Переход к менопаузе является важным событием в здоровье женщин. Данный период связан с появлением таких симптомов, как приливы, проблемы со сном, увеличение веса, а также сухость во влагалище, диспареуния и снижение выделения естественной смазки, что может отрицательно повлиять на качество жизни женщины. С менопаузой связано снижение функции яичников, что приводит к уменьшению уровня половых гормонов. Средний возраст наступления менопаузы составляет 51–52 года. Учитывая текущую продолжительность жизни, около 25 лет жизни женщины приходится на постменопаузальный период. Значит, не менее трети своей жизни женский организм проводит без эстрогенной поддержки, и избежать симптомов, связанных с эстрогенным дефицитом, без медикаментозной поддержки зачастую невозможно. По ряду причин многие женщины с симптомами не обращаются за лечением, а из тех, кто обращается, многие недовольны результатами проведенного лечения [1]. Поэтому таким пациенткам важно предлагать альтернативные методы для лечения симптомов менопаузы [2].
Не менее важной является проблема мочеполового синдрома менопаузы, ранее известного как вульвовагинальная атрофия, атрофический вагинит или урогенитальная атрофия, представляющая собой совокупность симптомов. Данное состояние связано со снижением уровня эстрогенов, что приводит к изменению в больших и малых половых губах, клиторе, влагалище, уретре и мочевом пузыре. Генитоуринарным менопаузальным синдромом (ГУМС) в той или иной степени страдают до 50% женщин в период менопаузы. Однако по сей день мочеполовой синдром менопаузы остается крайне недостаточно диагностируемым, несмотря на его высокую распространенность, главным образом, по причине нежелания женщин обращаться за помощью из-за смущения или в результате тенденции многих пациенток рассматривать его как нормальную особенность данного периода жизни [1, 3].
Важной отличительной особенностью мочеполовых климактерических расстройств является тот факт, что по прошествии времени симптомы не облегчаются, а значительно усиливаются, поскольку отражают реакцию «эстрогенно настроенных» тканей на отсутствие адекватной гормональной поддержки. Своевременное выявление и грамотно подобранная фармакологическая терапия являются важнейшими способами не только улучшения качества жизни пациенток с ГУМС, но и предотвращения нарастания таких симптомов, как сухость, жжение и раздражение половых органов, а также отсутствие смазки, дискомфорт и боль во время полового акта. Лечение первой линии состоит из негормональных методов лечения, таких как лубриканты и увлажняющие кремы, в то время как гормональная терапия местными препаратами эстрогена обычно считается «золотым стандартом». Однако, несмотря на высокий профиль безопасности, хорошо зарекомендовавшая себя и патогенетически обоснованная менопаузальная гормональная терапия имеет ряд ограничений. Помимо этого, значительно усложняет возможности назначения любых гормональных препаратов негативное отношение некоторых женщин к данному способу лечения. Поэтому поиск альтернативных методов лечения мочеполового синдрома менопаузы особенно важен [3, 4]. Среди различных многочисленных исследований отмечены попытки коллагенизации субмукозных слоев стенки влагалища как способа преодоления сухости путем увлажнения, повышения трофики, прочности, эластичности тканей. В Университетской клинике Сан-Хорхе в отделении урогинекологии в рамках научного исследования была оценена эффективность локальной инъекционной негормональной терапии факторами роста для активации синтеза коллагена и эластина в эпителиальном паттерне влагалища у пациенток, страдающих атрофией влагалища. Результатом данного исследования было не только купирование сухости, но и достижение увеличения толщины эпителия влагалища [5]. В рамках еще одного исследования оценивалось регенеративное влияние мезенхимальных стволовых клеток, полученных из костного мозга и жировой ткани, на атрофические изменения во влагалище в модели менопаузы у крыс. По результатам иммуногистохимических и гистологических исследований был сделан вывод о том, что данная терапия обладает значительным регенеративным потенциалом за счет индукции синтеза коллагена, ремоделирования стенки влагалища при атрофии [6]. На основании результатов приведенных исследований можно сделать вывод о том, что достижение коллагенизации влагалища является перспективным методом лечения ГУМС.
Целью данного исследования является оценка эффективности и безопасности применения биокомплекса «Живой Коллаген» компании «ПЕРВЫЙ ЖИВОЙ КОЛЛАГЕН» при ГУМС.
Материалы и методы
Проведенное исследование является неконтролируемым (before-after study) одноцентровым.
Критерии включения: женский пол, период постменопаузы, подтвержденный диагноз «постменопаузальный атрофический вагинит».
Критерии исключения: мужской пол, репродуктивный возраст, прием комбинированной оральной контрацепции, гестагенов и других гормональных препаратов, беременность и период лактации.
Все участницы исследования были пациентами клиники «Семейная», являющейся базой кафедры акушерства, гинекологии и перинатологии Сеченовского Университета. Сбор данных и оценка эффективности проводимой терапии осуществлялись в период с апреля по июнь 2023 г. Статистическая обработка продолжалась в течение 2 месяцев и была завершена в сентябре того же года.
В исследование включены 25 женщин от 50 до 68 лет (средний возраст составил 59,44±5,39 года) с длительностью постменопаузы 7±2,40 года, предъявляющих жалобы на сухость во влагалище, жжение, дизурические явления, диспареунию. Всем пациентам на основании лабораторных и инструментальных методов обследования был поставлен диагноз «постменопаузальный атрофический вагинит» (МКБ N95.2).
Исследование подразумевало оценку эффекта от перорального приема 15 г в день натощак за 30 минут до еды в течение 3 месяцев биокомплекса «Живой Коллаген» от компании «ПЕРВЫЙ ЖИВОЙ КОЛЛАГЕН», содержащего коллаген I, II, III типов, эластин, аминокислоты (глицин, аланин, валин, лейцин, изолейцин, оксипролин, серин, треонин, пролин, метионин, аспарагиновую, глутаминовую, γ-аминомасляную кислоты, лизин, оксилизин, аргинин, гистидин, тирозин, фенилаланин), макроэлементы (кальций, калий, натрий, магний), микроэлементы (цинк, железо, медь, алюминий, никель), жирные кислоты (пальмитиновую, стеариновую, олеиновую, линоленовую, линолевую). В производстве Живого Коллагена температура не превышает 59°С, он не лиофилизируется, благодаря чему не нарушается структура коротких пептидных цепочек и сохраняется трехспиральная модель вещества. В основе Живого Коллагена – экологически чистое коллагенсодержащее сырье птицы без применения антибиотиков; благодаря этому он обладает низкой иммуногенностью, высокой биосовместимостью и биоразлагаемостью [7].
Программа исследования состояла из 3 визитов пациентов в клинику «Семейная». Первый визит – сбор жалоб, первичный гинекологический осмотр, обследование на предмет симптомов ГУМС, определение критериев включения и исключения, подписание информированного добровольного согласия. Для объективизации состояния пациенткам дали заполнить вопросник с расчетом индекса женской сексуальности (Female Sexual Function Index, FSFI) до назначения биокомплекса «Живой Коллаген» сроком на 3 месяца [8]. Второй визит (день 45±2) – контроль отсутствия побочных эффектов, объективная оценка эффективности терапии живым коллагеном. Повторное заполнение пациентками промежуточного вопросника FSFI (контроль 1). Третий визит (день 90±2) – оценка безопасности и отдаленных результатов терапии. Заключительное заполнение вопросника FSFI (контроль 2).
На основании клинических рекомендаций «Менопауза и климактерическое состояние у женщины» (2021) [9] обследование включало сбор жалоб и анамнеза, осмотр наружных и внутренних половых органов, тщательную оценку состояния вульвы, слизистой оболочки влагалища на предмет атрофии эпителия, осмотр слизистой оболочки шейки матки. Для оценки жалоб использовались следующие критерии: сухость влагалища, боль при половом контакте (диспареуния), жжение, зуд, дизурические явления. Критерии для объективизации данных осмотра: общая увлажненность влагалища, его эластичность, секреция жидкости.
В данное исследование была добавлена адаптированная шкала R. Nappi (2019) [10, 11], используемая для объективизации жалоб и клинических симптомов ГУМС. Визуально-аналоговая шкала R. Nappi дает возможность оценить выраженность каждого из них от 0 до 10 баллов: 0 – нет симптомов; 1–3 – симптомы легкой степени; 4–7 – умеренные симптомы; 8–10 – симптомы тяжелой степени.
Для оценки сексуальной функции был использован вопросник для расчета индекса женской сексуальности – FSFI [8]. Использованный вопросник состоит из 19 вопросов по 6 аспектам половой жизни пациенток: половое влечение или желание, сексуальное возбуждение, выделение смазки, оргазм, удовлетворенность половой жизнью, боль при половом контакте. Каждый из критериев оценки сексуальной функции женщин подразумевает под собой ответы на 2–4 тематических вопроса, оцениваемые баллами от 0 до 6 для параметрического анализа. Для расчета индекса баллы суммировались. Минимальное количество баллов – 2, максимальное – 36. Индекс <26,5 балла указывает на наличие сексуальной дисфункции у исследуемых пациенток.
Таким образом, 6 баллов по каждому пункту свидетельствуют об отсутствии нарушений сексуального здоровья. Незначительный болевой синдром/отсутствие болевого синдрома оценивается максимальным количеством баллов (5–6), в то время как выраженный болевой синдром оценивается минимальным количеством баллов (0–1).
Статистический анализ
Для анализа данных использовалась программа IBM SPSS Statistics 26.0 (IBM, США).
Результаты
Средний возраст пациенток составил 59,4 года. Длительность постменопаузы в среднем составила 7 лет, а длительность возникновения симптомов ГУМС у разных женщин колебалась в пределах 2–10 лет (в среднем – 5,4 года).
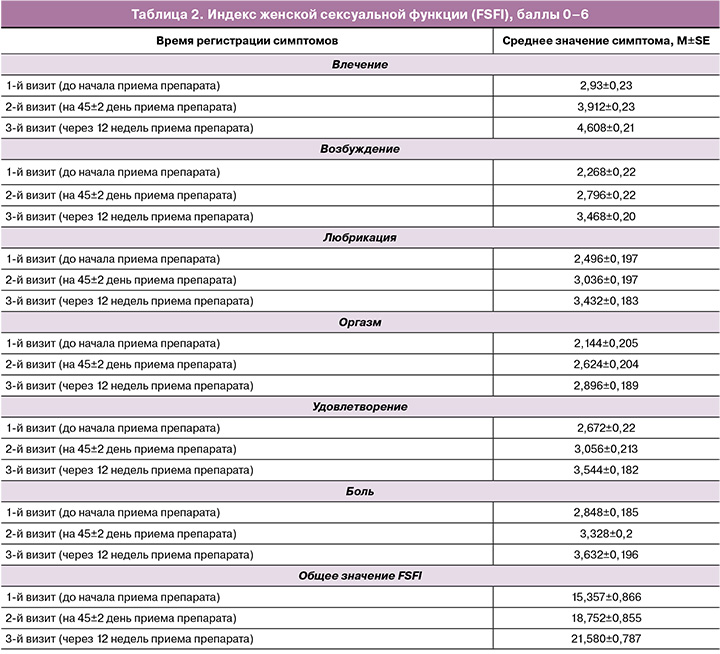
При первом визите наиболее частыми клиническими проявлениями среди женщин с ГУМС были сухость влагалища, зуд, жжение, дискомфорт в области наружных половых органов, диспареуния, кровянистые выделения из половых путей после полового акта, нарушение мочеиспускания по типу императивных позывов и недержания мочи согласно данным адаптированной шкалы R. Nappi. Согласно субъективным жалобам пациенток, наиболее выраженными и ухудшающими качество жизни были сухость, зуд, жжение, раздражение в области влагалища и вульвы. Меньший дискомфорт женщины отмечали при дизурических расстройствах и диспареунии. Среднее значение FSFI составило 15,4 балла. При объективном первичном осмотре зачастую регистрировались снижение общей увлажненности, секреции жидкости, эластичности, уменьшение толщины эпителия (атрофические явления), изменение цвета слизистых наружных половых органов.
При втором визите, на 45-й день приема Живого Коллагена, регистрировались как субъективные улучшения – снижение выраженности сухости, зуда, жжения в области влагалища и вульвы, так и объективные клинические – в ходе гинекологического осмотра было зарегистрировано повышение общей увлажненности влагалища, секреции жидкости, увеличение эластичности и толщины влагалищного эпителия, у некоторых пациенток отмечалось изменение цвета слизистых с бледного до розового. Нежелательных побочных эффектов, связанных с приемом Живого Коллагена, выявлено не было. Промежуточный подсчет FSFI показал положительную динамику – показатель увеличился с 15,4 до 18,8 балла, однако установленная нижняя граница нормы данного показателя (26,5) достигнута не была.
При третьем визите, после пройденного курса терапии в течение 12 недель, регистрировались улучшения в отношении всех клинических проявлений ГУМС. FSFI увеличился с 15,4 до начала терапии до 21,6 балла после приема препарата коллагена в течение 12 недель. Нежелательных побочных эффектов в течение всего периода приема Живого Коллагена зарегистрировано не было.
Кроме того, женщины, принимавшие Живой Коллаген, отметили улучшения в эмоциональной сфере, что может быть связано с улучшением качества жизни вследствие снижения выраженности симптомов ГУМС.
Результаты оценки субъективных (согласно данным адаптированной шкалы R. Nappi) и объективных симптомов представлены в таблице 1, а динамика FSFI – в таблице 2.
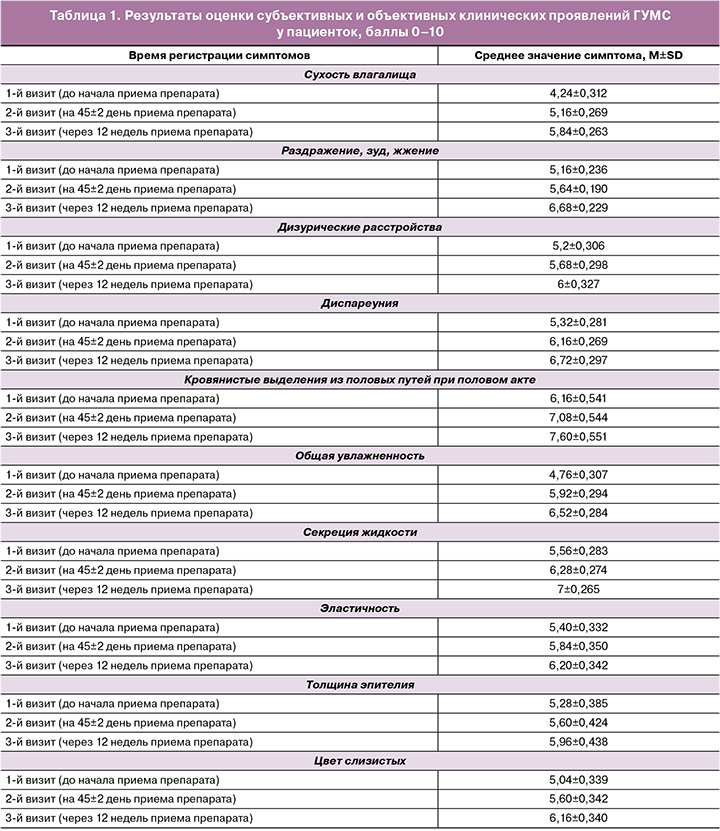
Ограничения исследования связаны с малой выборкой пациенток. Тем не менее, по нашим данным, полученным при анализе заполненных пациентками визуально-аналоговых шкал R. Nappi, отмечено снижение выраженности всех клинических симптомов ГУМС в 1,13–1,4 раза.
Следует отметить, что FSFI не достиг нижнего порогового значения нормы (26,5 балла), однако вырос в 1,4 раза; в отдельных его составляющих было отмечено повышение показателей в 1,6 раза и более, что, вероятно, связано не столько с сексуальной дисфункцией, сколько с особенностями состояния женщин постменопаузального периода с выраженными симптомами ГУМС.
Заключение
Для пациенток, страдающих от различных симптомов ГУМС и имеющих противопоказания к менопаузальной гормональной терапии, возможной альтернативой для повышения качества жизни может стать прием биокомплекса «Живой Коллаген» в дозе 15 г/день за 30 минут до еды в течение 12 недель.



