligate-anaerobic microorganisms in the gut microbiota composition is accompanied by a disorder in the production of short chain fatty acids (acetate, propionate, butyrate), which affect the condition of the colon, providing protection from inflammation and impairment of the barrier function.
Healthy epithelial cells of the large intestine deplete oxygen levels in its lumen, at the border of the mucous membrane, through beta-oxidation processes, and create an anaerobic environment. In case of inflammation they reduce the ability to beta-oxidation due to increased oxygen availability, which leads to dysbiotic changes associated with increased generation of proteobacteria, including enterobacteria [20, 21]. An increase in intestinal cell permeability is accompanied by an increase in LPS transport of the cell wall of gram-negative bacteria from the intestinal lumen to the bloodstream [22], causing endotoxemia. The decrease in the content of bacteroids in gut microbiota of patients who had spontaneous preterm birth in combination with an increase in the population of proteobacteria (Kl. pneumoniae), which was observed in our study, could lead to increased production of proinflammatory cytokines and prostaglandins and trigger a cascade of pathogenetic mechanisms.
Conclusion
The role of gut microbiota in the development of a wide range of obstetric pathologies, including large obstetric syndromes, is actively studied. The results of this research, along with numerous data from modern literature [10, 23, 24], are suggestive of the role of gut microbiota as one of the potential cofactors in the development of spontaneous preterm birth. Apparently, the depletion of gut microbiota in patients of the main group can cause a syndrome of systemic inflammatory response, which leads to the increased secretion of proinflammatory cytokines and prostaglandins, and consequently preterm birth [8]. The second issue under discussion is perinatal programming; the formation and maintenance of adequate functioning of the intestinal-brain axis [10] of the mother and fetus is impossible in situations of dysbiosis. Therefore, normalization of gut microbiota, restoration of its diversity and stability of its composition in pregnant women is one of the important preventive measures to reduce the frequency of preterm birth and neonatal complications.
References
- Lawn J.E., Cousens S., Zupan J.; Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005; 365(9462): 891-900. https://dx.doi.org/10.1016/S0140-6736(05)71048-5.
- NICE National Institute for Health and Care Excellence. Preterm labour and birth: NICE guideline. 20 November, 2015.
- Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet. Gynecol. Scand. 2008; 87(6): 590-600. https://dx.doi.org/10.1080/00016340802005126.
- Boyle A.K., Rinaldi S.F., Norman J.E., Stock S.J. Preterm birth: Inflammation, fetal injury and treatment strategies. J. Reprod. Immunol. 2017; 119: 62-6. https://dx.doi.org/10.1016/j.jri.2016.11.008.
- Hyman R.W., Fukushima M., Jiang H., Fung E., Rand L, Johnson B. et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014; 21(1): 32-40. https://dx.doi.org/10.1177/1933719113488838.
- Ходжаева З.С., Припутневич Т.В., Муравьева В.В., Гусейнова Г.Э., Горина К.А. Оценка состава и стабильности микробиоты влагалища у беременных в процессе динамического наблюдения. Акушерство и гинекология. 2019; 7: 30-8. [Khodzhaeva Z.S., Priputnevich T.V., Murav'eva V.V., Guseinova G.E., Gorina K.A., Mishina N.D. The composition and stability of the vaginal microbiota in pregnant women during dynamic observation. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (7):30-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.7.30-38.
- Bröms G., Granath F., Linder M., Stephansson O., Elmberg M., Kieler H. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm. Bowel Dis. 2014; 20(6): 1091-8. https://dx.doi.org/10.1097/MIB.0000000000000060.
- Dahl C., Stanislawski M., Mandal S., Lozupone C., Clemente J.C., Knight R., Stigum H., Eggesb M. Gut microbiome of mothers delivering prematurely shows reduced diversity and lower relative abundance of Bifidobacterium and Streptococcus. PLoS One. 2017; 12(10): e0184336. https://dx.doi.org/10.1371/journal.pone.0184336.
- Power S.E., O’Toole P.W., Stanton C., Ross R.P., Fitzgerald G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014; 111(3): 387-402. https://dx.doi.org/10.1017/S0007114513002560.
- Edwards S.M., Cunningham S.A., Dunlop A.L., Corwin E.J. The maternal gut microbiome during pregnancy. MCN Am. J. Matern. Child Nurs. 2017; 42(6): 310-7. https://dx.doi.org/10.1097/NMC.0000000000000372.
- Mokkala K., Röytiö H., Munukka E., Pietilä S., Ekblad U., Rönnemaa T. et al. Gut Microbiota richness and composition and dietary intake of overweight pregnant women are related to serum zonulin concentration, a marker for intestinal permeability. J. Nutr. 2016; 146(9): 1694-700. https://dx.doi.org/10.3945/jn.116.235358.
- Cani P.D., Osto M., Geurts L., Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012; 3(4): 279-88. https://dx.doi.org/10.4161/gmic.19625.
- Kashtanova D.A., Popenko A.S., Tkacheva O.N., Tyakht A.B., Alexeev D.G., Boytsov S.A. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition. 2016; 32(6): 620-7. https://dx.doi.org/10.1016/j.nut.2015.12.037.
- Kim C.J., Romero R., Chaemsaithong P., Kim J.S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015; 213(4, Suppl.): S53-69. https://dx.doi.org/10.1016/j.ajog.2015.08.041.
- DiGiulio D.B., Romero R., Kusanovic J.P., Gómez R., Kim C.J., Seok K.S. et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 2010; 64(1): 38-57. https://dx.doi.org/10.1111/j.1600-0897.2010.00830.x.
- Nuriel-Ohayon M., Neuman H., Koren O. Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 2016; 7: 1031. https://dx.doi.org/10.3389/fmicb.2016.01031.
- Vinturache A.E., Gyamfi-Bannerman C., Hwang J., Mysorekar I.U., Jacobsson B. Maternal microbiome – A pathway to preterm birth. Semin. Fetal Neonatal Med. 2016; 21(2): 94-9. https://dx.doi.org/10.1016/j.siny.2016.02.004.
- Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017; 279(1): 70-89. https://dx.doi.org/10.1111/imr.12567.
- Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R. et al. Enterotypes of the human gut microbiome. Nature. 2011; 473(7346): 174-80. https://dx.doi.org/10.1038/nature09944.
- Hughes E.R., Winter M.G., Duerkop B.A., Spiga L., Furtado de Carvalho T., Zhu W. et al. Microbial respiration and formate oxidation as metabolic signatures of inflammation-associated dysbiosis. Cell Host Microbe. 2017; 21(2): 208-19. https://dx.doi.org/10.1016/j.chom.2017.01.005.
- Litvak Y., Byndloss M.X., Tsolis R.M., Bäumler A.J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017; 39: 1-6. https://dx.doi.org/10.1016/j.mib.2017.07.003.
- Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008; 57(6): 1470-81. https://dx.doi.org/10.2337/db07-1403.
- Chu D.M., Seferovic M., Pace R.M., Aagaard K.M. The microbiome in preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 52: 103-13. https://dx.doi.org/10.1016/j.bpobgyn.2018.03.006.
- Younes J.A., Lievens E., Hummelen R., van der Westen R., Reid G., Petrova M.I. Women and their microbes: the unexpected friendship. Trends Microbiol. 2018; 26(1): 16-32. https://dx.doi.org/10.1016/j.tim.2017.07.008.
Received 06.07.2020
Accepted 05.08.2020
About the Authors
Ksenia A. Gorina, Junior researcher at the Department of Pathology of Pregnancy, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(926)649-77-32. E-mail: k_gorina@oparina4.ru.117997, Russia, Moscow, Akademika Oparina str., 4.
Zulfiya S. Khodzhaeva, M.D., Professor, Deputy Director for Research of Obstetrics Instituteof the Institution (Department of Obstetrics),
National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation.
Tel.: +7(916)407-75-67. E-mail: zkhodjaeva@mail.ru. 117997, Russia, Moscow, Akademika Oparina str., 4.
Vera V. Muravieva, Ph.D. in Biological sciences, senior research officer Laboratory Microbiology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-25-33. E-mail: v_muravieva@oparina4.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
Kamilla T. Muminova, Ph.D., Junior researcher at the Department of Pathology of Pregnancy, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(916)373-77-07. Е-mail: k_muminova@oparina4.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
Andrew Ye. Donnikov, Ph.D., Head of the Laboratory of Molecular Genetic Methods, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-49-51. Е-mail: a_donnikov@oparina4.ru.
117997, Russia, Moscow, Akademika Oparina str., 4.
Tatyana V. Priputnevich, M.D., Head of the Departament of Microbiology and Clinical Pharmacology, National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Healthcare of Russian Federation. Tel.: +7(903)264-12-57. Е-mail: priputl@gmail.com.
117997, Russia, Moscow, Akademika Oparina str., 4.
For citation: Gorina K.A., Khodzhaeva Z.S., Muravieva V.V., Muminova К.Т., Donnikov A.E., Priputnevich T.V. The role of maternal gut microbiota in spontaneous preterm birth.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 8: 64-71 (in Russian)
https://dx.doi.org/10.18565/aig.2020.8.64-71



