Синдром поликистозных яичников (СПКЯ) является одной из наиболее распространенных причин нарушений репродуктивного здоровья и обычно проявляется в подростковом возрасте [1]. Для пациенток с СПКЯ характерны множественные метаболические нарушения, изменяющиеся с возрастом – от инсулинорезистентности (ИР) у подростков до дислипидемии, ожирения, в особенности висцерального, артериальной гипертензии, жирового гепатоза, сахарного диабета второго типа и риска инфаркта миокарда в более зрелом возрасте [2–4]. У пациенток с СПКЯ в подростковом периоде частота выявления метаболических нарушений достигает 33% и в 3–5 раз превышает таковую в группе здоровых девушек аналогичного возраста и индекса массы тела [5].
На сегодняшний день растет число публикаций, посвященных взаимосвязи хронического системного воспаления и митохондриальной дисфункции, а также их значению в генезе СПКЯ и его отдаленных последствий [2, 6, 7]. Установлено, что развитие СПКЯ у пациенток репродуктивного периода сопровождается нарастанием оксидативного стресса на фоне митохондриальной дисфункции и снижения антиоксидантной защиты [7–9]. Показано, что в сравнении со здоровыми женщинами репродуктивного возраста наличие СПКЯ ассоциировано с повышенным содержанием в крови концентрации лимфоцитов и моноцитов, С-реактивного белка, провоспалительных цитокинов, продуктов перекисного окисления липидов и карбонилирования белков [10, 11].
Известно, что активация системного воспалительного ответа сопряжена с повышением продукции активных форм кислорода (АФК), что потенцирует митохондриальную дисфункцию, играющую ключевую роль в нарастании окислительного стресса и прогрессировании воспалительных реакций. В ответ на избыточную продукцию АФК происходит компенсаторное увеличение глютатиона (GSH) и закономерное повышение активности антиоксидантных ферментов – глутатионредуктазы, глутатионпероксидазы, супероксиддисмутазы (СОД) и каталазы [12]. У пациенток репродуктивного возраста с СПКЯ в сравнении со здоровыми женщинами выявлены более низкие концентрации восстановленного GSH и гаптоглобина в периферической крови, повышение активности глутатионпероксидазы, СОД и каталазы, в том числе при нормальном индексе массе тела [6–8].
Описанные закономерности определены для взрослой популяции пациенток СПКЯ, имеющей достаточно длительную историю заболевания и наличие метаболических осложнений, тогда как манифестация заболевания приходится на пубертатный период. В связи с этим гипотетически возможно отсутствие или менее выраженный системный воспалительный ответ и митохондриальная дисфункция у девочек-подростков с клиническими проявлениями СПКЯ. Подтверждение гипотезы может лечь в основу разработки профилактических мероприятий и терапии для предотвращения прогрессирования заболевания и его осложнений.
Цель исследования: изучение особенностей митохондриального функционирования у девочек пубертатного возраста с разными метаболическими фенотипами СПКЯ.
Материал и методы исследования
В исследование были включены 95 девочек в возрасте от 15 до 17 лет включительно, имеющих как минимум два из трех Роттердамских критериев СПКЯ. Кроме того, критериями включения были: указание на менархе не менее 2 лет назад; отсутствие иных эндокринных болезней; отсутствие приема лекарств в течение 3 месяцев и более до начала исследования; информированное согласие пациентки и ее законного представителя на включение в исследование. Критериями исключения служили: опухоли органов малого таза; обострение хронических и острых соматических и/или инфекционных заболеваний; психические заболевания; генетические синдромы и пороки развития. Группу контроля составили 30 сверстниц, отнесенных к первой группе здоровья, с регулярными менструациями без гинекологической и эндокринной патологии.
Всем участницам исследования было проведено общеклиническое обследование, включавшее подробный сбор анамнеза, изучение жалоб, измерение антропометрических показателей (рост, индекс массы тела (ИМТ), соотношение объема талии (ОТ) к объему бедер (ОБ)), оценку выраженности избыточного оволосения.
Всем участницам исследования определяли концентрацию в венозной крови общего белка, мочевой кислоты, креатинина, прямого и общего билирубина, глюкозы, Ca2+, Fe2+/3+ и высокочувствительного С-реактивного белка (СРБ). Оценку липидного состава крови проводили по показателям общего холестерина, триглицеридов (ТГ), липопротеинов низкой (ЛПНП) и высокой (ЛПВП) плотности, коэффициенту атерогенности (КА). Исследования проводили фотометрическим и турбидиметрическим методом на автоматических анализаторах BA-400 и A-25 с использованием реагентов Biosystems (Испания). Пероральный глюкозотолерантный тест (ПГТТ) проводили спустя 12–16 часов после последнего приема пищи. Уровень глюкозы и иммунореактивного инсулина определяли в цельной венозной крови натощак, и спустя 120 минут после приема 75 г глюкозы. Рассчитывали гомеостатический индекс НОМА-IR. Для косвенной оценки объема абдоминальной жировой ткани использовали индекс висцерального ожирения (Visceral Adiposity Index) [13] по формуле:
VAI=(ОТ÷(36.58+(1.89 × ИМТ))×(ТГ÷0.81)×(1.52 ÷ЛПВП),
где ОТ измеряется см, ИМТ – кг/м2, ТГ и ЛПВП – ммоль/л.
Всем девочкам было проведено ультразвуковое исследование органов малого таза на 3–5-й день спонтанного или индуцированного гестагенами менструального цикла и изучен расширенный гормональный профиль крови.
Анализ трансмембранного потенциала митохондрий проводили в мононуклеарных клетках периферической крови (МНК), выделенных из периферической венозной крови. МНК окрашивали флуоресцентным красителем JC1 (Life technologies, США) по рекомендованному производителем протоколу. Интенсивность окрашивания клеток в зеленом и красном канале оценивали на проточном цитофлюориметре BD FACSCaliburÔ (BD Biosciences, США). Измеряли долю МНК с высокополяризованными митохондриями, а также рассчитывали интегральный показатель энергопреобразующей функции по разности трансмембранных электрических потенциалов высокополяризованных митохондрий:
ΔΨ=(М красный1 ÷ М зеленый1) − (М красный2 ÷ М зеленый2)
где М красный1 и М зеленый1 – медианы интенсивности свечения на длинах волн 590 и 529 нм в интактной пробе, а М красный2 и М зеленый2 – в пробе после добавления разобщителя дыхания митохондрий − карбонилцианид-4-(трифторметил)-фенилгидразона в конечной концентрации 7 µМ (Sigma, США).
Концентрацию малонового диальдегида (МДА) как маркера перекисного окисления липидов определяли с использованием модифицированного метода Женча [14], основанного на реакции МДА с 2-тиобарбитуровой кислотой в результате которой образуется окрашенный комплекс с максимумом поглощения при длине волны в 535 нм.
Для оценки состояния антиоксидантной системы измеряли содержание общего глутатиона (tGSH) и соотношение концентраций его восстановленной и окисленной форм (GSH/GSSG) в образцах крови пациенток. Измерение проводили на спектрофотометре Agilent Cary 300 (Великобритания) по методике, описанной D. Giustarini и соавт. [15]. Активность антиоксидантных ферментов глутатионредуктазы и глутатионпероксидазы определяли по результатам оценки сопряженной работы ферментов в присутствии НАДФН. Измеряли оптическое поглощение НАДФН при длине волны 340 нм на спектрофотометре Agilent Cary 300. Ферментативную активность каталазы в плазме крови определяли методом полярографии высокого разрешения с помощью электрода Кларка по модифицированному методу Рота и Дженсена на оксиграфе (Hansatech, UK).
Статистическую обработку данных проводили в программах MS Excel и Statistica 8. Сравнение переменных, имеющих нормальное распределение, проводили методами дисперсионного анализа ANOVA. Сравнение множества групп попарно проводили пост-хок методом апостериорных множественных сравнений с подсчетом наименьшей значимой разницы Фишера (Least Significant Difference test, LSD). Показатели, для которых распределение значений в исследуемой выборке не соответствовало нормальному, сравнивали с использованием критерия Краскела–Уоллиса (Kruskal–Wallis rank tests). В последующем оценивали межгрупповые различия пост-хок тестом по критерию Данна или Сьегля–Кастеллана. Корреляции оценивали с использованием коэффициента ранговой корреляции Спирмена.
Результаты исследования
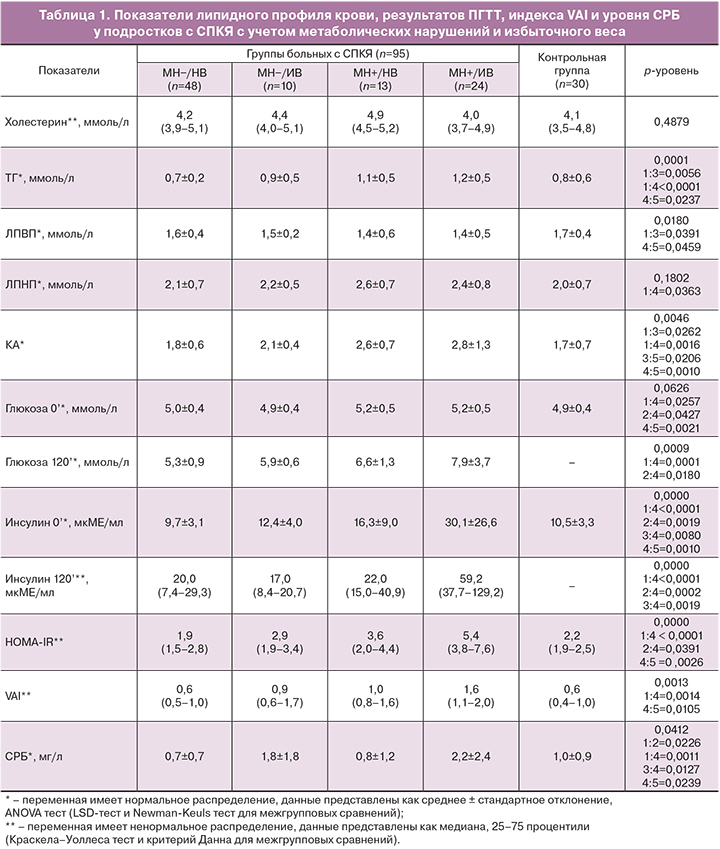
По результатам обследования было обнаружено, что 58 из 95 (61,1%) девочек с СПКЯ не имели отклонений ПГТТ и индекса НОМА-IR, тогда как у 37 (38,9%) подростков обнаружено нарушение толерантности к глюкозе и/или ИР (HOMA-IR≥3,46 у.е.). Первые были условно выделены в подгруппу без метаболических нарушений (МН−), вторые – составили подгруппу с метаболическими нарушениями (МН+). Притом, 15 из 37 (40,5%) девочек с МН+ были отнесены к подгруппе по сочетанию результатов ПГТТ и НОМА-IR, 14 (37,8%) – только по данным ПГТТ и 8 (21,6%) девочек только по данным НОМА-IR. Анализ ИМТ в подгруппах пациенток с СПКЯ выявил, что большинство (48 – 82,8%) девочек в группе без МН имели ИМТ в пределах возрастных нормативов (<24 кг/м2), в то время как в группе с МН+ только 13 (35,1%) девочек имели нормативные показатели ИМТ. В контрольной группе все 30 девочек не имели МН и были с нормативными для подростков показателями ИМТ и НОМА-IR.
Для независимого анализа влияния нарушений углеводного обмена и избыточной массы тела всех пациенток разделили на группы в зависимости от значения ИМТ. В результате такого разделения были сформированы 5 групп девочек. В первую группу включены 48 девочек с СПКЯ без МН с нормальным весом (СПКЯ и МН−/НВ), во вторую – 10 подростков с СПКЯ без МН с избыточным весом (СПКЯ и МН−/ИВ), третью – 13 пациенток с СПКЯ с МН и нормальным весом (СПКЯ и МН+/НВ), четвертую – 24 девочки с СПКЯ и МН и избыточным весом (СПКЯ и МН+/ИВ). Пятую группу составили 30 девочек контрольной группы (контроль и МН−/НВ).
Биохимический анализ крови позволил выявить значимые отличия пациенток из группы с СПКЯ и МН–/НВ от сверстниц контрольной группы, что выразилось более высоким содержанием общего белка (73,2±5,3 против 70,6±4,6, p=0,0431) и прямого билирубина (4,5±2,0 против 3,1±1,0, p=0,0251). В то же время девочки с СПКЯ и МН+/НВ значимо отличалась от пациенток СПКЯ и МН−/НВ только более высоким содержанием железа (23,8±8,0 против 16,6±6,8, p=0,0423), а от девочек контрольной группы – более высоким содержанием общего и прямого билирубина (19,0±5,5 против 11,1±4,2, p=0,0076 и 5,1±3,6 против 3,1±1,0, p=0,0155 соответственно) и железа (23,8±8,0 против 16,6±6,8, p=0,0343).
Анализ липидного профиля крови пациенток группы СПКЯ и МН–/НВ не выявил отличий от девочек контрольной группы по изученным показателям (табл. 1). Группа с СПКЯ и МН+/НВ отличалась от группы СПКЯ и МН−/НВ более высоким содержанием ТГ (p=0,0056) и значением КА (p=0,0262) и более низким уровнем ЛПВП (p=0,0391). Притом, значимое отличие от контрольной группы наблюдали только для показателя КА, который был повышен в группе девушек с СПКЯ и МН+/НВ (p=0,0206).
Пациентки с СПКЯ и МН+/ИВ в сравнении с девочками контрольной группы характеризовались, что было ожидаемо, не только значимо более высокими значениями НОМА-IR (р=0,0026) и глюкозы натощак (р=0,0021), но также более высоким уровнем ТГ (р=0,0237) и КА (р=0,0010) при более низком содержании ЛПВП (р=0,0459) и повышенным риском сердечно-сосудистой патологии по индексу VAI (р=0,0105). Как следует из данных табл. 1, пациентки с СПКЯ и МН+/ИВ имели аналогичные отличия и от группы с СПКЯ и МН−/НВ (табл. 1).
Из литературных данных известно, что у женщин с СПКЯ в репродуктивном возрасте наблюдают нарушения липидного состава крови и более высокие значения НОМА-IR не только при избыточном, но и при нормальном весе [1, 2]. Полученные данные исследуемой выборки свидетельствуют об отсутствии значимого влияния только избыточного веса на изученные показатели липидного спектра крови у девушек с СПКЯ, если развитие заболевания не ассоциировано с отклонениями углеводного обмена и инсулинорезистентностью. Напротив, сочетание избыточного веса и метаболических нарушений уже в подростковом периоде обусловливает появление достаточно выраженной дислипидемии и повышение атерогенности, а также увеличение сердечно-сосудистого риска.
При анализе маркера системного воспаления, СРБ, в группах с СПКЯ и избыточной массой тела обнаружено более высокое его содержание в плазме крови по сравнению с группами с нормативными показателями ИМТ, как на фоне МН, так и без (р=0,0127 и р=0,0226 соответственно) (табл. 1). Притом у подростков с СПКЯ и МН+/ИВ уровень СРБ был значимо выше по сравнению с больными с МН−/НВ (р=0,0011) и девочками контрольной группы (р=0,0239). По литературным данным, в том числе мета-анализов, у взрослых пациенток с СПКЯ имеется обусловленность более высокого уровня СРБ не фактом ожирения, а наличием самого заболевания [16, 17]. В представленной нами выборке девочек подросткового возраста не было обнаружено выраженного влияния фактора СПКЯ и наличия метаболических нарушений на уровень СРБ. По результатам корреляционного (r=0,29; р<0,05) и двухфакторного анализа (р=0,0028) значимое прямое влияние было подтверждено только для избыточного веса.
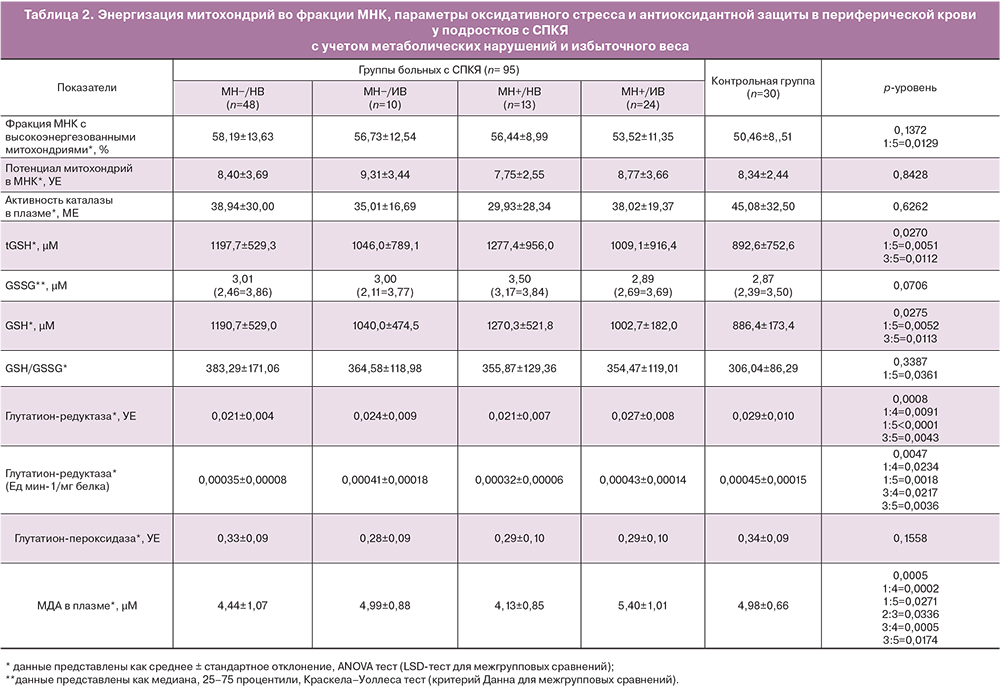
При анализе показателей оксидативного стресса и антиоксидантной активности на выборке девочек-подростков также выявлены закономерности, нехарактерные для взрослых женщин (табл. 2).
Так, у пациенток группы СПКЯ и МН−/НВ наблюдали более низкую концентрацию маркера перекисного окисления липидов – МДА (р=0,0271), более высокий уровень восстановленной формы антиоксиданта GSH (р=0,0051) и его отношения к окисленной форме (р=0,0361), пониженную активность антиоксидантного фермента глутатионредуктазы (р<0,0005) по сравнению с девочками контрольной группы. Наблюдавшиеся отличия свидетельствовали о сниженном уровне окислительного стресса у девочек в группе СПКЯ и МН–/НВ. Снижение активности глутатионредуктазы может объясняться высоким содержанием восстановленной формы GSH, то есть отсутствием необходимости в ее восполнении и соответствующим ингибированием фермента по типу обратной связи. Одновременно с этим у пациенток в данной группе СПКЯ наблюдали более высокую, по сравнению с девочками контрольной группы долю МНК с высокополяризованными митохондриями (р=0,0129), что свидетельствует о высоком уровне сопряжения митохондриального дыхания.
Подростки с СПКЯ и МН+/НВ достоверно не отличались от группы с СПКЯ и МН−/НВ по измеренным показателям окислительного стресса и антиоксидантной защиты. В группе СПКЯ и МН+/НВ также наблюдали тенденцию к поддержанию уровня перекисного окисления липидов на более низком уровне по сравнению со «здоровыми» девочками. Это проявлялось в более низком уровне МДА (р=0,0174), повышенном содержании восстановленной формы GSH (р=0,0112) и низкой активностью глутатионредуктазы (р=0,0042).
При этом для групп пациенток с СПКЯ с избыточным весом была характерна активация процессов перекисного окисления липидов – уровень МДА в плазме крови был значимо выше как у девочек с СПКЯ и МН−/ИВ в сравнении со сверстницами с СПКЯ и МН+/НВ (р=0,0336), так и в группе с СПКЯ и МН+/ИВ в сравнении с МН+/НВ (р=0,0005) и в сравнении с МН−/НВ (р=0,0002). Вместе с тем у девочек с более высоким ИМТ и МН+ отмечена активация антиоксидантного фермента глутатионредуктазы (р=0,0091) для восполнения уровня восстановленного антиоксиданта и нейтрализации перекисей при нарастании оксидативного стресса.
 Для анализа влияния факторов избыточного веса и наличия метаболических нарушений на измеренные показатели на фоне СПКЯ был проведен двухфакторный дисперсионный анализ. Было показано, что фактор избыточного веса оказывает независимое влияние на уровень МДА (р<0,0001), активность глутатионредуктазы (р=0,0088) и процент МНК с высокополяризованными митохондриями (р=0,0286) в плазме крови. В то же время фактор наличия метаболических нарушений не оказывал независимого влияния на изучаемые параметры. Однако было выявлено статистически значимое взаимодействие факторов избыточного веса и наличия метаболических нарушений на уровень МДА (р=0,0273) (рисунок). Таким образом, у девочек с избыточным весом присоединение метаболических нарушений сопровождалось более выраженным повышением уровня МДА, чем у аналогичной группы девочек, но без МН. Притом, девочкам с нормативными параметрами ИМТ наличие МН, напротив, обусловило более низкий уровень МДА, чем аналогичной группе с МН, что говорит о компенсаторном механизме регуляции оксидативного стресса в подростковом возрасте.
Для анализа влияния факторов избыточного веса и наличия метаболических нарушений на измеренные показатели на фоне СПКЯ был проведен двухфакторный дисперсионный анализ. Было показано, что фактор избыточного веса оказывает независимое влияние на уровень МДА (р<0,0001), активность глутатионредуктазы (р=0,0088) и процент МНК с высокополяризованными митохондриями (р=0,0286) в плазме крови. В то же время фактор наличия метаболических нарушений не оказывал независимого влияния на изучаемые параметры. Однако было выявлено статистически значимое взаимодействие факторов избыточного веса и наличия метаболических нарушений на уровень МДА (р=0,0273) (рисунок). Таким образом, у девочек с избыточным весом присоединение метаболических нарушений сопровождалось более выраженным повышением уровня МДА, чем у аналогичной группы девочек, но без МН. Притом, девочкам с нормативными параметрами ИМТ наличие МН, напротив, обусловило более низкий уровень МДА, чем аналогичной группе с МН, что говорит о компенсаторном механизме регуляции оксидативного стресса в подростковом возрасте.
При оценке влияния фактора наличия заболевания СПКЯ на изучаемые параметры и его взаимодействие с факторами повышенного веса и МН с помощью дисперсионного факторного анализа было установлено, что фактор СПКЯ оказывал независимое отрицательное влияние на активность глутатионредуктазы (р=0,0051) и положительное влияние на содержание восстановленной формы GSH (р=0,0026). Притом, факторы наличия СПКЯ и избыточного веса оказывали разнонаправленное влияние на активность глутатионредуктазы. У больных подростков с избыточным весом активность глутатионредуктазы была несколько выше, но не достигла значений у девочек из группы контроля.
Данные о влиянии избыточного веса и МН на изучаемые показатели подтверждались также результатами корреляционного анализа. В общей группе пациенток СПКЯ были выявлены положительные корреляции между ИМТ и активностью глутатионредуктазы (r=0,46; р<0,05, здесь и далее коэффициент ранговой корреляции Спирмена), а также между ИМТ и уровнем МДА (r=0,34; р<0,05). Положительную корреляцию между уровнем МДА и весом (r=0,41; р<0,05) наблюдали в том числе отдельно в группе с СПКЯ и МН−/НВ.
Также найдены отрицательные корреляции между параметрами ИМТ и отношением ОТ/ОБ, являющимся косвенным показателем висцерального ожирения, с уровнем восстановленного GSH (r=-0,30 и r=-0,35; р<0,05) у пациенток с СПКЯ. Следует подчеркнуть, что описанные выше зависимости не были характерны для контрольной группы девочек с нормативными показателями ИМТ, что говорит о реализации других механизмов поддержания гомеостаза в данной группе, отсутствии патологической активации оксидативного стресса и значимого сдвига антиоксидантной активности при повышении ИМТ.
Нарастание уровня оксидативного стресса и снижение емкости защитных антиоксидантных систем происходит не только при увеличении массы тела и нарастании степени висцерального ожирения, но и при присоединении нарушений углеводного обмена. Так, в общей группе пациенток с СПКЯ при повышении уровня инсулина на фоне ПГТТ отмечено увеличение уровня МДА (r=0,47; р<0,05). Выявлено, что дислипидемия у пациенток с СПКЯ в подростковом возрасте при более высоких концентрациях триглицеридов и коэффициента атерогенности ассоциирована со сниженной активностью защитного фермента, катализирующего восстановление перекисей – глутатионпероксидазы (r=-0,27 и -0,34; р<0,05 соответственно). Напротив, в группе контроля выявлена значимая обратная корреляция индекса НОМА-IR и уровня МДА (r=-0,27 и -0,34; р<0,05), то есть при повышении индекса ИР в норме срабатывает компенсаторный защитный механизм снижения интенсивности процессов перекисного окисления липидов и контроля развития оксидативного стресса.
Таким образом, в подростковом возрасте для пациенток с СПКЯ при нормативных значениях ИМТ характерно подавление уровня окислительного стресса и выраженное снижение перекисного окисления липидов за счет более высокого уровня антиоксидантной защиты по сравнению со здоровыми девочками. В то же время избыточный вес и нарушение углеводного обмена, в особенности их сочетание, потенцируют значимую активацию процессов перекисного окисления липидов и оксидативного стресса, при истощении уровня восстановленной формы антиоксиданта GSH и компенсаторном повышении активности антиоксидантных защитных ферментов.
Также выявлена положительная зависимость между повышенным весом и выраженностью системного воспаления, проявляющегося более высокими значениями СРБ в периферической крови (r=0,29; р<0,05) у пациенток с СПКЯ в целом. Важно отметить, что повышенный уровень СРБ у подростков с СПКЯ ассоциирован с накоплением МДА (r=0,45; р<0,05) при симультанной активации антиоксидантного фермента, направленного на нейтрализацию перекисей, каталазы (r=0,31; р<0,05) и снижения содержания восстановленной формы GSH с одновременным повышением концентрации его окисленной формы (r=-0,28; р<0,05). В группе здоровых девочек на момент исследования подобных корреляций обнаружено не было.
Таким образом, для пациенток с СПКЯ в подростковом возрасте характерна ассоциация процессов системного воспаления и оксидативного стресса на фоне митохондриальной дисфункции по типу положительной обратной связи, выраженная при отягощении течения СПКЯ избыточным весом и/или метаболическими нарушениями.
Обсуждение
На основании проведенного исследования можно заключить, что наблюдаемые изменения митохондриального функционирования при СПКЯ в подростковом возрасте имеют разные патогенетические механизмы у пациенток с нормальным и повышенным весом на фоне или в отсутствие МН. Полученные данные позволяют предположить, что у пациенток с СПКЯ с нормативными показателями ИМТ, но без МН срабатывает адаптивный компенсаторный механизм контроля и поддержания на низком уровне процессов перекисного окисления липидов и системного воспалительного ответа. Возможным механизмом реализации данной защитной модели на фоне нормального веса может быть исчерпание пула молекул НАДФ-Н в циклах синтеза и распада гема. Косвенным подтверждением участия билирубин-биливердинового цикла как защитного антиоксидантного механизма у пациенток с СПКЯ служат данные биохимического профиля крови. Выявлено, что концентрация билирубина в крови пациенток с СПКЯ на фоне нормативного ИМТ была значимо выше по сравнению со здоровыми девочками.
С другой стороны, митохондриальный контроль окислительного стресса и переключение стратегии клетки на исчерпание пула восстановленных молекул НАДФН в циклах синтеза и деградации гема обусловливает ограничение использования данного энергетического субстрата ферментом НАДФН-оксидазой иммунных клеток. Реализация данного защитного антиоксидантного механизма может обусловливать снижение показателей системного воспалительного ответа у пациенток с СПКЯ с нормативными показателями ИМТ в подростковом возрасте, наблюдаемое в нашей работе, и повышенную склонность девочек-подростков к неспецифическим инфекционным и соматическим заболеваниям, показанную в работе отечественных авторов [18].
Как известно, физиологический уровень продуктов перекисного окисления липидов и АФК, деликатный баланс про- и антиоксидантов на системном и локальном уровне необходим для развития и функционирования всех клеточных систем [19], в том числе для индукции пролиферации клеток, роста и созревания фолликулов, а также для овуляции [20, 21]. Параллельное поддержание физиологического диапазона уровня АФК и высоко энергетических молекул аденозинтрифосфата, мембранного потенциала митохондрий и уровня неповрежденной митохондриальной ДНК необходимо для запуска и обеспечения процессов клеточной пролиферации [22]. На сегодняшний день в культурах in vitro и на моделях животных выявлено, что избыточно низкое локальное содержание АФК в яичниках при повышенной концентрации антиоксидантов оказывает негативное влияние на процесс созревания и оплодотворения фолликула [20, 21]. С другой стороны, избыточная продукция АФК, превосходящая возможности антиоксидантной защиты, является проапоптотическим фактором и индуцирует повреждение клетки [8, 19]. Наблюдаемое в нашей работе чрезмерное подавление уровня оксидативного стресса у пациенток с СПКЯ с нормативными показателями ИМТ при отсутствии МН в сравнении с девочками контрольной группы может быть одним из механизмов нарушенного фолликулогенеза и ановуляции у данной группы пациенток.
В то же время при избыточном весе МН приводят к накоплению жирных кислот при одновременном снижении утилизации глюкозы. Известно, что накопление свободных жирных кислот в тканях, кроме прямого токсического действия, обусловливает повышение уровня перекисного окисления фосфолипидов и ТГ, увеличение проницаемости митохондрий и их дисфункцию, запуск каскадов реакций, приводящих к апоптозу [23]. Кроме того, свободные жирные кислоты оказывают прямое разобщающее действие на процессы дыхания и синтеза макроэргических молекул в митохондриях, снижая тем самым потенциал на мембране митохондрий. В связи с общностью происхождения митохондрий с бактериями попадающие в циркуляцию поврежденные белки матрикса митохондрий, компоненты митохондриальных мембран, молекулы митохондриальной ДНК рассматриваются иммунными клетками как чужеродные агенты, в ответ на которые происходит активация иммунного ответа [24].
Свободные жирные кислоты способны активировать сигнальный путь провоспалительного фактора NF-κB (nuclear factor kB) и индуцировать продукцию провоспалительных цитокинов МНК и клетками белой жировой ткани у пациенток с СПКЯ в репродуктивном возрасте [25, 26]. Кроме того, при гипергликемии на фоне ИР повышенное окисление глюкозы МНК в пентозофосфатном пути также сопровождается активацией сигнального пути NF-κB и повышением уровня провоспалительных цитокинов и АФК [2]. Интересно отметить, что у женщин с СПКЯ данные механизмы развития хронического системного воспаления описаны на фоне не только избыточного, но и нормального веса в отсутствии МН [25, 26].
Таким образом, в исследуемой выборке девочек с СПКЯ не было обнаружено активации системного воспаления и оксидативного стресса при нормативных показателях ИМТ в отсутствии МН. Однако чрезмерное снижение уровня АФК и оксидативного стресса в этой группе девочек может обусловливать нарушение межклеточного взаимодействия, необходимого для роста и развития фолликула и овуляции, наблюдаемое при СПКЯ.
При отягощении течения СПКЯ у пациенток подросткового возраста избыточным весом и МН выявлено разобщение митохондриального дыхания, активация оксидативного стресса и системного воспаления, что характерно и для взрослых пациенток с СПКЯ, видимо, в связи с перенапряжением деятельности гомеостатических систем и срывом адаптации. Понимание фундаментальных механизмов развития СПКЯ в начале формирования заболевания позволит разработать терапевтические подходы для предотвращения его дальнейшего прогрессирования.
Выводы
- У подростков с СПКЯ и нормальным весом реализуется адаптивный механизм снижения проявлений оксидативного стресса, опосредованный активацией защитного антиоксидантного цикла метаболизма гема в высоко сопряженных митохондриях.
- У подростков с СПКЯ и повышенным весом на фоне угнетения метаболизма гема выражены проявления оксидативного стресса, опосредованные разобщением митохондрий вследствие нарушения метаболизма холестерола и глюкозы, что усиливается при присоединении нарушений углеводного обмена.
- У подростков с СПКЯ и повышенным весом на фоне ИР митохондриальная дисфункция сопряжена с активацией системного воспалительного ответа.



