В мире наблюдается глобальная тенденция к снижению рождаемости [1]. При этом более 15% пар, желающих иметь ребенка, вынуждены обращаться за помощью к вспомогательным репродуктивным технологиям (ВРТ) [2, 3]. К сожалению, средний показатель успеха программы ВРТ около 30% [4]; из-за этого возникает дополнительная эмоциональная и финансовая нагрузка для пациентов.
В программах экстракорпорального оплодотворения (ЭКО) одним из важнейших факторов успешной беременности является качество эмбрионов, а значит, процесс отбора эмбрионов имеет критическое значение. В настоящее время отбор эмбрионов представляет собой ручной процесс, включающий оценку эмбрионов клиническими эмбриологами путем визуальной оценки морфологических признаков с использованием оптического микроскопа. Наиболее распространенной системой оценки, используемой эмбриологами, является шкала Гарднера [5]. Согласно данной классификации, эмбриолог оценивает степень развития эмбриона, качество зародышевого узла и качество трофобласта. Такие критерии допускают высокую вариабельность оценки эмбриона по данной шкале в зависимости от опыта и уровня квалификации эмбриолога [6, 7]. В связи с этим многие научные группы разрабатывают новые технологии, направленные на снижение вариабельности классификации эмбрионов и стандартизирование процедуры оценки, что позволит усовершенствовать процедуру ЭКО и привести к повышению ее эффективности.
На сегодняшний день технологии, использующие искусственный интеллект (ИИ) и машинное обучение, набирают популярность среди медицинского сообщества [8]. ИИ определяется как способность компьютера воспринимать и проявлять интеллектуальное поведение. Первые шаги к ИИ в медицине были сделаны еще в 1960-х гг., когда было введено использование наивного байесовского классификатора (простой вероятностный классификатор), за которым последовали нейронные сети, символическое (с использованием метода дерева принятия решений) и машинное обучение [9] и, наконец, нейронные сети глубокого обучения. Глубокое обучение позволяет компьютеру обнаруживать структурные зависимости в большом наборе данных благодаря использованию алгоритма обратного распространения.
Общая проблема в оценке ИИ и методов машинного обучения в медицинской отрасли заключается в том, что каждая клиническая область уникальна и требует специального подхода для решения данной проблемы. Существует ошибочная тенденция сравнивать точность ИИ в одной клинической области с другой или точность различных подходов ИИ в области, которая оценивает различные конечные точки. Это недопустимые сравнения, так как они не учитывают клинический контекст или значимость конечного результата. Необходимо соблюдать осторожность, чтобы понимать контекст, в котором работает ИИ, и преимущества, которые он обеспечивает в дополнение к текущим клиническим процессам.
Более 20 лет назад Kaufmann J. et al. предложили использовать в программах ВРТ программное обеспечение Cortex Pro, созданное на основе нейронных сетей и состоящее только из четырех критериев оценки (число полученных ооцитов, возраст пациентки, число перенесенных эмбрионов, число замороженных эмбрионов). Точность программы составляла 59%. С тех пор был достигнут значительный прогресс в использования ИИ и машинного обучения в области репродукции человека [10].
В 2018 г. на ежегодном конгрессе Европейского общества репродукции человека и эмбриологии (ESHRE) и Американского общества репродуктивной медицины (ASRM) было представлено несколько исследований на тему ИИ и машинного обучения в области репродукции человека [8].
Использование искусственного интеллекта для оценки качества сперматозоидов в программах ВРТ
Оценка качества спермы производится для изучения фертильности мужчины-партнера в бесплодной паре, а также для подбора донора. Множество работ посвящено использованию ИИ и нейронных сетей для предварительной оценки характеристик спермы и прогнозирования результатов анализа на основе базового опросника пациента. В работе Badura A. et al. описаны две модели нейронных сетей, разработанные на основе данных анкеты, включающей 11 вопросов. Одна из моделей направлена на прогнозирование общих показателей спермы, а вторая – на оценку концентрации сперматозоидов. Точность нейронной сети на тестовой выборке составила 80,95% и 85,71% соответственно. Вопросы в анкете главным образом нацелены на определение наличия негативных факторов в анамнезе пациента – употребление алкоголя и кофеина, курение, работа на вредном производстве и т.п. [11].
Аналогичное исследование показано в работе Ma J. et al. На основе данных о состоянии здоровья и привычек 100 добровольцев и их результатов анализа спермы сравнивали эффективность нейронных сетей различной архитектуры. Как показали авторы, наибольшую точность они получили, используя нейронные сети (НС) обратного распространения для качества спермы и генетический алгоритм для выбора наиболее входных признаков и оптимизации НС. Данный алгоритм авторы сравнивали с такими методами, как многослойный персептрон, метод опорных векторов, дерево принятия решений, сеть радиально-базисных функций [12].
Также стоит упомянуть работы Gil D. et al. и Helwan A. et al. Авторы также разрабатывают НС, определяющие факторы, оказывающие наиболее сильное влияние на качество семенной жидкости и способные прогнозировать качество спермы. Авторы полагают, что использование ИИ имеет большие перспективы в медицинской диагностике, особенно в тех случаях, когда нет возможности провести дополнительные дорогостоящие лабораторные исследования. Было показано, что созданные сети являются идеальным многомерным статистическим методом и являются превосходными прогностическими моделями. Следует подчеркнуть, что, хотя НС никогда не смогут заменить стандартное диагностическое обследование, они могут быть полезным инструментом для оценки состояния пациента, проводимой во время исследования фертильности потенциально бесплодной пары, а также для предварительного отбора доноров спермы [13, 14].
Отдельно стоит отметить, что многие научные группы ставят перед собой более сложную задачу – предсказать по анамнезу пациентов вероятность успешного использования ВРТ. В 2005 г. была описана программа ИИ, способная предсказать исход программы ЭКО/ИКСИ с использованием полученных хирургическим путем сперматозоидов [15]. В качестве входных параметров использовались возраст матери, тип спермы (свежая или криоконсервированная), этиология бесплодия и метод получения сперматозоидов. Обратный регрессионный анализ показал, что значимыми являются только два признака – материнский возраст и тип спермы. Эта программа способна прогнозировать с точностью 82% только неудачные попытки. В другой работе [16] авторы показали, что метод главных компонент позволяет выбрать наиболее важные характеристики пациентов, на основе которых можно смоделировать НС (авторы выбрали многослойный персептрон с несколькими скрытыми слоями), которая со 100% точностью будет предсказывать результат программы ИКСИ. Однако авторы использовали довольно ограниченную выборку – анализ включал всего 62 случая (из которых 29 – с удачным исходом). Для окончательной оценки эффективности предложенного метода необходимы дальнейшие исследования, однако уже сейчас ее можно использовать в качестве вспомогательного метода для корректировки методов лечения.
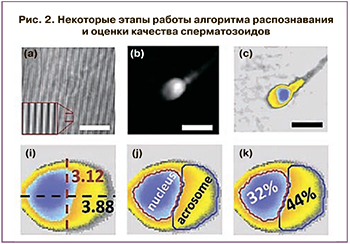
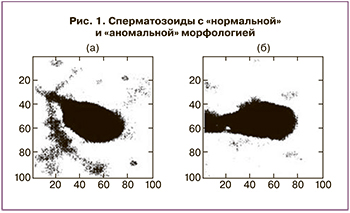 Важнейший в программе ВРТ процесс отбора сперматозоидов для ЭКО основан на субъективном качественном анализе клиническими эмбриологами с использованием неколичественных методов оптической микроскопии. Еще в 1994 г. была представлена работа по созданию НС для морфологической классификации сперматозоидов [17]. В работе были использованы микроскопические изображения препаратов эякулята, окрашенные по методу Папаниколау, прошедшие предварительную Фурье-обработку. Сперматозоиды на каждом изображении были классифицированы в независимых лабораториях как «нормальные» и «аномальные» (рис. 1). Авторы использовали НС прямого распространения с одним скрытым слоем; функция активации нейронов скрытого слоя – гиперболический тангенс.В работе Mirsky S. et al. продемонстрирован метод автоматизированного анализа сперматозоидов, визуализированных с помощью фазово-контрастной микроскопии. Метод основан на количественном фазовом анализе (световая волна проходит через образец с переменным показателем преломления и/или переменной толщиной, в результате чего волновой фронт искажается и меняется фазовое распределение; результирующее распределение фазы, отображающее максимумы и минимумы, изображается в виде фазовой карты). На основе изображений более 1400 сперматозоидов человека от 8 доноров разработан алгоритм распознавания головок сперматозоидов, способный оценивать их морфологию по 58 критериям. На рис. 2 показаны некоторые этапы работы алгоритма. На основе этих критериев производилось обучение классификатора сперматозоидов с хорошей и плохой морфологией методом опорных векторов (SVM). Точность метода SVM составила 90%. За основу алгоритма взяли морфологическую оценку по строгим критериям Крюгера. Авторы считают, что разработанный алгоритм визуализации и оценки может стать основой для объективного и автоматического отбора сперматозоидов в программах ЭКО, в частности для ИКСИ [18].
Важнейший в программе ВРТ процесс отбора сперматозоидов для ЭКО основан на субъективном качественном анализе клиническими эмбриологами с использованием неколичественных методов оптической микроскопии. Еще в 1994 г. была представлена работа по созданию НС для морфологической классификации сперматозоидов [17]. В работе были использованы микроскопические изображения препаратов эякулята, окрашенные по методу Папаниколау, прошедшие предварительную Фурье-обработку. Сперматозоиды на каждом изображении были классифицированы в независимых лабораториях как «нормальные» и «аномальные» (рис. 1). Авторы использовали НС прямого распространения с одним скрытым слоем; функция активации нейронов скрытого слоя – гиперболический тангенс.В работе Mirsky S. et al. продемонстрирован метод автоматизированного анализа сперматозоидов, визуализированных с помощью фазово-контрастной микроскопии. Метод основан на количественном фазовом анализе (световая волна проходит через образец с переменным показателем преломления и/или переменной толщиной, в результате чего волновой фронт искажается и меняется фазовое распределение; результирующее распределение фазы, отображающее максимумы и минимумы, изображается в виде фазовой карты). На основе изображений более 1400 сперматозоидов человека от 8 доноров разработан алгоритм распознавания головок сперматозоидов, способный оценивать их морфологию по 58 критериям. На рис. 2 показаны некоторые этапы работы алгоритма. На основе этих критериев производилось обучение классификатора сперматозоидов с хорошей и плохой морфологией методом опорных векторов (SVM). Точность метода SVM составила 90%. За основу алгоритма взяли морфологическую оценку по строгим критериям Крюгера. Авторы считают, что разработанный алгоритм визуализации и оценки может стать основой для объективного и автоматического отбора сперматозоидов в программах ЭКО, в частности для ИКСИ [18].
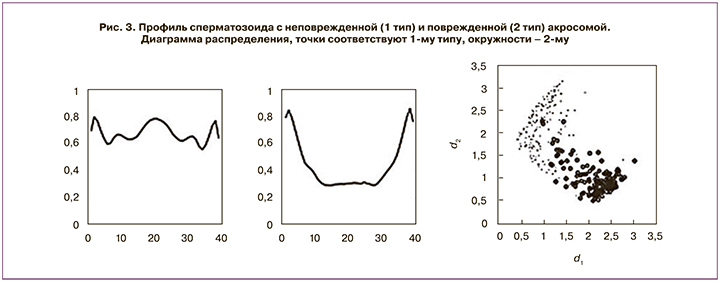
 В другой работе описан способ автоматического разделения сперматозоидов самца свиньи с поврежденной и неповрежденной акросомами [19]. Такая классификация важна для оценки потенциала к оплодотворению образца эякулята. Для анализа используются изображения сперматозоидов, полученные с помощью фазово-контрастного микроскопа. Изображения кадрируются и проходят предварительную обработку. В итоге для каждого сперматозоида вычисляется профиль, определяющий контур клетки (рис. 3). На этих данных авторы пробовали два метрических алгоритма – метод k ближайших соседей и классификатор на основе прототипов. Второй алгоритм показал достаточно высокую точность, несмотря на то, что данные для сперматозоидов с поврежденной и неповрежденной акросомами в некоторой степени перекрываются (рис. 3). Авторы обращают внимание, что целью работы системы является не классификация отдельных сперматозоидов, а достоверная оценка доли клеток с поврежденными акросомами.
В другой работе описан способ автоматического разделения сперматозоидов самца свиньи с поврежденной и неповрежденной акросомами [19]. Такая классификация важна для оценки потенциала к оплодотворению образца эякулята. Для анализа используются изображения сперматозоидов, полученные с помощью фазово-контрастного микроскопа. Изображения кадрируются и проходят предварительную обработку. В итоге для каждого сперматозоида вычисляется профиль, определяющий контур клетки (рис. 3). На этих данных авторы пробовали два метрических алгоритма – метод k ближайших соседей и классификатор на основе прототипов. Второй алгоритм показал достаточно высокую точность, несмотря на то, что данные для сперматозоидов с поврежденной и неповрежденной акросомами в некоторой степени перекрываются (рис. 3). Авторы обращают внимание, что целью работы системы является не классификация отдельных сперматозоидов, а достоверная оценка доли клеток с поврежденными акросомами.
В работе Bijar A. et al. описаны эвристический алгоритм и программа распознавания и сегментации сперматозоидов (рис. 4) на микроскопических изображениях на основе байесовского классификатора, обеспечивающего минимизацию вероятности ошибочной классификации. Данный метод может быть в дальнейшем использован для автоматической морфологической классификации сперматозоидов [20].
 Наконец, в 2019 г. был представлен портативный тестер на базе мобильного телефона, который позволяет оценить подвижность сперматозоидов в домашних условиях [21]. Система адаптирована к различным моделям смартфонов, состоит из микроскопа и микрофлюидных модулей. Полученные с ее помощью изображения сперматозоидов загружаются в облако, анализ подвижности сперматозоидов выполняется с применением технологий ИИ. Эта мобильная система может применяться в следующих областях: обнаружение бесплодия у мужчин, определение качества спермы во время подготовки к беременности и мониторинг лечения бесплодия. Частое домашнее тестирование обеспечит врачей большим набором данных для принятия более точных клинических решений.
Наконец, в 2019 г. был представлен портативный тестер на базе мобильного телефона, который позволяет оценить подвижность сперматозоидов в домашних условиях [21]. Система адаптирована к различным моделям смартфонов, состоит из микроскопа и микрофлюидных модулей. Полученные с ее помощью изображения сперматозоидов загружаются в облако, анализ подвижности сперматозоидов выполняется с применением технологий ИИ. Эта мобильная система может применяться в следующих областях: обнаружение бесплодия у мужчин, определение качества спермы во время подготовки к беременности и мониторинг лечения бесплодия. Частое домашнее тестирование обеспечит врачей большим набором данных для принятия более точных клинических решений.
Использование искусственного интеллекта для оценки морфологии и жизнеспособности эмбриона
Морфологический анализ является стандартным методом оценки качества эмбрионов. Существующие различные системы оценки допускают высокую вариабельность между оценками разных специалистов в зависимости от их опыта и количества совершаемых циклов ЭКО в год [6]. На рис. 5 показано расхождение в оценке эмбрионов (для оценки были выбраны 35 эмбрионов) эмбриологами различной квалификации. Эмбрионы оценивались по шкале Вика, в качестве контрольных значений использовалась оценка эмбрионов, сделанная непосредственно автором шкалы.

Разработка объективного метода оценки морфологии эмбрионов является задачей особой важности. В большинстве работ, посвященных этой проблеме, описывается схожая концепция решения: эмбриолога, проводящего визуальную оценку эмбриона, предлагается заменить на ИИ, которому для анализа будут передаваться изображения эмбрионов.
Работа ИИ по классификации биомедицинских изображений, как правило, основана на следующих шагах [22]: (1) сегментация (выбор информативной области на изображении) и предварительная обработка изображения (уменьшение шума, повышение четкости и контраста, коррекция яркости); (2) извлечение текстурных признаков при помощи дескрипторов, компактно представляющих исходное изображение; (3) выбор и обучение классификатора. Таким образом, система должна сочетать в себе хорошие текстурные дескрипторы с высокопроизводительными классификаторами. Архитектура системы схематизирована на рис. 6.

В работе Rocha J. et al. представлена полуавтоматическая программа Blasto3Q, которая производит оценку качества бластоцист крупного рогатого скота. В качестве входных данных в программу передается изображение бластоцисты. Далее программа оценивает, насколько данная бластоциста соответствует каждой из трех оценок: отлично, удовлетворительно, плохо. Работа программы основана на НС. Для обучения алгоритмов использовался набор изображений бластоцист (482 шт.); каждое изображение содержит один эмбрион, при этом плоскость фокуса соответствует наибольшему диаметру бластоцисты. Бластоцисты обучающего набора были оценены тремя опытными эмбриологами, при этом между их оценками были некоторые расхождения (индекс Каппа – 0,571). При помощи генетического алгоритма были выбраны три лучшие архитектуры НС, которые и вошли в итоговый вариант программы. НС демонстрируют одинаковую точность (76,4%), но допускают расхождения в классификации. Пользователь получает результат работы каждой из сетей и принимает решения в случае спорных результатов (рис. 7) [23].
По мнению авторов, Blasto3Q может стать отличным технологическим инструментом, который будет использоваться как в обучающих целях, так и в клинических лабораториях. На основе оценки программы будет возможно выбирать морфологически наилучший эмбрион для переноса в программах ВРТ, что повысит вероятность успешного шанса на беременность.
Другая модель ИИ – Life Whisperer – описана в работе VerMilyea M. et al. Эта модель предсказывает жизнеспособность эмбриона с точностью 64,3%. Для обучения системы использовался набор изображений 8886 эмбрионов 5-го дня из 11 независимых клиник США, Австралии и Новой Зеландии. В качестве критерия жизнеспособности выступал исход беременности. Авторы отмечают, что при использовании данного критерия максимально возможная точность ограничивается 80%, поскольку до 20% случаев ЭКО имеют отрицательный исход из-за факторов, не связанных с эмбрионом. Также на точность модели оказала влияние разница в исходных изображениях, полученных из разных клиник. Однако стоит отметить, что модель показала большую точность по сравнению с оценкой бластоцист эмбриологами, разница составила 30,8% [24].
По мнению авторов, расширение обучающей выборки и дополнительный анализ данных еще больше улучшат точность ИИ. Разработанная модель ИИ Life Whisperer была включена в облачное программное обеспечение. Программа Life Whisperer позволит эмбриологам или персоналу с аналогичной квалификацией загружать изображения эмбрионов с помощью любого компьютера или мобильного устройства и получать для них рассчитанную степень жизнеспособности. Преимущества такого подхода заключаются в его простоте и удобстве использования; система не требует установки сложного или дорогостоящего оборудования и каких-либо специальных вычислительных или аналитических знаний. Кроме того, использование этого инструмента не потребует каких-либо существенных изменений в стандартных рабочих процедурах, поскольку получение изображения эмбрионов обычно включено в стандартные лабораторные процедуры ЭКО. Описанное исследование показывает возможность использования модели Life Whisperer в качестве инструмента поддержки принятия клинических решений для прогнозирования жизнеспособности эмбрионов во время процедур ЭКО [24].
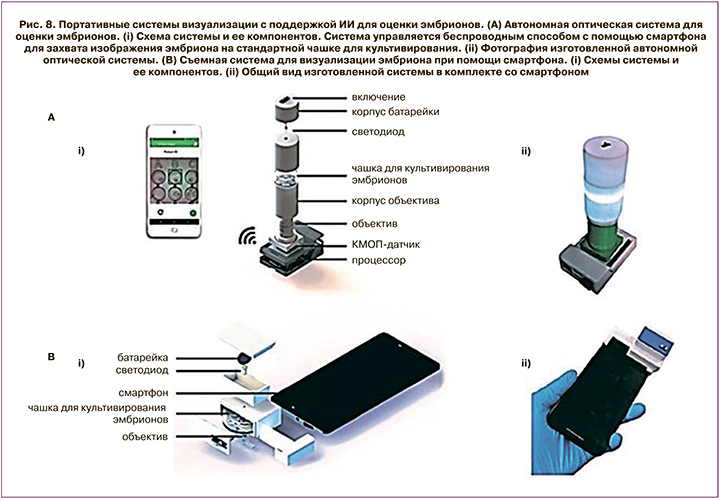
Еще одной интересной работой на стыке областей ИИ и клинической эмбриологии стало исследование группы американских авторов под руководством Kanakasabapathy М. Целью исследования было оценить, смогут ли портативные, недорогие оптические системы выдавать изображения подходящего качества, чтобы обеспечить автоматическую оценку эмбрионов с помощью ИИ, обученного на изображениях высокого качества, полученных на профессиональных системах визуализации [25].
В статье авторы описывают разработку двух недорогих (<100$ и <5$) платформ визуализации для быстрой, надежной и точной оценки морфологических качеств эмбриона. Обе системы показаны на рис. 8. Используя многоуровневый подход к обучению, авторы показали, что алгоритм, предварительно обученный на 2450 изображениях эмбрионов, полученных с помощью системы покадровой съемки, можно переобучить, используя данные с портативных оптических систем с относительно низким разрешением. Для каждой из разработанных систем авторы показали точность в классификации эмбрионов свыше 90%. Системы покадровой съемки доступны менее чем в 17% клиник, проводящих процедуру ЭКО, из-за их высокой стоимости. Разработанные портативные системы могут быть внедрены повсеместно без создания дополнительной финансовой нагрузки, что повысит эффективность и качество оценки жизнеспособности эмбрионов. Разработанная система обучена оценивать эмбрионы человека на 5-й день развития, но может быть адаптирована для любой стадии развития эмбрионов.
Коллектив авторов из Японии Miyagi Y. et al. в 2019 г. опубликовали результаты работы по созданию системы, использующей машинное обучение и ИИ, которая с высокой точностью (до 67%) оценивает вероятность положительного исхода беременности по изображению бластоцисты. Они сравнили шесть методов машинного обучения, на основе лучшего из них – логистическая регрессия с L2-регуляризацией создали программу-классификатор, которая показывает вероятность живорождения эуплоидного эмбриона. Для обучения алгоритма использовалось по 80 изображений бластоцист, приведших к положительному и отрицательному исходу беременности [26].
В исследовании Wong C. et al. продемонстрирован анализ 386 покадровых изображений бластоцисты, отобранной для переноса, точность прогнозирования живорождения составила 93% [27]. Авторы Conaghan J. et al. представили анализатор, в котором на основе получаемых в течение 3 дней изображений развития успешно оплодотворенных ооцитов удалось с высокой точностью произвести разделение бластоцист плохого и хорошего качества [28].
Заключение
Оценка морфологии и выбор наилучшего эмбриона для переноса в полость матки являются важнейшими этапами в программе ВРТ. Для достижения наилучшего результата необходима стандартизация процесса и большее число критериев оценки. В наше время развитие науки позволяет специалистам из разных областей создавать новейшие технологии, которые в скором будущем помогут с решением важнейших медицинских проблем. В сферах гинекологии, репродуктологии и эмбриологии разработки с использованием ИИ и машинного обучения помогут усовершенствовать программы ВРТ и привести к рождению здорового ребенка. Методы ИИ необходимо продолжать исследовать как перспективное средство для улучшения выбора эмбрионов и прогнозирования результатов имплантации и беременности.



