Using machine learning to analyze the lipid profile of culture medium and predict the efficacy of assisted reproductive technologies
Drapkina Yu.S., Makarova N.P., Chagovets V.V., Vasiliev R.A., Amelin V.V., Kalinina E.A.
Relevance: Determining the lipid profile of embryo culture medium is a modern and promising noninvasive method for predicting the effectiveness of assisted reproductive technology (ART) programs. The use of machine learning for the identification of the most significant lipid groups in the culture medium allows the processing of non-linear relationships within the data and the extraction of the most informative data from the input parameters.
Objective: To develop a method for predicting ART outcomes based on analyzing the lipid profile of embryo culture medium on day 5 post-fertilization using gradient boosting (GB), and to identify the lipids that contribute most to this prediction.
Materials and methods: Sixty couples seeking ART for infertility treatment were included in the study. Patients with tubal-peritoneal factor infertility underwent ovarian stimulation, following a protocol that included a gonadotropin-releasing hormone antagonist. On the day of embryo transfer, culture medium was collected, followed by cryopreservation of the medium samples. The lipid profile of the samples was determined using liquid chromatography-mass spectrometry (LC-MS). The data obtained were analyzed using GB.
Results: A GB model was developed to predict ART outcomes based on lipid profiles of the culture medium. The model achieved 79% accuracy (f1 score: 0.81) in identifying the lipid profiles associated with embryos that resulted in pregnancy. Among the lipids identified, triacylglycerols were found to contribute the most to determining embryo implantation potential.
Conclusion: Analyzing LC-MS data using GB allows for the identification of different classes of lipids in the embryo culture medium, which can serve as a noninvasive approach to assess embryo quality and implantation potential. This can also facilitate the development of a predictive testing system to determine the effectiveness of ART programs. Additionally, this information enables a more detailed investigation of the mechanisms of gamete damage in patients with various extragenital diseases, and can assist in developing methods for the selective transfer of the most promising embryos.
Authors' contributions: Drapkina Yu.S. – data analysis and interpretation, drafting of the manuscript; Makarova N.P., Kalinina E.A. – study conception and design, drafting of the manuscript; Chagovets V.V. – laboratory phase of the study, editing of the manuscript; Vasiliev R.A., Amelin V.V. – development of a mathematical model using gradient boosting.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Drapkina Yu.S., Makarova N.P., Chagovets V.V., Vasiliev R.A., Amelin V.V., Kalinina E.A. Using machine learning to analyze the lipid profile of culture medium and predict the efficacy of assisted reproductive technologies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (2): 91-99 (in Russian)
https://dx.doi.org/10.18565/aig.2024.280
Keywords
Infertility is one of the most pressing modern health issues worldwide. Among the effective strategies for overcoming infertility in married couples, the implementation of assisted reproductive technology (ART) programs stands out [1]. To date, numerous ovarian stimulation protocols have been proposed, enabling the retrieval of mature and high-quality oocytes from patients with diverse clinical and anamnestic backgrounds, as well as from women of various age groups. Furthermore, embryological techniques have been developed that significantly enhance the stages of embryo culture, fertilization of retrieved oocytes, and selective transfer of the highest-quality embryos [2].
Introduction of preimplantation genetic testing (PGT) into clinical practice has led to a decrease in the transfer frequency of embryos with chromosomal aneuploidies and a reduction in miscarriage rates. Additionally, it has contributed to fewer unsuccessful embryo transfer attempts required to achieve a successful pregnancy, due to improved selection of the most promising embryos [3]. Despite the undeniable advantages of PGT for optimizing embryo selection, studies by Kulakova E.V. et al. [4] and Dolgushina N.V. et al. [5] identified the most optimal and economically feasible scenarios for conducting PGT within a limited patient group, making it more applicable in practical healthcare. Consequently, the search for convenient, inexpensive, and non-invasive markers to select the highest-quality embryos appears to be a highly promising endeavor. One potential avenue for analyzing embryo quality is the culture medium, which serves as a unique study object containing information about the molecular biological profile and functional state of the intracellular signaling systems of blastocysts [6].
The composition of the embryo culture medium can be examined using mass spectrometry to identify additional diagnostically significant markers for selecting the most viable embryos. Among these markers, metabolites, which are the products of biochemical reactions occurring in the developing embryo, are particularly noteworthy. Metabolites include nucleotides, which form the basis of the genome and transcriptome; amino acids, which are essential for protein synthesis; and lipids, such as cholesterol, phospholipids, and triglycerides [7]. Lipids play crucial roles in providing energy to cells, forming plasma membranes, and acting as signaling molecules and regulators [8]. Excessive accumulation of intracellular lipids, such as fatty acids, can lead to oxidative damage to cells [9], whereas a deficiency of certain lipids negatively affects steroidogenesis [10].
Lipids detected in various biological samples can be used to assess the effectiveness of ART. A study by Fortygina Yu.A. et al. described both similarities and differences in the lipidome of blood plasma and follicular fluid, which could be employed to develop non-invasive methods for evaluating the state of oocytes and predicting the effectiveness of ART programs [11].
Moreover, studies have demonstrated that the lipid composition of follicular fluid correlates with the success of embryo implantation in ART programs [12–15]. Previous experimental research has also indicated a relationship between the lipid profile of blood serum and pregnancy rate [16].
It is important to highlight a 2022 study that revealed differences in the lipid composition of embryo culture media between groups with positive and negative ART outcomes [17]. Thus, investigating the lipidome of the embryo culture medium is an urgent task, as this medium serves as the "final" product reflecting embryo viability. Analysis of the lipidome of the embryo culture medium can contribute to predicting pregnancy rates in married couples undergoing ART and aid in identifying the most promising embryos.
Eldarov C. et al. demonstrated that the metabolomic profile of the culture medium reflects embryo quality, depends on culture time, and is influenced by the presence of chromosomal aneuploidies, providing potential for predicting implantation outcomes [7].
To process the data obtained on lipid profile changes, various mathematical models are utilized, each with distinct advantages and disadvantages, depending on the problem being addressed. The most commonly employed models include logistic and linear regressions. Regression analysis aids classification by indicating the probability that a given initial value belongs to a specific class. Typically, this type of analysis serves as a baseline algorithm against which all developed models are compared, owing to the simplicity of logistic regression, operational speed, and interpretability of results [18, 19]. However, this method requires normalization of features by "weight" to ensure equal contribution to the model, which can hinder performance on problems with a large number of features or complex data structures and may yield low accuracy if classes are not linearly separable.
Machine learning (ML) is an alternative method that can establish nonlinear dependencies of variables in models, extract the most informative features from input parameters, generalize information under conditions of incomplete data, and identify patterns hidden within extensive datasets. The experimental work of Drapkina Yu.S. et al. revealed that among various ML algorithms, gradient boosting (GB) – a gradient descent approach applicable not only to decision trees but also to other algorithms – demonstrates the highest accuracy and specificity for classification tasks [20].
Given the relevance and potential of using ML to create predictive models, the aim of this study was to predict ART outcomes based on the lipid profile of the embryo culture medium on the 5th day post-fertilization, utilizing GB for analysis.
Materials and methods
This prospective study included 60 married couples aged 24 to 39 years, each with a normal body mass index (up to 25 kg/m²), who sought infertility treatment using ART at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P. Written informed consent was obtained from each couple to participate in the study and to process their personal data. The inclusion criterion was infertility due to a peritoneal factor with preserved ovarian reserve. Exclusion criteria included uterine structural abnormalities, karyotype abnormalities, use of donor oocytes or sperm, severe forms of male infertility, and hereditary hypercholesterolemia.
The patients underwent ovarian stimulation according to a protocol using a gonadotropin-releasing hormone antagonist starting on the 2nd or 3rd day of the menstrual cycle. When the follicle diameter reached ≥17 mm, the patients were administered a trigger for final oocyte maturation, human chorionic gonadotropin (hCG). Transvaginal puncture was performed 35–36 hours after administration of the ovulation trigger, followed by oocyte collection and assessment of their quality according to the guidelines for evaluating oocytes and embryos in the RAHR ART laboratory. Fertilization of the collected oocytes was achieved by in vitro fertilization (IVF) or intracytoplasmic sperm injection. All culture stages were conducted in multi-gas incubators (COOK, Ireland) in 25 μl drops under oil (Vitrilife, Sweden) at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility.
After selective embryo transfer on the 5th day post-fertilization, 25 μl of the culture medium was collected in appropriately marked vials and stored at -80°C. Support during the post-transfer period was provided according to the standard protocol. Embryos meeting the morphological quality criteria (no lower than 3 BB quality) were vitrified. Blood was analyzed for beta-hCG 14 days after embryo transfer; if the result was positive, pelvic ultrasound examination was performed 21 days after the transfer.
Lipid analysis method
Lipid extracts were obtained using a modified Folch method. A chloroform-methanol solution (480 μl, 2:1, v/v) was added to a previously prepared sample (40 μl), and the mixture was incubated for 10 min with thorough mixing. In the next step, water was added to the solution. The mixture was then centrifuged for 10 min at 13,000 × G at ambient temperature. After centrifugation, the organic lower layer (150 μl) containing lipids was collected, and another 250 μl of chloroform-methanol solution (2:1, v/v) was added to the remaining part. The resulting mixture was mixed again, centrifuged under the same conditions, and another 300 μl of the lower layer was collected. The resulting organic phase was dried under a nitrogen stream and then dissolved in acetonitrile-2-propanol (200 μl, 1:1, v/v) for subsequent analysis. The resulting samples were analyzed using a Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) with a Maxis Impact qTOF mass spectrometric detector (Bruker Daltonics, Germany). The samples were separated on a Zorbax C18 column (150×2.1 mm, 5 μm, Agilent, USA) with a gradient from 15% to 45% eluent B for 2 min, and then from 45% to 99% for 15 min. Eluent A was an acetonitrile/water solution (60/40, v/v) with the addition of 0.1% formic acid and 10 mmol/L ammonium formate; eluent B was an acetonitrile/isopropanol/water solution (90/8/2 v/v/v) with the addition of 0.1% formic acid and 10 mmol/L ammonium formate. The elution flow rate was 35 μl/min and the volume of the injected sample was 0.5 μl. Mass spectra were acquired in positive ion mode in the m/z range of 100–1700 with the following settings: capillary voltage 4.1 kV, nebulizer gas pressure 0.7 bar, drying gas flow rate 6 l/min, drying gas temperature 200°C. For quantitative identification of lipids, tandem mass spectrometry was performed in data-dependent analysis (DDA) mode with a mass isolation window width of 5 Da. Eight lipid classes were the most abundant in the analyzed samples. Lipids were identified based on tandem mass spectra using the LipidMatch software.
Statistical Analysis
GB over decision trees was chosen as the ML method for lipidome analysis.
GB was introduced as an ensemble learning technique to build a predictive model. It combined several weak models (decision trees) to create a strong predictive model. The main idea was to train the models sequentially, with each model correcting the errors made by the previous models.
GB was built using the following steps.
- model initialization;
- building successive models: at each step, a new tree was built, learning from the errors made by the previous models;
- model update: a new model was added to the ensemble with a weight that minimized the error function;
- repeating the process: the process was repeated until the required model quality was achieved.`
A permutation test was used to adjust for the effects of false positives in the multiple comparisons.
- Initialization.
Let us have a training sample {(xi, yi)} n i=1, where xi are features and yi is the target variable. The model was initialized as F0(x) = arg min γ Xn i=1 L(yi, γ), where L is the loss function.
- Iterative construction.
For m=1, 2,…, M (where M is the number of iterations): 1 (a) Calculating gradients: calculating the residuals (gradients of the loss function): rim = -∂L(yi, F(xi)) ∂F(xi) F = Fm-1; (b) Training a new tree: training a new tree hm(x) on the residuals rim; (c) Step (update rate) determination: determine the optimal step γm: γm = arg min γ Xn i=1 L(yi, Fm-1(xi) + γhm(xi)); (d) Model updating: update the model: Fm(x) = Fm-1(x) + γmhm(x).
- Final model.
After performing M iterations, the final model appears as FM(x) = F0(x) + X M m=1 γmhm(x).
Comments.
- Loss function (L) determines how well the model predicts the target variable. In regression problems, this is often the mean squared error (MSE), and in classification problems, it is the logistic loss function.
- The slopes indicate the direction in which the model predictions should be adjusted to reduce errors.
- Decision trees are commonly used as weak models because they are easy to interpret and can be quickly trained.
Gradient boosting has proven effective in many ML problems owing to its ability to consistently correct errors and enhance model accuracy [20]. Gradient boosting was implemented using Python, with the data robustness determined, and the resulting data were validated on a test set of embryo culture medium lipid samples using the Scikit-learn library. To build the GB-based model, composition by induction was performed, where the outputs of the individual trees included in the model were assigned weights. The classifications from the first decision tree were weighted, and the data were then passed to the next tree. After numerous iterative cycles, boosting combines the weak classifiers into a powerful predictive algorithm. The quantitative values of lipid levels in all analyzed samples of the culture medium, obtained through mass spectrometry and input into an Excel table, were randomly divided into a training set (70%) and a validation set (30%).
Results
To analyze the quality metrics of the model, we introduced precision and recall criteria. Precision is the proportion of objects classified as positive by the classifier that are actually positive, whereas recall indicates the proportion of positive class objects identified by the algorithm out of all actual positive class objects. There are various methods to combine precision and recall into a single quality criterion, such as the F1-measure (generally represented as F_β), which is the harmonic mean of the precision and recall. In this study, we achieved an F1-measure value of 0.81, indicating high quality of the model. Another way to evaluate the model as a whole, without relying on a specific threshold, is the Area Under the Curve (AUC-ROC) metric, which measures the area under the ROC curve against the axis representing the proportion of false-positive classifications, as illustrated in the figure.
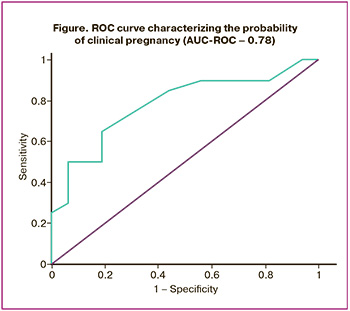
Based on the ART program outcomes, all patients were classified into two groups. Group 1 consisted of 24 patients with a positive treatment result (pregnancy +), whereas group 2 included 36 patients without pregnancy (pregnancy) within the ART protocol. A GB model was developed based on the changes in the lipid profile of the embryo culture medium obtained on the 5th day post-fertilization, which determines the "weight" of each lipid in predicting pregnancy rate. The contribution of each lipid class to the positive or negative prognosis of the ART program was evaluated using the Gini criterion, which measures the uncertainty or "purity" of a set of elements by assessing the probability of misclassification of a randomly selected element from the set. Previous stages of the study demonstrated that compared to regression analysis, GB over decision trees provides a more accurate forecast with higher quality metrics for the constructed predictive model [20].
Through construction of the GB model, we identified eight classes of lipids in the embryo culture medium that contributed most significantly to predicting the pregnancy rate (Table).
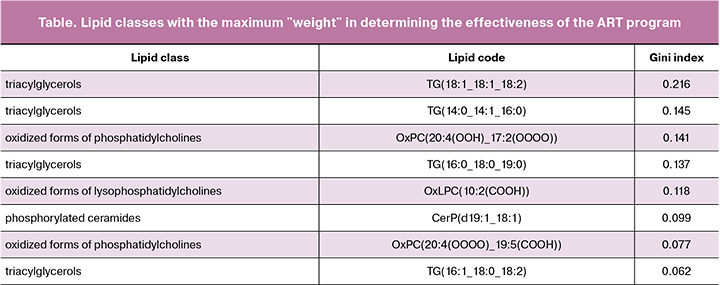
Among the lipids in the culture medium that significantly contributed to the GB model for determining the effectiveness of the ART program, we identified four lipids belonging to the triacylglycerol class (TG), one lipid from the oxylysophosphatidylcholine class, two lipids from the oxyphosphatidylcholine class, and one lipid from the ceramide-1-phosphate class (sphingolipids). Notably, the lipids that contribute the most to determining the embryo implantation potential are those classified as triacylglycerols.
Discussion
Studying the lipid composition of various biological fluids to predict the quality of gametes and embryos is crucial for identifying noninvasive, interpretable, and accurate markers of ART program effectiveness. The search for these molecules is important from both clinical and economic perspectives, as more accurate testing systems could reduce the number of IVF attempts required to achieve pregnancy. Lipids are a class of molecules that play a vital role in providing cells with energy [11–14]. In an article by Eldarov et al., the metabolomic profile of the embryo culture medium on the 3rd and 5th days after fertilization, depending on the implantation potential, is described. The profiles of culture medium samples from embryos that resulted in pregnancy differed significantly owing to changes in the levels of lipids and amino acids [7]. Previous studies have shown that lipids in the culture medium reflect embryo quality and developmental processes. Notably, a significant change in the profile of fatty acids, such as stearic, oleic, palmitic, and linoleic acids, was observed in blood plasma and follicular fluid [21].
Moreover, eicosanoids, which are derivatives of unsaturated fatty acids, can form at the blastocyst-endometrium interface and appear to act as key factors in decidualisation and implantation. Eicosanoids are oxidized derivatives of polyunsaturated fatty acids that influence vascular permeability during decidualization and implantation of blastocysts into the endometrium [22].
The findings of this study also highlight the significant role of oxidized forms of fatty acids in the culture medium in determining the implantation potential of the embryo (Gini index for the class of oxylysophosphatidylcholines is 0.1189491915, oxyphosphatidylcholines are 0.1417922541 and 0.07781558905). The accuracy of the obtained model was 0.79, with an f1 score of 0.81.
These results can be used to determine the optimal molecular composition of the culture medium for improving the functional properties of embryos during culture.
In addition, the obtained data are consistent with the results of Harden et al., who showed that oxidized forms of lipids, as well as lipids related to glycerophospholipids and linoleic fatty acid, play an active role in the processes of embryo implantation [23]. Thus, metabolomic profiling of embryo culture medium may be a promising diagnostic and prognostic method in combination with morphological criteria for assessing embryo quality. It is worth noting that, according to the results of the study by Eldarov et al., even two morphologically identical embryos may have different metabolic activities, and therefore different potentials for implantation [7]. In addition, an embryo of good and average quality may have a higher metabolic rate than an embryo of excellent quality, according to morphological assessment criteria. Metabolomic profiling of the blastocyst culture medium is crucial for selective embryo transfer into the uterine cavity.
In a study by Guan S.Y. et al., 11 markers of successful embryo implantation in blastocyst culture medium on the 5th day after fertilization in patients in the ART program were identified [24]. Among these markers, glycerophospholipid class lipids significantly contributed to the frequency of pregnancy and miscarriage. Glycerophospholipids are the main components of cell membranes and play important roles in the regulation of transport, signaling of molecules, and protein function [25]. In this study, a great weight in the final prognosis was obtained for lipids belonging to the class of oxylysophosphatidylcholines and oxyphosphatidylcholines. Previous studies have shown that the level of lysophosphatidylcholines decreases in patients with obesity and type 2 diabetes mellitus, and can act as a marker of obesity associated with poor nutrition and consumption of large amounts of fat [26]. Lysophosphatidylcholines are important mediators of fatty acid-induced insulin resistance and play a significant role in the transport, absorption, and utilization of glucose by cells [27]. The study found a contribution of this lipid, detected in the embryo culture medium on the 5th day after fertilization, to the implantation rate, which is consistent with the results of the study by Guan S.Y. et al. [24].
Similar changes in the lipidomic profile of follicular fluid described in previous studies indicate an integral and complex effect of lipidomics on the formation of high-quality embryos. For example, a decrease in the level of glycerophospholipids in the follicular fluid of patients with polycystic ovary syndrome leads to a violation of oocyte maturation in the ART program, and then to changes in the embryo itself and the receipt of blastocysts with an aneuploid set of chromosomes and a correspondingly reduced pregnancy rate in this group of patients, despite the large number of oocytes obtained during ovarian stimulation [24]. It is worth noting that ML allows us to identify nonlinear relationships between data and makes it possible to perform a higher-quality analysis of a dataset that has a direct correlation, such as age and anti-Müllerian hormone (AMH) level, in contrast to regression analysis, the results of which may become less accurate when using such data. Therefore, the interpretation of the data obtained during ML allows a more comprehensive examination of the value of traditional predictors of pregnancy in the ART program. GB conducted at the previous stages of the study showed that the parameter “pregnancy history from this partner” is a significant weight in determining embryo implantation along with the number of oocytes obtained during transvaginal puncture, AMH, and the patient's age [20, 28]. These study results may reflect complex, hidden molecular interactions necessary for embryo invasion into the endometrium, which are not captured by the number of oocytes obtained or AMH levels in patients undergoing infertility treatment with ART. Changes in the lipidome of the culture medium in young women with preserved ovarian reserves, which represents the most promising group for embryo implantation in the ART program, support this hypothesis. Using GB to analyze changes in the lipid profile of the culture medium allowed for the identification of four groups of lipids belonging to the TG class (triacylglycerolipids), one lipid from the oxylysophosphatidylcholine class, two lipids from the oxyphosphatidylcholine class, and one lipid from the ceramide-1-phosphate class (sphingolipids). According to the constructed model, these lipids can be used to create an integrated system for predicting ART outcomes and a decision support system for selective embryo transfer into the uterine cavity.
Conclusion
To improve the predictive ability of the model, it is essential to employ not only integrated mathematical systems that can capture "hidden" trends in large datasets but also unique markers that determine the quality of gametes and embryos in patients undergoing infertility treatment with ART. A detailed description of the contributions of various lipid classes in the embryo culture medium on the 5th day after fertilization, as indicated by the constructed GB model, enables further investigation into the mechanisms of gamete damage in patients with various extragenital diseases and helps identify the most important lipid groups for the selective transfer of the most promising embryos.
References
- ESHRE Add-ons working group; Lundin K., Bentzen J.G., Bozdag G., Ebner T., Harper J., Le Clef N. et al. Good practice recommendations on add-ons in reproductive medicine. Hum. Reprod. 2023; 38(11): 2062-104. https://dx.doi.org/10.1093/humrep/dead184.
- Armstrong S., Bhide P., Jordan V., Pacey A., Marjoribanks J., Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst. Rev. 2019; 5(5): CD011320. https://dx.doi.org/10.1002/14651858.CD011320.pub4.
- ESHRE PGT Consortium Steering Committee; Carvalho F., Coonen E., Goossens V., Kokkali G., Rubio C., Meijer-Hoogeveen M. et al. ESHRE PGT Consortium good practice recommendations for the organisation of PGT. Hum. Reprod. Open. 2020; 2020(3): hoaa021. https://dx.doi.org/10.1093/hropen/hoaa021.
- Кулакова Е.В., Михайлов И.А., Макарова Н.П., Драпкина Ю.С., Калинина Е.А., Назаренко Т.А., Трофимов Д.Ю. Клинико-экономический анализ эффективности преимплантационного генетического тестирования у пациентов с различными формами бесплодия в программах вспомогательных репродуктивных технологий. Гинекология. 2022; 24(3): 181-5. [Kulakova E.V., Mikhailov I.A., Makarova N.P., Drapkina Ju.S., Kalinina E.A., Nazarenko T.A., Trofimov D.Iu. Clinical and economic analysis of the effectiveness of pre-implantation genetic testing in patients with various types of infertility in assisted reproductive technology programs. Gynecology. 2022; 24(3): 181-5. (in Russian)]. https://dx.doi.org/10.26442/20795696.2022.3.201708.
- Долгушина Н.В., Коротченко О.Е., Бейк Е.П., Абдурахманова Н.Ф., Ильина Е.О., Кулакова Е.В. Клинико-экономический анализ эффективности преимплантационного генетического скрининга у пациенток позднего репродуктивного возраста. Акушерство и гинекология. 2017; 11: 56-61. [Dolgushina N.V., Korotchenko O.E., Beik E.P., Abdurakhmanova N.F., Ilyina E.O., Kulakova E.V. Clinical and cost-effectiveness analysis of preimplantation genetic screening in patients of late reproductive age. Obstetrics and Gynegology. 2017; (11): 56-61 (in Russian)]. https://dx.doi.org/10.18565/aig.2017.11.56-61.
- Timofeeva A., Drapkina Y., Fedorov I., Chagovets V., Makarova N., Shamina M. et al. Small noncoding RNA signatures for determining the developmental potential of an embryo at the morula stage. Int. J. Mol. Sci. 2020; 21(24): 9399. https://dx.doi.org/10.3390/ijms21249399.
- Eldarov C., Gamisonia A., Chagovets V., Ibragimova L., Yarigina S., Smolnikova V. et al. LC-MS analysis revealed the significantly different metabolic profiles in spent culture media of human embryos with distinct morphology, karyotype and implantation outcomes. Int. J. Mol. Sci. 2022; 23(5): 2706. https://dx.doi.org/10.3390/ijms23052706.
- Комедина В.И., Юренева С.В., Чаговец В.В., Стародубцева Н.Л. Особенности липидного состава сыворотки крови женщин в период менопаузального перехода. Акушерство и гинекология. 2022; 6: 90-7. [Komedina V.I., Yureneva S.V., Chagovets V.V., Starodubtseva N.L. Changes in serum lipid profile during the menopausal transition. Obstetrics and Gynecology. 2022; (6): 90-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.6.90-97.
- Бурдули А.Г., Кициловская Н.А., Сухова Ю.В., Ведихина И.А., Иванец Т.Ю., Чаговец В.В., Стародубцева Н.Л., Франкевич В.Е. Фолликулярная жидкость и исходы программ вспомогательных репродуктивных технологий (обзор литературы). Гинекология. 2019; 21(6): 36-40. [Burduli A.G., Kitsilovskaya N.A., Sukhova Yu.V., Vedikhina I.A., Ivanets T.Yu., Chagovets V.V., Starodubtseva N.L., Frankevich V.E. Follicular fluid and assisted reproductive technology programs outcomes (literature review). Gynecology. 2019; 21(6): 36-40. (in Russian)]. https://dx.doi.org/10.26442/20795696.2019.6.190663.
- Tokareva A.O., Chagovets V.V., Kononikhin A.S., Starodubtseva N.L., Nikolaev E.N., Frankevich V.E. Comparison of the effectiveness of variable selection method for creating a diagnostic panel of biomarkers for mass spectrometric lipidome analysis. J. Mass Spectrom. 2021; 56(3): e4702. https://dx.doi.org/10.1002/jms.4702.
- Фортыгина Ю.А., Макарова Н.П., Драпкина Ю.С., Новоселова А.В., Гамисония А.М., Чаговец В.В., Франкевич В.Е., Калинина Е.А. Сравнительный анализ липидного профиля крови и фолликулярной жидкости женщин, проходящих лечение бесплодия методами вспомогательных репродуктивных технологий. Акушерство и гинекология. 2024; 4: 93-102. [Fortygina Yu.A., Makarova N.P., Drapkina Yu.S., Novoselova A.V., Gamisonia A.M., Chagovets V.V., Frankevich V.E., Kalinina E.A. Comparative analysis of blood and follicular fluid lipid profiles in women undergoing infertility treatment with assisted reproductive technologies. Obstetrics and Gynecology. 2024; (4): 93-102 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.62.
- Ding Y., Jiang Y., Zhu M., Zhu Q., He Y., Lu Y. et al. Follicular fluid lipidomic profiling reveals potential biomarkers of polycystic ovary syndrome: A pilot study. Front. Endocrinol. (Lausanne). 2022; 13: 960274. https://dx.doi.org/10.3389/fendo.2022.960274.
- Núñez Calonge R., Guijarro J.A., Andrés C., Cortés S., Saladino M., Caballero P. et al. Relationships between lipids levels in blood plasma, follicular fluid and seminal plasma with ovarian response and sperm concentration regardless of age and body mass index. Rev. Int. Androl. 2022; 20(3): 178-88. https://dx.doi.org/10.1016/j.androl.2021.02.004.
- Shehadeh A., Bruck-Haimson R., Saidemberg D., Zacharia A., Herzberg S., Ben-Meir A. et al. A shift in follicular fluid from triacylglycerols to membrane lipids is associated with positive pregnancy outcome. FASEB J. 2019; 33(9): 10291-9. https://dx.doi.org/10.1096/fj.201900318RR.
- Zarezadeh R., Mehdizadeh A., Leroy J.L.M.R., Nouri M., Fayezi S., Darabi M. Action mechanisms of n-3 polyunsaturated fatty acids on the oocyte maturation and developmental competence: Potential advantages and disadvantages. J. Cell. Physiol. 2019; 234(2): 1016-29. https://dx.doi.org/10.1002/jcp.27101.
- Jamro E.L., Bloom M.S., Browne R.W., Kim K., Greenwood E.A., Fujimoto V.Y. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF. Reprod. Biomed. Online. 2019; 39(4): 665-73. https://dx.doi.org/10.1016/j.rbmo.2019.06.004.
- Guan S.Y., Liu Y.Y., Guo Y., Shen X.X., Liu Y., Jin H.X. Potential biomarkers for clinical outcomes of IVF cycles in women with/without PCOS: Searching with metabolomics. Front. Endocrinol. (Lausanne). 2022; 13: 982200. https://dx.doi.org/10.3389/fendo.2022.982200.
- Wang Q.Q., Yu S.C., Qi X., Hu Y.H., Zheng W.J., Shi J.X. et al. [Overview of logistic regression model analysis and application]. Zhonghua Yu Fang Yi Xue Za Zhi. 2019; 53(9): 955-60. (in Chinese). https://dx.doi.org/10.3760/cma.j.issn.0253-9624.2019.09.018.
- Uddin S., Khan A., Hossain M.E., Moni M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med. Inform. Decis. Mak. 2019; 19(1): 281. https://dx.doi.org/10.1186/s12911-019-1004-8.
- Драпкина Ю.С., Макарова Н.П., Васильев Р.А., Амелин В.В., Франкевич В.Е., Калинина Е.А. Изучение аналитической обработки клинико-анамнестических и эмбриологических данных пациентов в программе вспомогательных репродуктивных технологий различными методами машинного обучения. Акушерство и гинекология. 2024; 3: 96-107. [Drapkina Yu.S., Makarova N.P., Vasilev R.A., Amelin V.V., Frankevich V.E., Kalinina E.A. Application of various machine learning techniques to the analysis of clinical, anamnestic, and embryological data of patients undergoing assisted reproductive technologies. Obstetrics and Gynecology. 2024; (3): 96-107 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.281.
- Mirabi P., Chaichi M.J., Esmaeilzadeh S., Ali Jorsaraei S.G., Bijani A., Ehsani M. et al. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis. 2017; 16(1): 18. https://dx.doi.org/10.1186/s12944-016-0396-z.
- Kennedy T. Interactions of eicosanoids and other factors in blastocyst implantation. In: Hilier K., ed. Eicosanoids and Reproduction. MTP Press Limited; Vancouver, BC, Canada; 2012; 73.
- Harden S.L., Zhou J., Gharanei S., Diniz-da-Costa M., Lucas E.S., Cui L. et al. Exometabolomic analysis of decidualizing human endometrial stromal and perivascular cells. Front. Cell Dev. Biol. 2021; 9: 626619. https://dx.doi.org/10.3389/fcell.2021.626619.
- Guan S.Y., Liu Y.Y., Guo Y., Shen X.X., Liu Y., Jin H.X. Potential biomarkers for clinical outcomes of IVF cycles in women with/without PCOS: Searching with metabolomics. Front. Endocrinol. (Lausanne). 2022; 13: 982200. https://dx.doi.org/10.3389/fendo.2022.982200.
- Jia C., Xu H., Xu Y., Xu Y., Shi Q. Serum metabolomics analysis of patients with polycystic ovary syndrome by mass spectrometry. Mol. Reprod. Dev. 2019; 86(3): 292-7. https://dx.doi.org/10.1002/mrd.23104.
- Li F., Jiang C., Larsen M.C., Bushkofsky J., Krausz K.W., Wang T. et al. Lipidomics reveals a link between CYP1B1 and SCD1 in promoting obesity. J. Proteome Res. 2014; 13(5): 2679-87. https://dx.doi.org/10.1021/pr500145n.
- Han M.S., Lim Y.M., Quan W., Kim J.R., Chung K.W., Kang M. et al. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J. Lipid. Res. 2011; 52(6): 1234-46. https://dx.doi.org/10.1194/jlr.M014787.
- Драпкина Ю.С., Макарова Н.П., Васильев Р.А., Амелин В.В., Калинина Е.А. Сравнение прогностических моделей, построенных с помощью разных методов машинного обучения, на примере прогнозирования результатов лечения бесплодия методом вспомогательных репродуктивных технологий. Акушерство и гинекология. 2024; 2: 97-105. [Drapkina Yu.S., Makarova N.P., Vasiliev R.A., Amelin V.V., Kalinina E.A. Comparison of predictive models built with different machine learning techniques using the example of predicting the outcome of assisted reproductive technologies. Obstetrics and Gynecology. 2024; (2): 97-105 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.263.
Received 02.11.2024
Accepted 14.02.2025
About the Authors
Yulia S. Drapkina, PhD, Senior Researcher at the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Centerfor Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, yu_drapkina@oparina4.ru,
https://orcid.org/0000-0002-0545-1607
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher at the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research
Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, np_makarova@oparina4.ru,
https://orcid.org/0000-0003-1396-7272
Vitaliy V. Chagovets, PhD, Head of the Laboratory for Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., vvchagovets@gmail.com, https://orcid.org/0000-0002-5120-376X
Robert A. Vasiliev, Head of the Laboratory of Applied Artificial Intelligence Z-union, Vice-President of the Association of Laboratories for the Development of Artificial Intelligence, PhD student at the Moscow Institute of Physics and Technology (MIPT), Master of the Department of Applied Physics and Mathematics of the Moscow Institute of Physics and Technology, Master of Economics, Bachelor’s degree at the Research University «Moscow Institute of Electronic Technology».
Vladislav V. Amelin, Technical Director of the Laboratory of Applied Artificial Intelligence Z-union, Expert in machine learning. Master’s degree from Moscow State University (Faculty of Computational Mathematics and Cybernetics, Department of Mathematical Methods), Bachelor’s degree from the National Research University «Moscow Institute of Electronic Technology».
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kalinina@oparina4.ru,
https://orcid.org/0000-0002-8922-2878



