Predictors of spontaneous puberty and premature ovarian insufficiency in girls with Turner syndrome
Turchinets A.I., Uvarova E.V., Kumykova Z.Kh., Mamedova F.Sh.
Background: Turner syndrome is one of the most common sex chromosome abnormalities associated with premature ovarian insufficiency. Phenotypic manifestations often do not correlate with the karyotype of Turner syndrome; 20% of girls exhibit timely sexual development, and 5–10% experience spontaneous menstruation. Therefore, identifying significant predictors of preserved ovarian function in this cohort of patients is essential.
Objective: To investigate the prognostic markers of spontaneous puberty and premature ovarian insufficiency in girls with Turner syndrome, focusing on age and karyotype.
Materials and methods: This retrospective study included 122 girls with Turner syndrome, aged 9–17 years, who were observed in the 2nd Gynecological Department of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Patients were divided into groups based on age and karyotype variants, with subsequent rankings according to the preservation of ovarian function. Clinical and medical history data, serum hormone levels, and ultrasound findings of the gonads and mammary glands were also assessed.
Results: Spontaneous puberty was observed in 49% of the patients, with 11% experiencing menarche at a mean age of 12.62 (0.37) years; however, 41% displayed no signs of puberty. During the examination, premature ovarian insufficiency was diagnosed in 81% of the patients. No association was found between the absence of spontaneous sexual development and the set of chromosomes, including X monosomy; however, the risk of premature ovarian insufficiency was associated with ploidy of the X chromosome. The risk of detecting FSH levels greater than 25 IU/L had an inverse correlation with ovarian volume, increasing at a value of less than 1.29 cm³.
Conclusion: Despite the karyotype and characteristics of sexual development, ovarian reserve should be assessed in patients with Turner syndrome starting from prepubertal age. This assessment should consider the dynamics of blood hormone levels and ovarian volume measured using ultrasound, which serves as a valuable marker for the risk of ovarian insufficiency in this cohort of patients.
Authors’ contributions: Uvarova E.V., Turchinets A.I. – conception and design of the study, data analysis; Mamedova F.Sh., Kumykova Z.Kh. – data collection and processing; Turchinets A.I. – drafting of the manuscript; Uvarova E.V.,
Kumykova Z.Kh. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Turchinets A.I., Uvarova E.V., Kumykova Z.Kh., Mamedova F.Sh.
Predictors of spontaneous puberty and premature ovarian insufficiency in girls with Turner syndrome.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 86-93 (in Russian)
https://dx.doi.org/10.18565/aig.2024.239
Keywords
The most common cause of premature ovarian insufficiency (POI) in adolescent girls is sex chromosome abnormalities, including Turner syndrome (TS), which has an incidence of 1 in 2,500 live births in females [1, 2]. In half of the girls with TS, the karyotype is X-monosomy (45,X); about 15–25% are due to 45,X/46,XX mosaicism, while the remainder are attributed to structural abnormalities of the X chromosome and various mosaic variants (such as 45,X/46,X,i(Xq); 45,X/47,XXX; 45,X/46,X,del(Xp); 45,X/46,X,r(X); 45,X/46,XY, etc.) [3]. The likelihood of spontaneous puberty (SP) in TS is low: only 20% of girls experience spontaneous thelarche, 16% experience menarche, and only 5–10% achieve regular menstruation [4–6]. The probability of spontaneous pregnancy in women with TS is approximately 2% or higher with a mosaic karyotype; however, anecdotal reports of spontaneous pregnancy in women with X-monosomy, including patients without a history of SP, suggest that ovarian reserve (OR) may be reduced regardless of the peripheral karyotype [7–9].
Over the past 14 years, various laboratory markers of OR in patients with TS and their threshold values for predicting SP and POI have been actively studied globally [10–15]. However, according to the 2024 clinical guidelines for managing patients with TS, the results of pelvic ultrasound examinations in girls with TS are yet to demonstrate their usefulness as markers for SP. Moreover, there are no publications assessing ovarian volume as a predictor of POI in TS [3, 15, 16]. Many studies have presented conflicting data regarding the effect of karyotypes on the frequency of SP. Lunding S.A. et al. (2015) [12] and Hankus M. et al. (2018) [16] reported that girls with X-monosomy have a significantly lower chance of spontaneous puberty than those with other karyotype variants. In contrast, Wang J. et al. (2023) found no correlation between karyotypes and the presence of SP in patients from this cohort [15]. The recommended timing for assessing OR and counseling regarding reproductive options also varies. Oktay K. et al. (2016) recommend diagnostics as early as possible, starting in the neonatal and infancy periods [17], while Fitz V.W. et al. (2021) suggest assessment at age 10–12 years [18]. The Russian clinical guidelines of 2014 indicate that tests for follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels should be performed at ages 13–14 years before initiating estrogen therapy [19]. In contrast, the 2024 European Association of Endocrinologists guidelines proposed assessing gonadotropin and anti-Müllerian hormone (AMH) levels as early as 8–9 years, or even earlier [3].
Therefore, studying the predictors and timing of POI in this patient cohort is an urgent task to optimize the assessment of the reproductive prognosis in girls with TS.
The aim of this study was to evaluate the prognostic markers of SP and POI in girls with TS according to age and karyotype.
Materials and methods
A retrospective study was conducted on 122 girls aged 9 to 17 years, all with confirmed TS without a Y chromosome in their karyotype, based on cytogenetic results. The participants had no history of ovarian surgery and had not received hormonal or gonadotoxic therapy. All girls underwent an inpatient examination to determine the necessity of initiating sex steroid hormone therapy in the 2nd Gynecological Department of the V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia in the period from 2001 to 2021.
To assess the relationship between preserved ovarian function, age, and karyotype, the patients were divided into groups: group A included girls with a monosomal karyotype <12 years (n=8); group B included girls with a mosaic karyotype or structural abnormalities of the X chromosome <12 years (n=7); group C included girls with a monosomal karyotype ≥12 years (n=53); and group D included girls with a mosaic karyotype or structural abnormalities of the X chromosome ≥12 years (n=54)). The rates of different karyotypic variants in groups B and D were represented by a mosaic karyotype with 45,X/46,XX cell clones with a structural anomaly of one X chromosome in 50% (n=31); 46,XX karyotype with a structural anomaly of one X chromosome in 32% (n=20); mosaic karyotype without structural anomalies of the X chromosome (45,X/46,XX, 45,X/47,ХХХ, 45,X/46,XX/47,ХХХ) in 18% (n=11). The first stage included statistical analysis of the data in the selected groups, followed by a comparison of the indicators between the groups of patients comparable by age or karyotype (A–B, A–C, B–D, and C–D).
In the second stage, to identify clinical and instrumental prognostic signs of primary ovarian insufficiency (POI), all girls were further divided into three groups: group I included girls with spontaneous puberty (SP) and FSH<25 IU/L (n=23); group II included girls with a history of SP but with arrested puberty and FSH>25 IU/L (n=36); and group III included girls without the initiation of SP and FSH>25 IU/L (n=62) [20, 21].
The patients underwent evaluation of clinical and anamnestic data (life and disease history, clinical examination, sexual development according to Tanner stages, anthropometric data, and examination of the external genitalia), serum hormone analysis (LH, FSH, estradiol, AMH) via electrochemiluminescence on the automated analyzer Cobas e411, and ultrasound evaluation of the pelvic organs on the Vivid-q device using a linear and convex sensor with a frequency of 1.8–6.0 MHz. Thelarche was confirmed by visualization of the stroma and glandular tissue, as indicated by ultrasound findings of the mammary glands on the Vivid-q device.
Statistical analysis
Statistical analysis was performed using MS Excel and Prism version 9. Student's t-test was used for normally distributed variables, while the Mann–Whitney U-test was used for non-normally distributed variables to calculate the p-value of significance when comparing independent samples. Pearson’s correlation coefficient was used to assess dependent data. For categorical data, a contingency table analysis was performed using Fisher's exact test for small sample sizes. The threshold for continuous data was determined by constructing a ROC curve. Correlation and ROC analyses were conducted in 102 patients who submitted complete laboratory and instrumental research protocols. Differences in statistical values were considered significant at p<0.05 (95% confidence interval).
Results
The mean age of 15 patients in groups A and B was 10.43±0.68 years, while the mean age of 107 girls in groups C and D was 15.14±1.55 years. The mean age at karyotyping and diagnosis was 11.22±0.42 years, and the mean age at consultation with a gynecologist was 15.03±0.19 years.
The mean values of FSH, LH, estradiol, and ovarian volume in groups A–D are presented in Table 1. In younger girls from groups A and B, FSH levels were significantly lower than those in groups C and D, regardless of the karyotype (p>0.05). In the case of monosomy, the difference between the younger and older age groups was two-fold, whereas in the groups with other karyotype variants, it was four-fold (p=0.0079, p=0.0117). Furthermore, the FSH level in girls with monosomy from group C was 18% higher than in group D (100.90 IU/L vs. 83.56 IU/L, respectively, p=0.0423). When assessing LH values, a significant difference was observed between the patients in groups B, A, and D (p=0.0380, p=0.0001). However, as shown in Figure 1A, there was no correlation between the FSH levels (p>0.05). The estradiol level in all groups did not exceed the lower limit of the reference value, and in 55% of patients (n=67), it was below the sensitivity threshold of the reagent used (73.40 pmol/L), regardless of age. No statistically significant difference was noted between estradiol levels in the selected groups (p>0.05). Total estradiol levels were negatively correlated with FSH values (correlation coefficient -0.37, p=0.0181), mainly in older groups C and D (p=0.0270, p=0.0483), with no correlation observed in younger girls (Fig. 1B). Based on the medical history data, AMH levels could be clarified only in 45 girls, of whom 67% (n=30) were below the sensitivity limit of the reagent used, while 33% (n=15) were detectable, averaging 0.03 ng/ml. Fisher's exact test was used to compare the detectability of AMH in serum, and no significant difference was found between patients in the study groups (p>0.05). Ovaries were not visualized during ultrasound examination (US) in 41% of the girls (n=50). The ovarian volume in all groups was lower than the lower limit of the reference value for this age group (Table 1). Additionally, the ovarian volume of younger girls from group A was almost twice as large as that of their peers from group B with other karyotype variants (p=0.0433). At the same time, older girls in group D showed a threefold increase in ovarian volume compared to younger patients in group B (p=0.0061), with no dependence of the indicator on age among girls with a monosomic karyotype (p>0.05). A significant inverse correlation was found between FSH levels and ovarian volume (correlation coefficient -0.37, p=0.0001), mainly in older age groups C and D (p=0.0080, p=0.0044) (Fig. 1C).
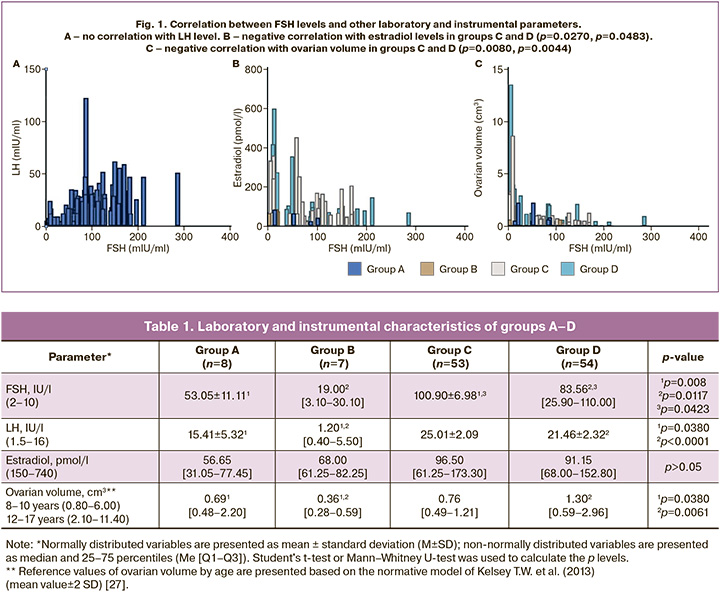
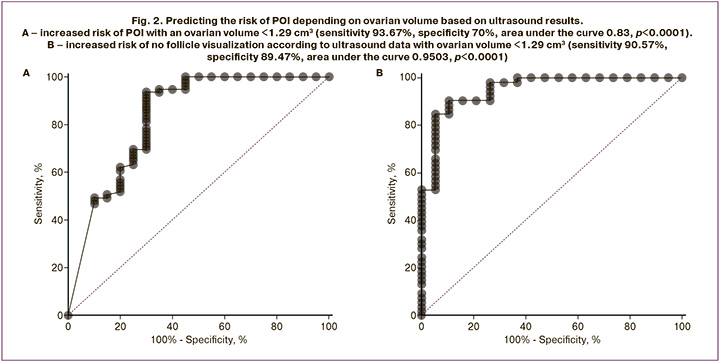
During ultrasound examination, ovarian follicles were detected in only 16% of the patients (n=19). A significant relationship was found between ultrasound visualization of follicles and the presence of minimal but laboratory-detectable AMH levels in the blood serum of girls with TS (p<0.001). As shown in Figure 2, the analysis of the ROC curve indicated that the risk of detecting a serum FSH level >25 IU/L increased with an ovarian volume of <1.29 cm3 (sensitivity 93.67%, specificity 70.00%, area under the curve 0.83, p<0.0001). With the same ovarian volume, the risk of no follicle visualization according to ultrasound data had a sensitivity of 90.57% and specificity of 89.47% (area under the curve 0.95, p<0.0001).
Considering the literature indicating that a significant increase in serum FSH levels is observed from the age of 10 years, which is considered the initial age for detecting SP, the assessment of ovarian function preservation (groups I–III) was performed in 121 girls aged ≥10 years [20, 22, 23]. In group I, consisting of girls with ongoing SP (n=23), the mean age was 13.48±0.55 years. Among these girls, 15 exhibited sexual development corresponding to Tanner stages 2–3, while eight patients with spontaneous menstruation were at stages 3–5, with a mean age of menarche of 13 [12–14] years. All girls with ongoing menstruation reported menstrual cycle disorders such as abnormal uterine bleeding and/or oligomenorrhea. In group I, 7 of 23 (30%) patients had a monosomic karyotype, and 6 of 23 (26%) had a mosaic karyotype (45.X/46.XX, or 45.X/47.XXX, or 45.X/46.XX/47.XXX) without structural abnormalities of the X chromosome (Table 2). Notably, trisomy on the X chromosome did not worsen the prognosis of ovarian function. The average ovarian volume in this group was 2.96 cm³, and follicles were detected in 65% of the girls (n=15) based on ultrasound findings. Furthermore, the analysis of mean serum hormone levels in group I revealed a moderately elevated FSH level (11.63 IU/L) alongside low AMH (median 0.06 ng/ml), with 5 girls having an undetectable AMH level (Table 3).
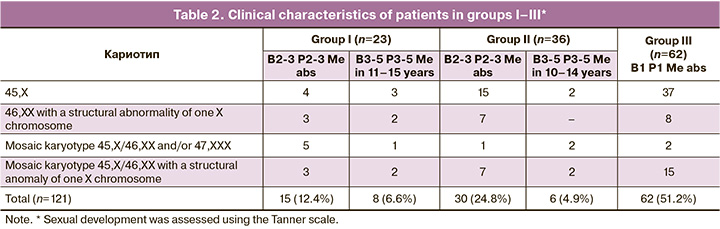
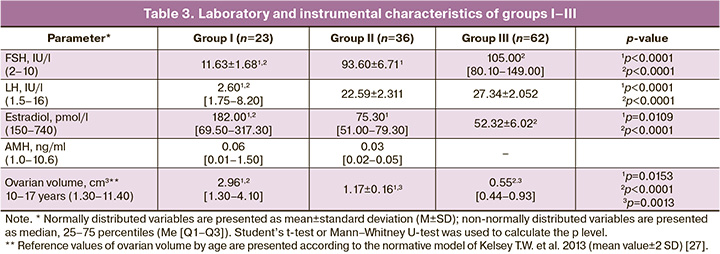
Group II included 36 girls aged 14.70±0.28 years who had initiated SP but experienced subsequent delayed puberty or secondary amenorrhea, with spontaneous menarche occurring at a mean age of 12.50 [11.50–13.25] years. In group II, 30 girls ceased puberty at Tanner stages 2–3. The duration of spontaneous menstruation in the 6 girls ranged from 4 months to 4 years. Half of the patients in group II had a 45,X karyotype (n=17), and three (8%) exhibited 45,X/46,XX and/or 47,XXX mosaicism without structural abnormalities of the X chromosome. All girls in this group had confirmed hypergonadotropinemia with a mean estradiol level of 75.30 pmol/L, which was below the laboratory reference interval. Among them, three girls had a detectable AMH level not exceeding 0.05 ng/ml. The mean ovarian volume at the time of examination in group II was 1.17±0.16 cm³.
In group III, all patients (n=62) exhibited no signs of sexual development, with a mean age at the time of assessment of 14.83±0.27 years. More than half of the girls in this group had an X-monosomal karyotype (n=37), while only two (3%) had a karyotype with a 46,XX cell line. During the examination, hypergonadotropic hypogonadism was confirmed in all patients in group III; the AMH level was undetectable, and the average ovarian volume was 0.55 [0.44–0.93] cm³.
When comparing patients across the studied groups, it was found that the mean age of girls in group I, who were undergoing SP, was over one year younger than that of girls in groups II and III (p=0.0321, p=0.0188). However, no statistically significant age difference was observed between groups II and III or between patients with different karyotypes across the three groups (p>0.05). According to the results of the correlation analysis, no correlation was found between FSH levels and ovarian volume in groups I–III. As indicated in Table 3, the mean serum hormone values in groups II and III did not differ significantly (p>0.05), but the ovarian volume in group II was significantly larger (1.7 times) than that in group III (p=0.0013). An assessment of the influence of karyotype on the preservation of ovarian function in groups I–III using Fisher's exact test revealed that the presence of a monosomic karyotype was not a risk factor for the absence of SP (thelarche and menarche). However, it increased the risk of developing POI (p=0.08, p=0.034), whereas the presence of the 46,XX cell line (n=10) in the karyotype was significantly associated with both the presence of SP and the absence of POI (p=0.013, p<0.01).
Discussion
On average, 3.5 years elapses between diagnosis and the first visit to an obstetrician-gynecologist, during which time the ovarian function was not assessed. Clinical and anamnestic examinations of girls with TS over 10 years of age revealed spontaneous thelarche in 48.7% (n=59) and menarche in 11% (n=13); however, at the time of admission to the department, only 19% of the patients (n=23) had ongoing SP. The mean age of menarche in groups I and II was 12.62±0.37 years, corresponding to the mean population values among healthy girls, and did not differ between girls with X monosomy and other karyotype variants studied (p>0.05), which is in agreement with studies [10, 24]. However, the clinical and diagnostic characteristics of patients in group I, specifically menstrual dysfunction, excessive FSH levels, and low AMH levels, indicate ovarian dysfunction and a risk of POI.
Among all the studied OR parameters in patients with TS over 12 years of age, a significant negative correlation was noted between FSH levels and both estradiol and ovarian volume, whereas no relationship was observed with LH values, as reported by Carpini S. et al. (2018) [20]. Until 2019, the study of AMH and estradiol levels in the groups was limited by the low sensitivity of the reagents.
When comparing FSH, estradiol, and AMH levels in the blood serum of girls with TS under 12 years of age, no significant differences were found based on the karyotype. Given the lack of correlation between estradiol, ovarian volume, and FSH in groups A and B, their use as OR markers at ages up to 12 years without dynamic monitoring of changes is likely to be limited. However, since girls with a mosaic karyotype and/or structural abnormalities of the X chromosome exhibit a greater increase in FSH levels and ovarian volume with age, it is probable that ovarian function depletion in patients with X monosomy occurs at a faster rate. This assumption is supported by the fact that the FSH level in the group of patients with X monosomy aged 12 years and older was significantly higher than that in the group with other karyotypes.
When assessing the distribution of girls in groups I–III based on age and karyotype, it was also found that X monosomy was not associated with the risk of absence of SP but significantly increased the risk of POI up to 18 years. This finding aligns with the results of Fitz V.W. et al. (2021), in which linear regression analysis indicated that an increase in the number of 45,X cells in the peripheral karyotype by 1 percentage point was highly likely associated with a decrease in the age of POI by 0.09 years [18]. In contrast, the presence of the 46,XX cell line in peripheral blood cells was significantly associated with both the presence of SP and preserved ovarian function. This is consistent with the conclusion of Lunding et al., who reported in 2015 that most girls with a 45,X/46,XX mosaic karyotype retained ovarian function after reaching 18 years of age [12]. However, despite notable phenotypic differences, the study confirmed that POI was detected in 81% of patients who visited a doctor at the age of 15 years, with more than half of these cases occurring before the onset of SP. A limitation in establishing the exact age of ovarian function exhaustion in our study was the lack of retrospective data on the dynamics of individual laboratory and instrumental markers of POI due to the late presentation of patients. In 2019 and 2021, Fitz V.W. et al. reported that, according to their retrospective observation (n=79), the average age of POI with a monosomic karyotype was 10 years, whereas for other karyotypic variants, it ranged from 13 to 15 years. The period between the diagnosis of Turner syndrome (TS) and ovarian exhaustion was 2 years [18, 25]. In their longitudinal study, Lunding et al. (2015), AMH decreased to undetectable values in girls with TS over a period of 0.5 to 3 years [12].
Considering the strong correlation between ovarian volume and FSH level among all studied patients, as well as the absence of this correlation in groups I–III, divided by the liminal FSH value, ovarian volume was assessed as a POI risk marker by constructing an ROC curve. An ovarian volume of less than 1.29 cm³ was found to be a predictor of ovarian depletion, with a sensitivity of 93.67% and a specificity of 70.00%. Notably, in girls under 12 years of age, no correlation was observed between FSH levels and ovarian volume, which may limit the use of this criterion in prepubertal age. Conversely, a statistically significant difference in ovarian volume between patients in groups II and III indicated the presence of temporary ovarian function during the pubertal period. In one of the few studies assessing ovarian volume in girls with TS, the mean value in girls with SP was reported to be 1.40±0.90 cm³, which is similar to the threshold value of our result [26].
Ultrasound visualization of follicles is closely associated with the detectability of AMH in blood serum. When the ovarian volume exceeds 1.29 cm³, the probability of detecting follicles significantly increases, demonstrating a sensitivity of 90.57% and a specificity of 89.47%. Therefore, ultrasound assessment of ovarian volume can serve as a valuable diagnostic marker of POI risk in girls with TS.
Conclusion
In the presence of preserved ovarian function, sexual development in girls with TS occurs concurrently with that in the general population and is unaffected by karyotype variants. The risk of POI in TS increases with a monosomic karyotype up to the age of 18 years and decreases in the presence of the 46,XX cell lineage in peripheral blood lymphocytes. A decline in ovarian function in most girls with TS was observed before the age of 15 years, with half of the cases occurring before the age of SP. This decline occurs earlier in X monosomy cases than in other karyotypic variants. An ultrasound ovarian volume of less than 1.29 cm³ is a predictor of POI in girls with TS during puberty. Therefore, it is essential to evaluate ovarian function in girls with TS by the age of 10 years, the age of expected SP, and to monitor it dynamically. This evaluation should consider the combined determination of POI markers significant for this condition: FSH levels, presence of AMH, and ovarian volume based on ultrasound data.
References
- Dowlut-McElroy T., Shankar R.K. The care of adolescents and young adults with Turner syndrome: a pediatric and adolescent gynecology perspective. J. Pediatr. Adolesc. Gynecol. 2022; 35(4): 429-34. https://dx.doi.org/10.1016/ j.jpag.2022.02.002.
- Ye M., Yeh J., Kosteria I., Li L. Progress in Fertility preservation strategies in Turner syndrome. Front. Med. (Lausanne). 2020; 7: 3. https://dx.doi.org/10.3389/fmed.2020.00003.
- Gravholt C.H., Andersen N.H., Christin-Maitre S., Davis S.M., Duijnhouwer A., Gawlik A. et al.; International Turner Syndrome Consensus Group; Backeljauw P.F. Clinical practice guidelines for the care of girls and women with Turner syndrome. Eur. J. Endocrinol. 2024; 190(6): G53-G151. https://dx.doi.org/10.1093/ejendo/lvae050.
- Pasquino A.M., Passeri F., Pucarelli I., Segni M., Municchi G. Spontaneous pubertal development in Turner’s syndrome. J. Clin. Endocrinol. Metab. 1997; 82(6): 1810-3. https://dx.doi.org/10.1210/jcem.82.6.3970.
- Borgström B., Hreinsson J., Rasmussen C., Sheikhi M., Fried G., Keros V. et al. Fertility preservation in girls with Turner syndrome: prognostic signs of the presence of ovarian follicles. J. Clin. Endocrinol. Metab. 2009; 94(1): 74-80. https://dx.doi.org/10.1210/jc.2008-0708.
- Жахур Н.А., Марченко Л.А., Курило Л.Ф., Карселадзе А.И., Бутарева Л.Б., Строганова А.М. Мозаицизм половых хромосом в гонадах у больных с преждевременной недостаточностью яичников. Акушерство и гинекология. 2011; 6: 70-5. [Zhakhur N.A., Marchenko L.A., Kurilo L.F., Karseladze A.I., Butareva L.B., Stroganova A.M. Sex chromosome mosaicism in the gonads of patients with premature ovarian failure. Obstetrics and Gynecology. 2011; 6: 70-5. (in Russian)].
- Bernard V., Donadille B., Zenaty D., Courtillot C., Salenave S., Brac de la Perrière A. et al.; CMERC Center for Rare Disease. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum. Reprod. 2016; 31(4): 782-8. https://dx.doi.org/10.1093/humrep/dew012.
- Mortensen K.H., Rohde M.D., Uldbjerg N., Gravholt C.H. Repeated spontaneous pregnancies in 45,X Turner syndrome. Obstet. Gynecol. 2010; 115(2 Pt 2): 446-9. https://dx.doi.org/10.1097/AOG.0b013e3181cb5b2a.
- Белоконь И.П., Уварова Е.В., Киселева И.А., Яворовская К.А., Ходжаева З.С. Реализация репродуктивной функции у женщин с первичным дефицитом эстрогенов гонадного генеза. Акушерство и гинекология. 2012; 4-2: 109-15. [Belokon I.P., Uvarova E.V., Kiseleva I.A., Yavorovskaya K.A., Khodzhayeva Z.S. Reproductive function realization in patients with primary gonadal estrogen deficiency. Obstetrics and Gynecology. 2012; 4-2: 109-15.(in Russian)].
- Hagen C.P., Aksglaede L., Sørensen K., Main K.M., Boas M., Cleemann L. et al. Serum levels of anti-müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J. Clin. Endocrinol. Metab. 2010; 95(11): 5003-10. https://dx.doi.org/10.1210/jc.2010-0930.
- Visser J.A., Hokken-Koelega A.C., Zandwijken G.R., Limacher A., Ranke M.B. et al. Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum. Reprod. 2013; 28(7): 1899-907. https://dx.doi.org/10.1093/humrep/det089.
- Lunding S.A., Aksglaede L., Anderson R.A., Main K.M., Juul A., Hagen C.P. et al. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 Turner syndrome patients. J. Clin. Endocrinol. Metab. 2015; 100(7): E1030-8. https://dx.doi.org/10.1210/jc.2015-1621.
- Hamza R.T., Mira M.F., Hamed A.I, Ezzat T., Sallam M.T. AntiMüllerian hormone levels in patients with turner syndrome: Relation to karyotype, spontaneous puberty, and replacement therapy. Am. J. Med. Genet. A. 2018; 176(9): 1929-34. https://dx.doi.org/10.1002/ajmg.a.40473.
- Hagen C.P., Fischer M.B., Mola G., Mikkelsen T.B., Cleemann L.H., Gravholt C.H. et al. AMH and other markers of ovarian function in patients with Turner syndrome – a single center experience of transition from pediatric to gynecological follow up. Front. Endocrinol. (Lausanne). 2023; 14: 1173600. https://dx.doi.org/10.3389/fendo.2023.1173600.
- Wang J., Lan T., Dai X., Yang L., Hu X., Yao H. The cut-off value of serum Anti-Müllerian hormone levels for the diagnosis of Turner syndrome with spontaneous puberty. Int. J. Endocrinol. 2023; 2023: 6976389. https://dx.doi.org/10.1155/2023/6976389.
- Hankus M., Soltysik K., Szeliga K., Antosz A., Drosdzol-Cop A., Wilk K. et al. Prediction of spontaneous puberty in Turner syndrome based on mid-childhood gonadotropin concentrations, karyotype, and ovary visualization: a longitudinal study. Horm. Res. Paediatr. 2018; 89(2): 90-7. https://dx.doi.org/10.1159/000485321.
- Oktay K., Bedoschi G., Berkowitz K., Bronson R., Kashani B., McGovern P. et al. Fertility preservation in women with Turner syndrome: a comprehensive review and practical guidelines. J. Pediatr. Adolesc. Gynecol. 2016; 29(5): 409-16. https://dx.doi.org/10.1016/j.jpag.2015.10.011.
- Fitz V.W., Law J.R., Peavey M. Karyotype is associated with timing of ovarian failure in women with Turner syndrome. J. Pediatr. Endocrinol. Metab. 2021; 34(3): 319-23. https://dx.doi.org/10.1515/jpem-2020-0304.
- Волеводз Н.Н. Федеральные клинические рекомендации «Cиндром Шерешевского–Тернера (СШТ): клиника, диагностика, лечение. Проблемы эндокринологии. 2014; 60(4): 65-76. [Volevodz NN. Federal clinical practice guidelines on the diagnostics and treatment of Shereshevsky-Turner syndrome. Problems of Endocrinology. 2014; 60(4): 65-76. (in Russian)]. https://dx.doi.org/10.14341/probl201460465-76.
- Carpini S., Carvalho A.B., de Lemos-Marini S.H.V., Guerra-Junior G., Maciel-Guerra A.T. FSH may be a useful tool to allow early diagnosis of Turner syndrome. BMC Endocr. Disord. 2018; 18(1): 8. https://dx.doi.org/10.1186/s12902-018-0236-4.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Женское бесплодие. M., 2024. [Ministry of Health of the Russian Federation. Clinical guidelines. Female infertility. Moscow; 2024.(in Russian)].
- Conte F.A., Grumbach M.M., Kaplan S.L. A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J. Clin. Endocrinol. Metab. 1975; 40: 670-4. https://dx.doi.org/10.1210/jcem-40-4-670.
- Yen S.S., Vicic W.J. Serum follicle-stimulating hormone levels in puberty. Am. J. Obstet. Gynecol. 1970; 106(1): 134-7. https://dx.doi.org/10.1016/0002-9378(70)90139-0.
- Милушкина О.Ю., Попов В.И., Скоблина Н.А., Бокарева Н.А., Асташкевич Е.В., Захарова А.А., Скоблина Е.В. Влияние фактора миграции на становление менструальной функции у девочек. Вестник РГМУ. 2022; 2: 83-7. [Milushkina O.Yu., Popov V.I., Skoblina N.A., Bokareva N.A., Astashkevich E.V., Zakharova A.A., Skoblina E.V. The influence of migration factor on the establishment of menstrual function in girls. Bulletin of RSMU. 2022; (2): 83-7. (in Russian)]. https://dx.doi.org/10.24075/vrgmu.2022.017.
- Fitz V.W., Law J.R., Peavey M. Characterizing ovarian function by karyotype in a cohort of women with turner’s syndrome. Fertil. Steril. 2019; 111(4 Suppl.): E27-E28. https://dx.doi.org/10.1016/j.fertnstert.2019.02.074.
- Haber H.P., Ranke M.B. Pelvic ultrasonography in Turner syndrome: standards for uterine and ovarian volume. J. Ultrasound. Med. 1999; 18(4): 271-6.https://dx.doi.org/10.7863/jum.1999.18.4.271.
- Kelsey T.W., Dodwell S.K., Wilkinson A.G., Greve T., Andersen C.Y. et al. Ovarian volume throughout life: a validated normative model. PLoS One. 2013; 8(9): e71465. https://dx.doi.org/10.1371/journal.pone.0071465.
Received 22.09.2024
Accepted 28.11.2024
About the Authors
Anna I. Turchinets, PhD student, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(926)522-87-39, Ponomarevaanna28@gmail.com, https://orcid.org/0000-0002-4478-9133Elena V. Uvarovа, Corresponding Member of the Russian Academy of Sciences, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology,
I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University); Head of the Department of Pediatric and Adolescent Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, elena-uvarova@yandex.ru, https://orcid.org/0000-0002-3105-5640
Zaira Kh. Kumykova, PhD, Senior Researcher at the Departament of Pediatric and Adolescent Gynecology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, zai-kumykova@yandex.ru, https://orcid.org/0000-0001-7511-1432
Fatima Sh. Mamedova, PhD, Physician at the Department of Ultrasound Diagnostics of the Department of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Teaching Assistant at the Department of Radiation Diagnostics of Pediatric Age, RMACPE,
Ministry of Health of Russia, f_mamedova@oparina4.ru, https://orcid.org/0000-0003-1136-7222
Corresponding author: Anna I. Turchinets, Ponomarevaanna28@gmail.com



