Predicting impairment of pre–lactation transformation and preventing lactogenesis failure in metabolic syndrome
Objective. To identify the features of pre-lactation transformation and the initiation of lactation in the metabolic syndrome to develop a new pathogenetically based method for predicting and preventing early hypogalactia.Tezikov Yu.V., Lipatov I.S., Pechkurov D.V.,Tyutyunnik V.L., Kan N.E., Protasov A.D., Kovyazina I.O.
Material and methods. To develop a predictive model of the risk of early hypogalactia and evaluate the effectiveness of a staged non-pharmacological complex of preventive measures, including prenatal colostrum expression and phototherapy with visible infrared polarized polychromatic non-coherent light in the metabolic syndrome, 184 pregnant women with metabolic syndrome were examined; 35 healthy pregnant women were enrolled in the control group. Diagnostic evaluation included ultrasound examination, biochemical and immunological testing. The effectiveness of the method for preventing early hypogalactia was evaluated by the methodological standards of evidence-based medicine.
Results. The performance of the predictive model of impaired lactogenesis in women with metabolic syndrome, based on the characteristics of the pre-lactation transformation of the mammary glands, confirmed the advantage of the standardized multicomponent approach over individual predictors – AUC = 0.74; standard error of AUC = 0.08; p = 0.006. The quality of prediction of early hypogalactia using the predictive model with the separation point d = 0.78: Se = 74%, Sp = 81% (p < 0.05). Key indicators of the effect of preventive intervention in relation to early hypogalactia confirmed the high efficiency of the proposed method: the rate of early hypogalactia and lactostasis decreased from 75% to 10.4% and from 22.7% to 2%, respectively, the NNT was 2 (95% CI 1–2,
p < 0.05), OR 0.04 (95% CI 0.02–0.1, p < 0.05).
Conclusion. The developed staged prediction and prevention of incomplete gestational mammogenesis and impaired lactation in women with metabolic syndrome is based on a complex pathogenetic approach to support breastfeeding, thus optimizing medical management aimed to improve lactopoiesis.
Keywords
Breastfeeding is a postnatal equivalent of the trans-placental passage of nutrients, i.e., the hemotrophic nutrition, which realizes epigenetic inheritance of health programming [1, 2]. Initial imbalance of neuro-endocrine-immune-metabolic processes, in particular, metabolic syndrome (MS) significantly increases the likelihood of an abnormal puerperal period, resulting in incomplete gestational mammogenesis and, as a result, the impaired lactogenesis affects the entire subsequent stage of natural feeding [3, 4].
MS has been proven to be associated with abnormal changes in pregnancy with characteristic alterations in carbohydrate and lipid metabolism, pro-inflammatory status, intense vascular-platelet hemostasis, increased coagulation potential result in either the state of “a norm of compensated pathology” with preserved autoregulation, or disruption of adaptation mechanisms with the development of obstetric complications, which changes the structural and functional transformation of the body of the puerperal women in the postpartum period [4, 5]. Early hypogalactia is a typical complication of the postpartum period that is pathogenetically linked to MS. MS exemplifies the role of the fetal programming of lactation, which is accompanied by an increase in the incidence of early hypogalactia.
The main cause of late breastfeeding cessation in women with MS is hypogalactia and associated diseases of neonates [6]. Therefore, the development of pathogenetically based effective methods for predicting and preventing early lactation failure is relevant. The optimal timing of the intervention is the end of gestation and lactogenesis, that is, the first seven days postpartum. Pharmacotherapy for lactation failure is associated with adverse effects that exacerbate hypothalamic dysfunction, and are used after the diagnosis is made by the end of lactogenesis, which is not very effective for MS [7, 8]. The solution to the problem of preventing hypogalactia in MS should be sought in the development of non-pharmacological approach and the timely start of prevention. In recent years, studies investigating non-pharmacological treatments with a regulatory mechanism of action have been increasingly focused on phototherapy with visible, infrared polarized polychromatic non-coherent light (VIP-light). The biologic mechanism that underlies VIP-light is the molecular mechanisms of activating enzyme complexes (NADPH oxidase, NO synthase) with the formation of highly reactive radicals and molecules. A cascade of active forms of oxygen and nitric oxide modifies cell receptor complexes and transmembrane translation of biochemical signals, which results in systemic and local effects [7].
The above justifies the need to modernize the prediction and prevention of lactogenesis impairment in MS.
This study was aimed to identify the features of pre-lactation transformation and the initiation of lactation in the metabolic syndrome to develop a new pathogenetically based method for predicting and preventing early hypogalactia.
Material and methods
The study comprised 219 pregnant women, of whom 184 had MS. Group I included 88 pregnant women with MS, who received only basic measures to support breastfeeding [WHO (2003), Order No 572n of the Ministry of Health of the Russian Federation of 2012]. Because the initiation of lactation is affected not only by MS but also by pregnancy complications, the women in Group I were subdivided into those who had an uncomplicated (subgroup IA, n = 46) and complicated (subgroup IB, n = 42) pregnancy. Group II consisted of 96 pregnant women with MS, who in addition to the basic measures underwent the correction of the impaired lactation initiation using the integrated non-pharmacological treatment. Similarly to Group I, Group II was divided into subgroup IIA (n = 52) and subgroup IIB (n = 44) comprising women with uncomplicated and complicated pregnancy, respectively. Thirty-five healthy pregnant women were enrolled in Group III (control group).
The inclusion criteria for selection of the study patient with MS were as follows: spontaneous pregnancy, the absence of severe comorbidities including diabetes mellitus, uncontrolled arterial hypertension, bulimia, infectious and inflammatory diseases, and polycystic ovary syndrome. Exclusion criteria: absolute and relative contraindications for breastfeeding, pathological changes in the mammary glands, antenatal fetal death, critical placental insufficiency (PI), severe pre-eclampsia (PE), extremely early and early preterm labor (before 34 weeks’ gestation).
PI was diagnosed using a comprehensive grading scale with the calculation of the total score of PI [9], and the severity of PE was assessed by WHO criteria (2011). Diagnostic evaluation included measuring unconjugated estriol (UE), progesterone, placental lactogen (PL) with ELISA (Hoffman Le Roche, Switzerland), markers of vascular endothelial dysfunction – platelet count and their maximum amplitude of aggregation (MAAP, Payton aggregator, USA), circulating endothelial cells (CEC), placental growth factor (PGF) – ELISA (ELISA), fibronectin (FN), ( NVO-Immunotech, Russia); parameters of lipid and energy metabolism – total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C) with the calculation of TG/HDL-C ratioratio and atherogenicity coefficient [AC = (total cholesterol – HDL)/HDL), thermostable alkaline phosphatase (TSAP) using Stat Fax 4500+ analyzer (USA), leptin (DSL, USA), placental α-1 microglobulin (PAMG-1) – PAMG Fertitest (RF); markers of inflammatory reaction and apoptosis – tumor necrosis factor alpha (TNFα), C-reactive protein (CRP) – (Protein contour, the RF), lymphocytes with the phenotype CD95 + (L CD95 +), the total reactive potential of the body (LSI — blood leukocyte shift index , IA – allergy index). A morphological study of placentas was performed according to the criteria offered by A.P. Milovanov (2009).
Ultrasound examination and Doppler ultrasound (DUS) of the mammary glands were performed at 37–39 weeks’ gestation, 4–6 days postpartum. The criteria for the completion of mammogenesis at the end of pregnancy and full lactogenesis were the ultrasound breast morphotypes “pregnancy” and “lactation”, respectively. The DUS of lateral thoracic arteries (S/D) allows the extent of blood flow deficit in the mammary glands to be identified in patients with MS during pre-lactation transformation.
The initiation of lactation was assessed by the daily volume and qualitative composition of milk, taking into account the need of newborns, breast thermometry, blood levels of prolactin (PRL) (ELISA, Hoffman Le Roche), Switzerland), pro-and anti-inflammatory cytokines of interleukin IL-6, IL-10 (Bender-Medsystems GmbH, Austria), leukocyte intoxication index (LII).
Prevention of early hypogalactia in Group II was carried out using a comprehensive non-pharmacological method, including prenatal colostrum expression and VIP-light phototherapy using the Bioptron Compact lamp (Zepter, Switzerland; registration certificate FS No. 2006/372 dated March 30, 2006). The first component of the method, colostrum expression, was performed in the settings of a maternity clinic or the Department of Pregnancy Pathology starting from 37 weeks before giving birth with five to ten minutes of expressing on each breast three times a day. This preventive modality forms a reflectory mechanism and induces milk secretory and production functions [10]. Phototherapy was administered on day two postpartum, three times daily with an interval of at least 4 hours: after treatment with an Oxy – spray, the lamplight was directed at a right angle at a distance of 3–4 cm on the area above the areola of the nipple of the left, then the right breast with 8 minute exposure at every point. The treatment course ranged from 10 to 16 sessions.
We used descriptive statistics, Spearman correlation analysis, logistic regression, ROC analysis. The predictive values of predictors of early hypogalactia were calculated using univariate logistic regression. To form a group of patients with a high risk for impaired lactation among women with MS, a logistic regression model was constructed using multivariate analysis. The effectiveness of preventive measures was evaluated by the methodological standards of evidence-based medicine.
Results and discussion
The mean age of women in groups I, II, and III was 26.5 ± 4.2, 27.5 ± 3.6, and 25 ± 3.8 years, respectively, p> 0.05. Group I and Group II were comparable (p > 0.05) by employment status (74.4 and 70.9% were employees), parity (67.8 and 71.8% were primiparous), the rates of non-obstetric (97.8 and 99, 1%) and gynecological (19.0 and 21.1%) comorbidities, pregnancy complications, mode of delivery (55.6 and 61.8% had vaginal delivery), clinical status of newborns (hypotrophy 28.4 and 28, 1%, asphyxia – 18.2 and 18.8%). The body weight of women with MS before pregnancy ranged from 84 to 114 kg. The mean body weight in Groups I and II was 92.5 ± 7.4 and 94.3 ± 6.5 kg, respectively (p> 0.05). The women with MS had a body mass index (kg/m2) ranging between 30 and 35; the mean was 33.2 ± 1.6 and 33.6 ± 1.1 (p> 0.05). The waist circumference ranged from 89–114 cm, with the mean being 101.5 ± 5.2 cm and 103.2 ± 6.4 cm (p> 0.05). In Groups I and II, 48% (42/88) and 49% (47/96) of women, respectively, had controlled arterial hypertension. Blood pressure (mm Hg) characteristics in Groups I and II, respectively, were as follows: systolic blood pressure ranged from 126 to 135 (mean 128 ± 5) and from 127 to 136 (mean 129 ± 4); diastolic blood pressure – from 65 to 82 (77 ± 4) and 67 to 84 (mean 77 ± 5), p> 0.05. At baseline, women in Groups I and II had an atherogenic lipid profile: total cholesterol (mmol/l) was 6.1 [5.2–7.0] and 6.2 [5.3–6.9], TG (mmol/l) 1.84 [1.77–2.67] and 1.86 [1.76–2.69], HDL (mmol/l) – 1.0 [0.88–1.16] and 0.98 [0.9–1.12], the TG/ HDL-C ratio was 1.62 [1.33–2.61] and 1.60 [1.35–2.58]; AC was 5.1 [4.7–5.5] and 5.3 [4.9–5.8], p> 0.05. Therefore, most of the patients in Groups I and II were obese and had impaired lipid profile and high blood pressure.
The rates of pregnancy complications in subgroups IB and IIB were comparable: PI of varying severity was observed in 81% (34/42) and 79.5% (35/44) of women; fetal growth restriction in 59.5% (25/42) and 61.4% (27/44); chronic fetal hypoxia in 38.1% (16/42) and 40.9% (18/44); moderate PE in 23.8% (10/42) and 27.3% (12/44); preterm birth in 11.9% (5/42) and 13.6% (6/44), p˃0. 05. The absence of significant clinical and laboratory differences between Groups I and II substantiates the correct comparison of early hypogalactia rates in women with MS, depending on what type of the preventive measures they underwent.
An analysis of the features of pre-lactation transformation in the pregnant women with MS in Group I is described in Table 1.

The levels of trophic-adaptive hormones, reflecting the state of the hormonal-metabolic activity of the fetal-placental unit (FPU) and inducing structural changes in the epithelial and connective tissue of the mammary glands, confirmed the condition of the FPU, diagnosed using the comprehensive grading scale of PI The levels of PL, progesterone, and UE were 1.6, 1.8, and 1.6 fold lower than in the controls, indicating an imbalance in the neuroendocrine regulation of gestational mammogenesis.
The total reactive potential of the body, the lipid profile and the associated leptin in pregnant women with MS at the third trimester of pregnancy showed unidirectional changes in those who had MS only (IA, IIA) and those who had it concurrently with obstetric complications (IB, IIB) compared with the controls. In these patients, TC, TG, TG/HDL-C ratio, AC, and leptin were 20%, 50%, 26%, 1.9 times, 1.6 times, and 2.8 times higher, respectively, and HDL-C, 26% lower than among controls; these variables had a strong positive correlation with markers of vascular endothelial dysfunction, nonspecific inflammatory response, apoptosis and hormone levels (or from 0.85 to 0.96, with p <0.05). All these results highlight the role of impaired lipid and energy metabolism and MS in general in the pathogenesis of adverse gestational and perinatal outcomes and may be considered as an important link in the disrupted reciprocal connections in the “placenta-hypothalamus-hypophysis-mammary glands” system.
Breast ultrasound in the pregnant women in Group I revealed a “reproductive” ultrasound morphotype in 76.1% of cases [IA – 63% (29/46), IB – 90.5% (38/42)], an ultrasound morphotype “pregnancy” in 23.9% [IA – 37% (17/46), IB – 9.5% (4/42)]. Among puerperal women, 71.6% had “pregnancy with dilated ducts” morphotype [(IA – 65.2% (30/46), IB – 78.6% (33/42)], while the “lactation” morphotype was diagnosed only in 28.4% (25/88) of women. DUS of the lateral thoracic arteries revealed that women with MS had a statistically significantly higher S/D in both breasts, both before delivery and in the postpartum period. These findings represent a manifestation of endothelial dysfunction typical for MS, which also affects the mammary vessels, depriving them of the priority of blood supply. These ultrasound and DUS data confirm the reduction of neuroendocrine support of mammogenesis, decline in mammary gland blood supply and indicate an incomplete morphological and functional transformation for subsequent lactation in MS. The revealed pattern is consistent with the research by A.N. Strizhakov et al. [11], S.M. Anderson [12].
There was a strong positive correlation (r from 0.81 to 0.92, p <0.05) between the markers of lipid metabolism, cellular energy supply, endothelial hemostatic dysfunction, pro-inflammatory and general reactive potential, the state of the FPU and the indicators of pre-lactation preparation of the mammary glands (Ultrasound morphotype, S/D of lateral thoracic arteries). These findings indicate that changes typical of MS imbalance of endocrine regulation, disadaptation of the endothelial system, increased dyslipidemia and general reactive potential with microcirculation disorder in the mammary glands, activation of immunopathological processes, manifested by trophoblast-induced programmed cell death lymphocytes, pro-inflammatory state, changes in the regulation of cellular transformation and energy exchange have a pathogenetic role in the delay in structural and functional restructuring of mammary glands during gestation (incomplete mammogenesis) and after delivery (pathological lactogenesis). The comparability of the results of laboratory tests and diagnostic examinations of pregnant women in Groups I and II (Table 1) confirmed the similarity of the study groups of women with MS.
Evaluation of the secretory function of the mammary glands revealed a decrease in daily breast milk supply among women in Group I both on day 4 (182 ± 29 ml) and day 6 (265 ± 42 ml) postpartum. On day 6, the mother’s breast milk supply in healthy puerperal women (532 ± 32 ml) was 1.8 and 2.6–fold higher than in the subgroup IA (304 ± 38 ml, p <0.05) and subgroup IB (205 ± 34 ml, p <0.05). Insufficient breast milk supply was observed in 75%, 67.4% (31/46), and 83.3% (35/42) of women in Group I, subgroup IA and IB, respectively. In subgroup IA and IB, actual breast milk supply in subgroup IA and IB made up 23% and 38% of required, respectively. A similar pattern was observed in milk composition regarding the content of protein, milk fat, and lactose in the mammary gland secretion: women with MS had statistically significantly 1.4 and 1.3 and 1.2 times lower levels of macronutrients, respectively, compared to the controls [2.6% (2.4–2.8), 3.3% (3.1–3.5), and 5.1% (4.7–5.5)], with the most pronounced changes in subgroup IB. Diagnosis of the clinical course of lactation in Group I revealed that 72.7% of women had objective (inadequate breast engorgement, in a newborn – urinary frequency less than 6 times a day, “hungry stools”) and 59% had subjective (no feeling of milk “tide” and „warming” in the breasts, the newborn not falling asleep after feeding, the interval between feedings less than 1 hour) symptoms of impaired lactogenesis. Statistical differences between axillary and mammary temperatures were detected only in the control group at 5–6 days after delivery, which is evidence of impaired microcirculatory and metabolic processes in women with MS, whose mammary glands are not prepared for lactation.
There was also a marked increase in the level of PRL in healthy puerperal women by days 5–6 (Table 2). The women with MS showed less significant changes in PRL levels, which were 1.4 and 1.7 times lower in subgroups IA and IB, respectively, on days 5–6 compared with the controls. The impaired release of this leading lactation regulator is explained by the insufficiency of the synthesizing and receptor units in hypothalamic-pituitary dysfunction, which is manifested by the quantitative and qualitative insufficiency of the mammary gland secretion.
In the postpartum period (the 2nd, 5–6th days), Group I was found to have pro-inflammatory status with an increase in IL-6 (68 pg/ml [53–77] and 53 pg/ml [41–62]) LII (2.09 [1.89–2.16] and 1.56 [1.43–1.62]) with a concurrent decrease in the anti-inflammatory cytokine IL-10 (12.8 pg/ml [9.6–14, 9] and 16.4 pg/ml [14.7–18.8]), which reflects the imbalance between inflammatory and proliferative processes in the tissues of the mammary glands [13, 14].
The rates of impaired lactation initiation in the patients with MS in Group I, subgroups IA and IB were 75% (66/88), 83.3% (35/42), and 67.4% (31/46), respectively.
Analysis of the mother’s breast milk supply in relation to the needs of the newborn showed the following structure of the severity of early hypogalactia: most MS patients without obstetric complication had grade I hypogalactia (77.4% versus 22.6% of grade II hypogalactia); among MS patients with obstetric complication the rate of grade II and grade III hypogalactia was comparable to the rate of grade I hypogalactia (54.3% versus 45.7%). The correlation analysis showed a strong (IA) and moderately strong (IB) positive correlation between the presence/absence of obstetric complications in women with MS and the severity of hypogalactia (r = 0.84 for IA; r = 0.72 for IB, p <0.05). These findings objectify the clinical and pathogenetic parallels between the nature of the course of pregnancy in patients with MS and functional insufficiency of the mammary glands with its leading role in the impairment of gestational mammogenesis and lactogenesis.
Existing predictors of impaired lactation initiation have low prognostic significance, as they reflect only some of the mechanisms of the pre-lactation transformation of the mammary glands. Greater accuracy of the prediction of early hypogalactia should be sought in a unified, standardized multi-component approach to predicting [2, 5]. The prognostic value of the selected indicators of the pre-lactation transformation was calculated using the univariate logistic regression analysis and ROC-analysis. At the end of the third trimester of gestation, the following indicators had the greatest informative value in relation to the effect on the development of early hypogalactia: an increase in S/D in the lateral thoracic arteries (AUC = 0.72, OR = 8.94 [95% CI 1.57–50.87], p = 0.014) a “reproductive” ultrasound morphotype (AUC = 0.66, OR = 3.69 [95% CI 1.09–12.48], p = 0.035), an increase in leptin level (AUC = 0.68, OR = 3.94 [95% CI 1.46–8.27], p = 0.013), a decrease in PL levels (AUC = 0.71, OR = 1.75 [95% CI 1.13–2.69], p = 0.011) and UE (AUC = 0.70, OR = 1.40 [95% CI 1.10–1.78], p = 0.006). The use of a comprehensive grading scale of PI severity with the calculation of total score was also found informative (AUC = 0.71, OR = 1.45 (95% CI 1.02–2.05), p = 0.039).
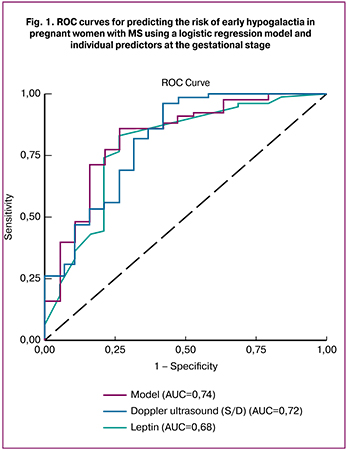 In a multivariate analysis, including indicators of gestational pre-lactation transformation, MS, pro-inflammatory status and state of the FPU, a multivariate binary logistic regression model was developed. The calculation of the resultant integrated model (Z) based on the laboratory test results and diagnostic examination data of the pregnant woman and the regression fits the probability functions of the following form: Z = – 4.263 × EPD + 0.098 × Leptin +1.426 × TG / HDL-C ratio + 0.042 × TNFα – 0.853 × PL + 2.077 × Reproductive ultrasound morphotype + 2,634 × S/D lateral thoracic arteries – 33.246. The high prognostic significance of the model is confirmed: AUC = 0.74; standard error of AUC = 0.08; p = 0.006. The quality of prediction of early hypogalactia using the gestational model with the separation point d = 0.78: Se = 74%, Sp = 81% (p <0.05). Based on the Z value, the probability of occurrence of early hypogalactia (Р = ez/1 + ez) is calculated: the value d> 0.79 indicates a high risk of developing this condition and the need for preventive measures. The accuracy of individual predictors and predictive model of impaired lactogenesis is presented in Fig. 1.
In a multivariate analysis, including indicators of gestational pre-lactation transformation, MS, pro-inflammatory status and state of the FPU, a multivariate binary logistic regression model was developed. The calculation of the resultant integrated model (Z) based on the laboratory test results and diagnostic examination data of the pregnant woman and the regression fits the probability functions of the following form: Z = – 4.263 × EPD + 0.098 × Leptin +1.426 × TG / HDL-C ratio + 0.042 × TNFα – 0.853 × PL + 2.077 × Reproductive ultrasound morphotype + 2,634 × S/D lateral thoracic arteries – 33.246. The high prognostic significance of the model is confirmed: AUC = 0.74; standard error of AUC = 0.08; p = 0.006. The quality of prediction of early hypogalactia using the gestational model with the separation point d = 0.78: Se = 74%, Sp = 81% (p <0.05). Based on the Z value, the probability of occurrence of early hypogalactia (Р = ez/1 + ez) is calculated: the value d> 0.79 indicates a high risk of developing this condition and the need for preventive measures. The accuracy of individual predictors and predictive model of impaired lactogenesis is presented in Fig. 1.
The study findings expand the scientific and practical knowledge of the realization of the adaptive-trophic function in the postpartum period in women with MS. The impairment of pre-lactation transformation is realized through the insufficiency of the “placenta-mammary glands” system co-occurring with hypothalamic-pituitary dysfunction.
Based on the pathogenesis of MS and the features of the neonatal period, a non-pharmacological approach to the prevention of early hypogalactia is obvious. The method is notable for availability, safety, and efficiency. The control of lactogenesis in the puerperal women in Group II, who received the comprehensive non-pharmacological preventive treatment, was carried out according to the algorithm of examination of puerperal women in Group I.
Breast ultrasound on days 4–6 of preventive treatment showed a marked positive trend: the lactation morphotype was found in 91% (87/96) of women versus 28.4% (25/88) in Group I ; S/D in the lateral thoracic arteries (right breast 1.62 [1.49–1.72], left breast 1.63 [1.52–1.74]) was close to these parameters in the control group (right breast – 1,51 [1.43–1.62], left breast 1.55 [1.47–1.64]), p> 0.05 and significantly differed from these parameters in Group I (right breast – 2.14 [1, 79–2.63], left breast 2.19 [1.84–2.71]). These findings indicate a beneficial effect of the preventive treatment on endocrine regulation, metabolic, proliferative processes, the blood supply in the mammary glands resulting in their complete functional transformation.
Women in Group II were found to have a significant increase in the secretory activity of the mammary glands, both on day 4 (303 ± 30 ml) and day 6 (501 ± 36 ml) of the postpartum period. On days 4 and 6, the mother’s breast milk supply in Group II was 1.6 (p <0.05) and 1.6-fold (p <0.05) higher than in Group I, respectively, and was maximally close to the secretory activity in the control group (p> 0.05).
The analysis of the qualitative composition of the mammary gland secretion showed statistically significant differences in the levels of protein, milk fat and lactose in Groups I and II, while there were no significant differences in these parameters between Group II and the controls. We also noted a marked positive change in the clinical course of the lactogenesis period: on days 5–6 puerperal women in Group II had less pronounced subjective (4.2% versus 59%) and objective (3.1% vs. 72.7%) clinical signs of impaired lactogenesis. The results of thermometry on days 5–6 confirmed that breast temperature in women in group II was statistically significantly higher than the axillary temperature, which was similar to the control group. These findings indicate an active functional transformation of the mammary glands resulting from the hormonal, proliferative, metabolic, microcirculatory actions of the complex of corrective measures.
There were statistically significant differences in the basal level of PRL (Table 2) in favor of women in Group II; the difference between the level of PRL stimulated by VIP-light and Group I at 5–6 days was 1710 mIU/L, the difference in Group II the between days 2 and 5–6 was 3093 mIU/L (an increase of 98% versus 72% in Group I). These results indicate the positive synergistic effect of the components of the method of preventing early hypogalactia on the level of this hormone. At the same time, the analysis of basal and induced levels of PRL confirmed the physiological nature of the proposed VIP-light phototherapy, since its stimulating effect is manifested only at low basal levels of PRL and does not produce this effect in patients with normal basal levels of this hormone.
Evaluation of the pro-inflammatory status and reactive potential of puerperal women at days 5–6 confirmed a pronounced therapeutic effect of VIP-light, which was manifested by a decrease in IL-6 and LII levels in Group II (which were 2.4 and 1.3-fold lower for IL-6 and LII, respectively, than in Group I, p <0.05), while the level of anti-inflammatory IL-10 was 3.2-fold higher than in Group I (p <0.05) and 4.4-fold higher than in the controls (p <0.05). These findings confirm the significant anti-inflammatory and immunomodulatory effects of VIP-light as a component of the developed prophylactic complex.
The rates of early hypogalactia in women with MS in Group II was 10.4% (10/96) (χ2 = 78.59, p <0.01, compared to Group I), 5.8% (3/52) in subgroup IIA (χ2 = 42.79, p <0.01, compared with subgroup IA), and 15.9% (7/44) in subgroup IIB (χ2 = 36.38, p <0.01, compared with subgroup IB). The rates of early hypogalactia showed a 64.6% (7.5-fold), 61.6% (11.6-fold), and 67.4% (5.2-fold) decrease in Group II and subgroups IIA and IIB, respectively. At the same time, in Group II, grade I hypogalactia was observed in 8.4% of women (versus 60.6% in Group I), and grade II hypogalactia just in 2% (vs. 28.8%), and no grade III hypogalactia was diagnosed (in Group I – 10.6%), which demonstrates a significant improvement in lactation function in MS patients undergoing the staged comprehensive non-pharmacological prophylaxis. Noteworthy is the decrease in the rates of lactostasis in mothers in group II (2% vs. 22.7% in Group I, i.e.11-fold lower).
Therefore, prenatal colostrum expression and VIP-light phototherapy in puerperal women with MS at the stage of lactogenesis contributes to the normalization of both the quantity and the qualitative composition of milk, reducing the rates and severity of early impairment of lactation. Improvement of secretory activity occurs as a result of neuro-hormonal, metabolic, immunomodulating and reflex mechanisms of action of the prophylactic non-pharmacological complex, which is confirmed by the changes in the basal and stimulated PRL level, breast thermometry, subjective and objective clinical symptoms of hypogalactia, and the balance of pro-and anti-inflammatory cytokines. These effects were achieved not only due to an increase in the water component, which is confirmed by the analysis of protein, fat and lactose content. Overall, this result is encouraging with respect to the favorable course of lactopoiesis in puerperal women with MS.
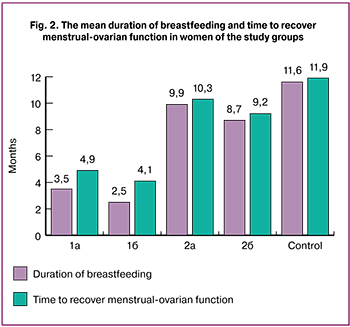 In Group II, the vast majority of women (95.8%) continued breastfeeding for more than 6 months. The reasons for refusal to continue breastfeeding included mainly (72.9%) the mother’s return to work or education, or the child achieving the age of one year. The mean duration of breastfeeding by mothers in Group II (Fig. 2) was 9.4 ± 1.3 months. (6.4 months longer than in Group I).
In Group II, the vast majority of women (95.8%) continued breastfeeding for more than 6 months. The reasons for refusal to continue breastfeeding included mainly (72.9%) the mother’s return to work or education, or the child achieving the age of one year. The mean duration of breastfeeding by mothers in Group II (Fig. 2) was 9.4 ± 1.3 months. (6.4 months longer than in Group I).
The time to recover menstrual-ovarian function in Group II was 9.8 ± 0.6 vs. 4.5 ± 0.5 months in Group I. These findings assert the physiological nature and non-aggressiveness of the preventive therapy for early hypogalactia and the absence of adverse effect on the interdependent neuro-hormonal processes of the body.
An assessment of the health status of infants, whose mothers underwent corrective measures during the first year of life showed a 1.7-fold lower rate of respiratory diseases and 2.4-fold lower rate of manifestations of specific immunological sensitivity.
The use of methodological standards of evidence-based medicine to assess the effectiveness of the developed method for the prevention of early hypogalactia allowed us to obtain the following characteristics. At the stage of lactogenesis: EER = 10%, CER = 75%, RRR = 87% (CI 95% 76–94, p <0.05), ARR = 65% (CI 95% 53–77, p <0.05), NNT = 2 (CI 95% 1–2, p <0.05), OR = 0.04 (CI 95% 0.02–0.1, p <0.05). At the stage of lactopoiesis (taking into account the duration of breastfeeding for more than 6 months): EER = 4%, CER = 90%, RRR = 95% (CI 95% 86–98, p <0.05), ARR = 86% (CI 95% 79–95, p <0.05), NNT = 1 (CI 95% 1–1.17, p <0.05), OR = 0.004 (CI 95% 0.0008–0.009, p < 0.05). Higher key indicators of the effect of preventive intervention at the stage of lactopoiesis indisputably prove the leading role of the stages of lactation initiation – the completion of mammogenesis and the physiological course of lactogenesis – in the full realization of natural breastfeeding.
Conclusion
Lactation is a phylogenetic reaction of the mammary glands after the disintegration of the mother-placenta-fetus system. Changes in the adaptation mechanisms restoring the postpartum newborn-mother bonding in women with MS through natural breastfeeding indicate a serious neuro-endocrine-metabolic dysfunction, which manifests as an impaired lactation.
Women with MS undoubtedly belong to a high-risk group of obstetric patients due to impaired lactation, which is associated both with an insufficient pre-lactation transformation of the mammary glands and a delay or inadequate development of secretory activity during lactogenesis. The created predictive model of the risk of developing early hypogalactia is highly informative, which allows its use for planning preventive measures. The findings of this study suggest that the developed comprehensive staged non-pharmacological preventive treatment of early lactation impairment is more effective than standard basic measures aimed to support breastfeeding in patients with this condition.
The method suggests a possibility to affect the pathogenesis of early hypogalactia (the formation of a reflex component, stimulation of milk production and lactation, improvement of microcirculation, normalization of basal and induced PRL levels, pro-inflammatory, immunomodulating and metabolic effects), thus significantly improving lactation in women with such risk factor for developing early hypogalactia, as MS; it is affordable, safe and can be recommended for routine clinical application.
References
1. Thomas W., Hale W.T., Hilary E. Medications and Mothers’ Milk 2017. Springer Publishing Company. 2017; 17: 1096.
2. Хамошина М.Б., Руднева О.Д., Захарова Н.И., Союнов М.А., Лебедева М.Г., Лукаева Д.Д. Организация грудного вскармливания после самопроизвольных родов. Вестник Российского университета дружбы народов. 2016; 2: 39-46. [Hamoshina M.B., Rudneva O.D., Zaharova N.I., Soyunov M.A., Lebedeva M.G., Lukaeva D.D. Organizaciya grudnogo vskarmlivaniya posle samoproizvol’nyh rodov. Vestnik Rossijskogo universiteta druzhby narodov. 2016; 2: 39-46. (in Russian)]
3. Chowdhury R. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015; 4: 96-113.
4. Серов В.Н. Беременность и метаболический синдром. Омск: АдамантЪ; 2013:159. [Serov V.N. Beremennost’ i metabolicheskij sindrom. Omsk: Adamant”; 2013:159. (In Russian)]
5. Victora C.G., Bahl R., Barros A.J.D. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet Breastfeeding Series Group. 2016; 387: 475-490.
6. Riddle S.W., Nommsen-Rivers L.A., Ochsner J. A Case Control Study of Diabetes During Pregnancy and Low Milk Supply. Cincinnati Children’s Hospital Medical Center. 2016; 16: 4: 511-524.
7. Дындарь М.А., Бенюк В.А. Состояние лактации у женщин с избыточной массой тела и метаболическим синдромом. Охрана материнства и детства. 2014; 1:23: 31-34. [Dyndar’ M.A., Benyuk V.A. Sostoyanie laktacii u zhenshchin s izbytochnoj massoj tela i metabolicheskim sindromom. Ohrana materinstva i detstva. 2014; 1:23: 31-34. (In Russian)]
8. Cluet de Rodríguez I, Rossell-Pineda Mdel R, Álvarez de Acosta T, Chirinos R. Comparison between levels of prolactin of mother in relactation and exclusive breast feeding mothers. Arch Latinoam Nutr. 2014; 64: 1: 1-8.
9. Стрижаков А.Н., Липатов И.С., Тезиков Ю.В., Шарыпова М.А. Стандартизация диагностики и клиническая классификация хронической плацентарной недостаточности. Вопросы гинекологии, акушерства и перинатологии. 2014; 13: 3: 5-12. [Strizhakov A.N., Lipatov I.S., Tezikov Yu.V., Sharypova M.A. Standartizaciya diagnostiki i klinicheskaya klassifikaciya hronicheskoj placentarnoj nedostatochnosti. Voprosy ginekologii, akusherstva i perinatologii. 2014; 13: 3: 5-12. (In Russian)]
10. Углицких А.К., Стенина О.И. Гипогалактия: пути решения проблемы. Практика педиатра. 2013; 3: 22-24. [Uglickih A.K., Stenina O.I. Gipogalaktiya: puti resheniya problemy. Praktika pediatra. 2013; 3: 22-24. (In Russian)]
11. Стрижаков А.Н., Тимохина Е.В., Игнатко И.В., Белоцерковцева Л.Д. Патофизиология плода и плаценты. М.: ГЭОТАР-Медиа; 2015: 176. [Strizhakov A.N., Timohina E.V., Ignatko I.V., Belocerkovceva L.D. Patofiziologiya ploda i placenty. M.: GEHOTAR-Media; 2015: 176. (In Russian)]
12. Anderson S.M. Lactation and its Hormonal Control. Knobil and Neill’s Physiology of Reproduction. 2015; 1: 2055-2105.
13. Van Klompenberg M.K. Regulation and localization of vascular endothelial growth factor within the mammary glands during the transition from late gestation to lactation. Horm Behav. 2016; 77: 167-181.
14. Шафиева К.А., Мальгина Г.Б., Пестряева Л.А. Особенности становления лактационной функции у пациенток после сверхранних преждевременных родов. Акушерство и гинекология. 2016; 2; 83-86. [Shafieva K.A., Malgina GB, Pestryaeva L.A. Features of the formation of lactational function in patients after early premature birth. Akusherstvo i ginekologiya. 2016; 2: 83-86. (In Russian)]
Received 17.02.2018
Accepted 02.03.2018
About the Authors
Tezikov, Yuriy V., PhD, MD, head of department of obstetrics and gynecology № 1 of the Samara state medical University Ministry of Health of Russia.(443099, Samara, 89 Chapaevskaya str.). Tel.: +7-846-9-58-24-18. E-mail: yra.75@inbox.ru. ORCID ID 0000-0002-8946-501Х.
Lipatov, Igor S., PhD, MD, head of department of obstetrics and gynecology № 1 of the Samara state medical University Ministry of Health of Russia.
(443099, Samara, 89 Chapaevskaya str.). Tel.: +7-846-9-58-24-18. E-mail: i.lipatoff2012@yandex.ru. ORCID ID0000-0001-7277-7431.
Pechkurov, Dmitry V., PhD, MD, head of the department of children’s diseases of the Samara state medical University Ministry of Health of Russia.
(443099, Samara, 89 Chapaevskaya str.). Tel.: +7-846-9-59-45-11. E-mail: dmpechkurov@yandex.ru.
Tyutyunnik Victor L., PhD, MD, the head of the obstetric physiological department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-903-969-50-41. E-mail: tioutiounnik@mail.ru.
Number Researcher ID B-2364-2015.ORCID ID 0000-0002-5830-5099.
Kan, Natalia E., PhD, MD, the head of the obstetric department of the National Medical Research Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov Ministry of Health of Russia (117997, Moscow, Ac. Oparina, 4 str.). Tel.: +7-926-220-86-55. E-mail: kan-med@mail.ru.
Number Researcher ID B-2370-2015. ORCID ID 0000-0001-5087-5946.
Protasov, Andrey D., PhD, associate professor, department of microbiology, immunology and allergology of the Samara state medical University Ministry of Health of Russia. (443099, Samara, 89 Chapaevskaya str.). Tel.: +7-846-9-58-24-18. E-mail: crosss82@mail.ru.
Kovyazina, Irina O., postgraduate student of department of obstetrics and gynecology № 1 of the Samara state medical University Ministry of Health of Russia.
(443099, Samara, 89 Chapaevskaya str.). Tel.: +7-846-9-58-24-18.
For citations: Tezikov Yu.V., Lipatov I.S., Pechkurov D.V.,Tyutyunnik V.L., Kan N.E., Protasov A.D., Kovyazina I.O. Predicting impairment of pre-lactation transformation and preventing lactogenesis failure in metabolic syndrome. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 60-8. (in Russian)
https://dx.doi.org/10.18565/aig.2018.11.60-68



