Structural and functional state of the lower uterine segment myometrium in postpartum women at risk for infection
Aim. To conduct morphological and immunohistochemical analyses of the structure of the lower uterine segment myometrium in postpartum women at risk for infection. Material and methods. Eighteen tissue samples of the lower uterine segment myometrium from 10 patients at risk of infection (study group) and 8 in the control group were analyzed. The analysis included verification and distribution of CD3, CD20, CD68, and actin in myometrial tissue. Results. In patients at risk of infection, the relative area of CD3 in the tissue was 21% greater than in the control group, along with a reduction in the number of CD68 (CD68 area was 19.9% smaller). In the study group, the actin area in the circular myometrium of the lower uterine segment was 5.5% (p <0.05) smaller than in the control group. Conclusion. In pregnant women at risk of infection, an increase in the number of CD3 in the myometrial tissue of the lower uterine segment along with the decrease in the protective function of CD68, and the reduction in the amount of actin in smooth muscle cells by 5.5% (p <0.05) are associated with an impaired postpartum uterine activity.Barinov S.V., Tirskaya Yu.I., Kadtsyna T.V., Lazareva O.V., Medyannikova I.V., Chulovsky Yu.I.
Keywords
The normal course of the postpartum period is mostly determined by the uterine involution and myometrial wound regeneration. Delayed involution of the uterus, especially in the first postpartum week, is associated with the risk of septic complications. Insufficient uterine involution, especially of its lower segment, facilitates transmission of vaginal bacteria into the uterus, thus creating conditions for the development of postpartum endometritis. Infectiousriskfactorsactingatthepregravid stage and during pregnancy create certain conditions, the so-called «premorbid background», which becomes the basis for the subsequent inflammatory processes. Many studies have investigated the state of the postpartum endometrium, including that already affected by the inflammatory process [1–6]. Analysis of the literature showed that the least explored part of the uterus is the myometrium. This fact is attributed to the difficulties in performing a morphological study of myometrial tissue during pregnancy and childbirth. There have been many studies investigating changes in hemodynamics and myometrial thickness [3, 7]. Still, concerning the morphologyofmyometrium, researchershavebeenmainly focused on such problems as placenta accreta spectrum, preeclampsia, and postpartum hemorrhage [8–10].
This study aimed to conduct morphological and immunohistochemical analyses of the structure of the lower segment of the uterine myometrium in postpartum women at risk for infection.
Materials and methods
To accomplish this task, 18 samples of lower uterine segment myometrium were collected. The study group of infectious risk (n = 10) included pregnant women with foci of acute and chronic of extragenital and genital infections, an exacerbation of the infectious process during pregnancy and with a complicated obstetric history including miscarriages, missed miscarriage, preterm birth, postpartum endometritis, and sepsis during previous pregnancies.
The control group (n = 8) comprised healthy primigravida women with a normal course of pregnancy.
All patients included in the study were delivered by elective cesarean sections. Indications for abdominal delivery in the study group included fetal growth restriction and chronic fetal hypoxia. In the control group, the indications for cesarean section included large fetus and breech presentation. A laparotomy was performed through a transverse suprapubic incision. Cesarean section was performed by the transverse lower uterine segment incision. Following the delivery of the fetus, a 10 x 5 mm uterine myometrial tissue specimen was taken in the center of the lower uterine segment incision.
The exclusion criteria for this study were the presence of a uterine scar after a previous cesarean section and an acute inflammatory process of any location.
Surgical specimens were fixed using the generally accepted technique. They were kept in a 10% neutral solution of formalin, then washed with water, dehydrated with alcohol, and embedded in paraffin. For morphological analysis, we used ≤7 μm thick serial sections stained with hematoxylin and eosin.
Immunohistochemical analysis included verification and investigation of the distribution of proteins CD3 (T-lymphocytes), CD20 (B-lymphocytes), CD68 (macrophages), and actin in myometrial tissue. We used monoclonal antibodies to macrophages CD68, CD3, CD20 (Dako), a set of monoclonal antibodies directed against smooth muscle actin (Actin, Skeletal Muscle Ab-2. Clone: '5C5.F8.C7; alpha-Sr-1), murine monoclonal antibodies to CD3 (Mouse anti CD3; peptide CD3egd /CD3w; Clone: F7.2.38; isotype: IgG1, dilution 1:30), mouse monoclonal antibodies to CD20 (Mouse anti CD20; BALB / C; Clone: L26; isotype: IgG2a, Kappa ), mouse monoclonal antibodies to CD68 (Mouse anti CD68, macrophage; Clone: KP1; isotype: IgG1).
The results were evaluated quantitatively by computer-assisted microscopic image analysis, including a Nikon Eclipse E400 microscope, a Nikon DXM1200 digital camera, a personal computer Intel Pentium 6, AST-1 software version 2.12 and Videotest-Morphology 5.0. We chose five most representative fields of view and took photographs at an optical magnification of 400 × (40× objective with a 10× eyepiece).
Statistical analysis
Statistical analysis was performed using the «STATISTICA 6.0» software. Quantitative variables, including means, standard deviation, median, and quartiles Q1 and Q3, were calculated and tested for normality of distribution. The groups were compared by nonparametric analyses. The Kolmogorov-Smirnov test was used for comparing continuous variables between groups. Differences between the groups were considered statistically significant at p<0.05. Data were reported as the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format.
Results
Histological examination of the study group specimens often showed zones of marked vascular congestion and proliferation of connective tissue in the myometrial tissue of the lower uterine segment of puerperal patients at risk of infection. Such changes were not typical for puerperal women in the control group.
There were no signs of acute inflammation with infiltration of muscle tissue with leukocytes and lymphocytes in the myometrial tissue specimens from the patients of the compared groups. Only insignificant perivascular accumulations of these cells were noted.
Morphometric analysis was carried out using the ImageJ 1.46 program on discriminated image masks of nuclei of different shapes. Comparative analysis of all specimens in the study group and the control group (50 fields of view each) with the determination of the central trend (median) showed that the numerical density of nuclei in myometrial tissue of the lower uterine segment of the compared groups did not differ significantly (Table 1).
Therefore, conventional and morphometric histological analysis showed the structural preservation of the myometrial tissue of the lower uterine segment of the puerperal patients of both compared groups; the differences were found in the blood vessel congestion and proliferation of connective tissue.
In the course of immunohistochemical analysis, verification and investigation of the distribution of CD3 positive (T-lymphocytes), CD20 (B-lymphocytes), CD68 (macrophages) cells, and actin in the myometrium were carried out.
CD3-positive structures were detected in all myometrial tissue specimens. Patients in the compared groups had different distribution and number of labeled T-lymphocytes. There were more CD3 cells in the myometrial tissue of the lower uterine segment in patients of the study group; these cells were often seen in groups located between and within myometrial layers.
Most of T-lymphocytes were detected near large or small vessels in the form of single cells or infiltration. Infiltrate-like accumulations of T-lymphocytes were more often detected in myometrial tissue of the lower uterine segment of puerperal patients at risk of infection.
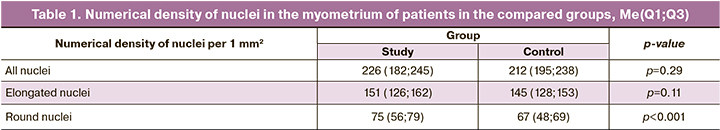
Morphometric analysis of immunohistochemical preparations was performed using the ImageJ 1.46 software. On 8-bit images, the histogram thresholds corresponding to the marked cells were indicated (Fig. 1).
Further analysis was carried out on the obtained black and white images. In each group, 70 fields of view were analyzed at a magnification of x10 (objective), the matrix of a digital camera DCM500: 2592 x 1944 = 5,040,000 pixels, and the 2.2 μm pixel size. CMOS image sensor 1/2.2".
Using the tools of the ImageJ 1.46 program, the number and relative area of particles (%) in the field of view were determined. This approach allowed a morphometric analysis of high accuracy.
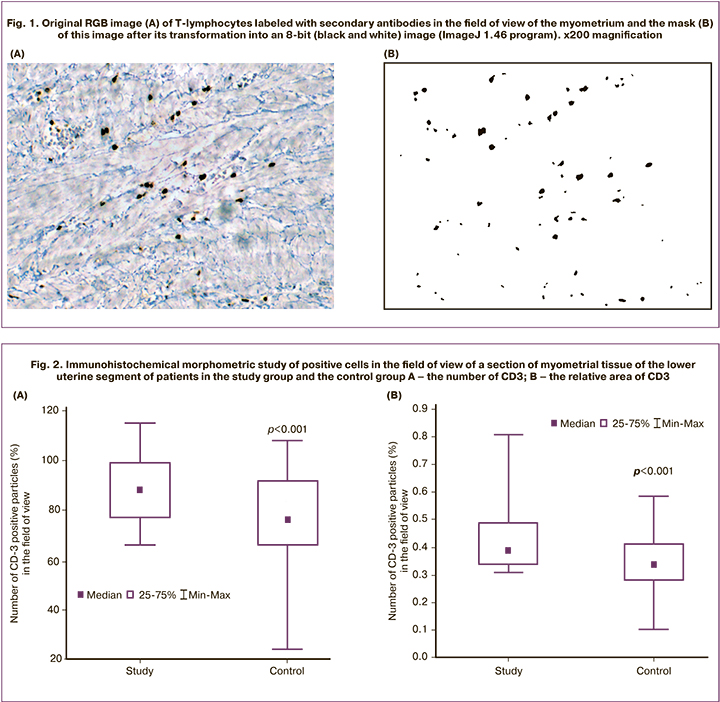
The number of CD3-positive cells in the field of view of myometrial tissue of the lower uterine segment in the study group patients was 17.0% higher than in patients of the control group (Fig. 2). This indicator reflects the numerical density of CD3-positive cells per unit section area. However, it should be noted that some of the cells have a complex shape due to the overlap of cells on top of each other (Fig. 2A), thus somewhat reducing the cell density index.
For comparison, the most reliable indicator is the relative area (%) of particles, which fully corresponds to the area of T-lymphocytes. The relative area (%) of CD3-positive cells (T-lymphocytes) in the field of view of a section of myometrial tissue of the lower uterine segment of patients in the study group was 21.0% higher than in patients of the control group (Fig. 2B).
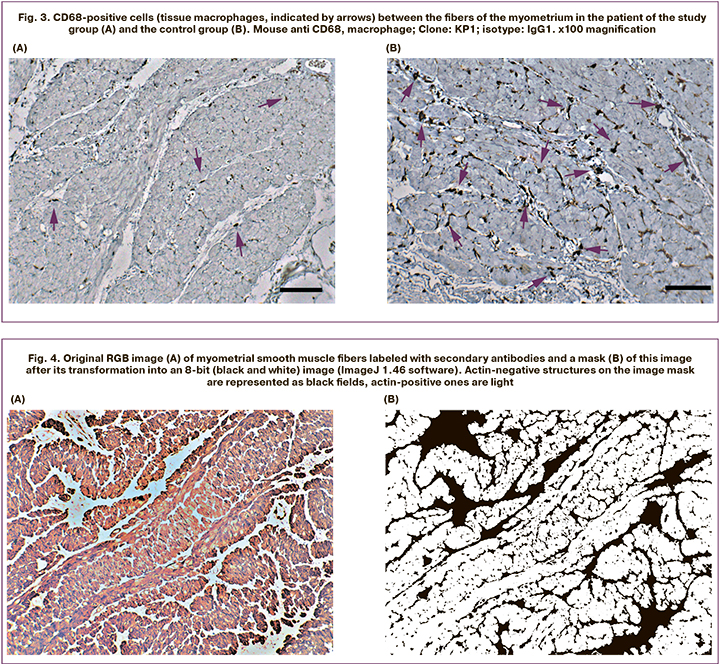
CD68 (macrophages). The protein is expressed on blood monocytes and tissue macrophages. Besides, it is present on lymphocytes, fibroblasts, and endothelial cells. Tissues macrophages (histiocytes), in contrast to T-lymphocytes, have a more complex shape and pronounced processes. These processes, stained during the immunohistochemical reaction, penetrate between the fibers of the myometrium. The vessels showed rounded CD68-positive cells (probably monocytes and lymphocytes).
Myometrial tissue of the lower uterine segment of puerperal patients in the study group contained significantly fewer visually detected CD68 marks (Fig. 3). More accurate data about the distribution of CD68-positive cells were obtained by morphometric examination using the ImageJ 1.46 software.
As in the study of CD68, 70 fields of view were analyzed in each group at a magnification of x10 (objective), the matrix of a digital camera DCM500: 2592 x 1944 = 5,040,000 pixels, and the 2.2 μm pixel size. However, in contrast to round T lymphocytes, different introductory conditions were used. All particles were taken into account. This allowed estimation of the area of not only CD68-positive bodies, but also cell processes (macrophages).
The analysis findings showed that the relative area (%) of CD68-positive cells (macrophages) and their processes in the field of view of the sections of the lower uterine segment of puerperal patients in the study group was 19.9% lower than in women in the control group.
CD20 (B-lymphocytes). The ligand of the CD20 receptor is not known, the protein is involved in providing an optimal B-lymphocytic immune response, in particular against T-lymphocyte-independent antigens. It regulates the activation and proliferation of B-lymphocytes, expressed on B-lymphocytes. In our study, B-lymphocytes were detected, in small numbers, mainly in the vessels. Morphometric analysis showed no statistically significant differences between the groups in terms of the number or area of CD20-positive material. Thus, according to the data of immunohistochemical morphometric analysis, the structural and functional state of the population of CD3- and CD68-positive cells in the myometrial tissue of the lower uterine segment of patients in the compared groups was statistically significantly different. A complicated past medical history of patients in the infectious risk group was associated with the activation of T-lymphocytes and a decrease in the activity of tissue macrophages. This indicates that after delivery, the conditions for restoring the circular myometrium of the lower segment of the uterus will also differ. In women of the infectious risk group, the nonspecific reaction (diferon – macrophages) to damage decreases, and cellular immunological reactivity increases. The fundamental basis of humoral immunity (B-lymphocytes) probably does not change.
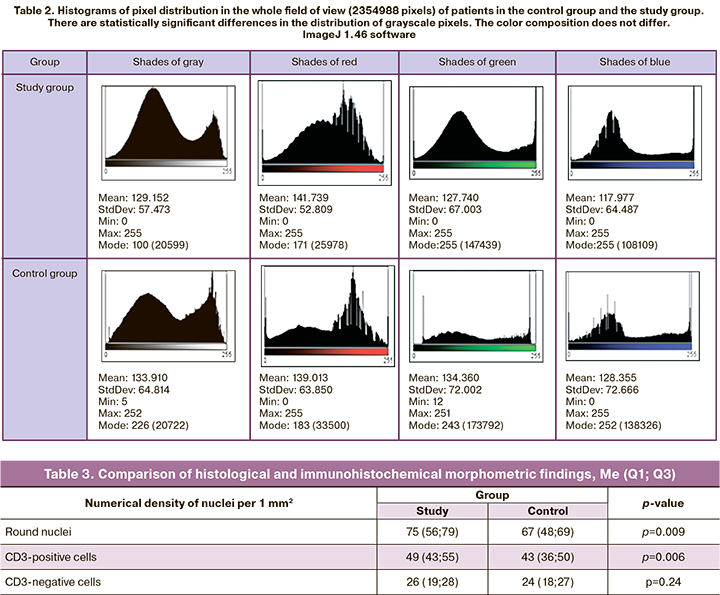
Immunohistochemical analysis of actin allowed an accurate verification and location of the main component of the contractile apparatus of smooth muscle fibers of the myometrium and to separate these fibers from the surrounding connective tissue and vessels (Fig. 4A).
The ImageJ 1.46 software provides the possibility to clearly distinguish between actin-positive and actin-negative structures (zones) of the myometrium (Fig. 4B), as well as to determine their relative area.
The indicator of the area of actin-positive structures varied significantly in different fields of vision and different patients from 15 to 82% and from 50 to 80% in the study group and the control group, respectively. The central tendencies (median) of this indicator differed slightly (by 5.5%), but statistically significantly (p<0.05). All this indicated that patients with infectious risk had impaired structural and functional basis of the contractile function of the circular myometrium of the lower uterine segment. This group had less actin per unit area/volume of the myometrium than the control group. The extreme values of the variation series were notably different (by 37%).
Therefore, the immunohistochemical morphometric analysis of the distribution and amount of actin in the visual field also does not confirm the null statistical hypothesis (belonging to the same general population). This can be caused either by an increase in the content of connective tissue in the myometrium of the lower uterine segment, which is actin-negative or by a change in the quality of actin itself.
We determined the qualitative changes in actin (actin complex – labeled secondary antibodies) on immunohistochemical preparations by analyzing histograms of the pixel composition of images in different zones of actin accumulation (ImageJ 1.46 software). To begin with, we compared different zones of actin-positive structures (muscle fibers) in the tested, visual field in the study group, which did not disprove the null statistical hypothesis - the zones are identical, actin-positive structures in the degree of binding to antibodies belong to the same general population. In specimens from the control group patients, similar data were obtained. This indicates the homogeneity of the myometrium in different parts of the lower segment.
There were some visible differences in the histograms of small areas of the myometrial tissue of the lower uterine segment preparations from different groups. However, no statistically significant differences were found between the groups in the quantitative indicators of the pixel composition of the histograms of different myometrial zones. This observation indicates the identity of the properties (binding of antibodies) of the myometrium in the patients of the compared groups (Table 2).
When comparing entire visual fields, rather than individual tissue fragments of the lower uterine segment myometrium, statistically significant differences in the content of light/dark pixels were revealed. Patients of the control group had more dark pixels (the mode – 100), corresponding to actin – positive muscle tissue. The color composition of the image pixels did not differ (Table 2). Consequently, these patients had less connective tissue in the circular myometrium (light pixels closer to shade 255).
A comparison of the two types of morphometric studies showed that statistically significant differences between the groups of patients were due to the different content of CD3-positive cells (T-lymphocytes) (Table 3). These data indicate a difference in the reaction of leukocyte and lymphocytic blood differones in patients with an uncomplicated and complicated medical history.
Analysis of histograms of pixel distribution in muscle tissue zones on preparations stained with hematoxylin and eosin showed an almost complete identity of indicators (mean, mode) for grayscale and primary colors (RGB) in patients of the study group and the control group. These findings confirm the data of the analysis of immunohistochemical preparations and testify to the identical tinctorial properties of circular muscle fibers in the compared groups.
Discussion
Postpartum inflammatory complications account for 11% of maternal deaths. Pregnant women with foci of chronic genital and extragenital infection were included in the group at risk for infectious complications during pregnancy. The hormonal and immunological changes that occur throughout pregnancy are necessary to support a healthy pregnancy, but also dramatically affect female susceptibility to autoimmune and infectious diseases. [11]. Therefore, the combination of infection and pregnancy is especially unfavorable [12].
Mild, persistent and asymptomatic infection in a pregnant woman can lead to severe complications of pregnancy (miscarriages, missed miscarriageы, preterm birth, placental attachment abnormalities, fetal growth restriction) and childbirth (prelabor rupture of membranes, hypotonic uterine action, postpartum purulentseptic complications) [13–15].
Of particular importance are acute and exacerbation of chronic infectious diseases during pregnancy, a large number (high index) of inflammatory processes, their recurrence, duration, and severity.
The development of the inflammatory process in the postpartum uterus depends on many factors. The involution and pro-inflammatory processes occurring in the uterus in the postpartum period can be influenced by many factors. Contamination with microorganisms, the patient's health state, the characteristics of the course of pregnancy and the birth, and the integrity of the uterine wall are also critical. The state of the uterine mucosa in the presence of an infectious process has been studied quite widely [1–6]. At the same time, studies investigating effects the structure of the myometrium are lacking. In the literature, there are isolated experimental studies of the histo- and cytostructure of the myometrium of the postpartum uterus in animals [16]. In these works, the state of myometrial cells was studied using electron microscopy, but the cellular composition and state of the primary contractile protein actin were not studied. In a study by Ivanisevic M., conducted in 2010, a panel of antibodies specific for T-cells, monocytes, natural killer cells, and B cells were assessed immunohisto-chemically. However, the findings of pregnant and non-pregnant patients were compared without determining the predisposition to postpartum inflammatory complications [17].
In our study, an attempt has been made to investigate the structure of the uterine myometrium in terms of the susceptibility for the inflammatory process in postpartum women at risk of infection.
The findings of morphometric analysis of histological and immunohistochemical preparations of the patients of the infectious risk group and the control group indicated the presence of statistically significant differences in the content of T-lymphocytes, macrophages, the area of connective tissue and muscle fibers. In patients of the infectious risk group, small foci of accumulation of T-lymphocytes were more often detected. There were no signs of inflammation in the compared groups. All this indicated that at the time of taking the myometrium section (during the cesarean section), the structural and functional state of the lower myometrium segment and its reactivity in patients of both groups were statistically significantly different. In patients at risk of infection, the relative area of CD3-positive structures in the tissue was 21% higher than in the control group against the background of a decrease in the protective function of tissue macrophages (the relative area of CD68-positive structures was 19.9% less than in the control group). In the study group, the area of actin-positive structures in the circular myometrium of the lower segment was 5.5% (p<0.05) smaller than in the control group. A decrease in the structural and functional basis of the contractile function of myocytes in puerperal women of the infectious risk group in the postpartum period can lead to a reduction of the contractile ability of the myometrium of the lower segment, and, consequently, to impair uterine involution.
The study showed that patients at risk of infection risk had impaired structural and functional basis of the myometrial contractile function. Impaired postpartum uterine involution in puerperal women creates the potential for the transmission of pathogens into the uterine cavity and the development of postpartum endometritis.
Conclusion
Compared with the control group, the myometrial tissue of the lower uterine segment of puerperal patients at risk of infection contains a significantly (p<0.05) higher number of T-lymphocytes (CD3) along with the decrease in the protective function of tissue macrophages (CD68) and a 5.5% reduction in the amount of the contractile protein actin in smooth muscle cells (p<0.05). In the postpartum period, these changes may lead to an impaired uterine activity and the development of postpartum endometritis.
References
- Стрижова Н.В., Кутенко А.Н., Гарвиленко А.С. Сходство и различия субинволюции матки и послеродового эндометрита. Акушерство и гинекология. 2005; 1: 30-4. [Strizhova N.V., Kutenko A.N., Garvilenko A.S. Similarities and differences in subinvolution of the uterus and postpartum endometritis. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2005; 1: 30-4. (in Russian)].
- Абрамова В.Н., Гайдуков С.Н., Тайц А.Н. Значение иммуногистохимического исследования при патологии эндометрия у пациенток с неудачами программах вспомогательных репродуктивных технологий. Педиатр. 2017; 8(1): 82-8. [Abramova V.N., Gajdukov S.N., Tajc A.N. Importance of an immunohistochemical study in endometrial pathology in patients with failures in assisted reproductive technology programs. Pediatr/ Pediatrician. 2017; 8(1): 82-8. (in Russian)].
- Логутова Л.С., Титченко Л.И., Тареева Т.Г., Баринова И.В., Титченко Ю.П., Барыкина О.П., Федотова А.В. Новые ультразвуковые технологии в диагностике стертых форм послеродового эндометрита. Российский вестник акушера-гинеколога. 2006; 6(4): 23-6. [Logutova L.S., Titchenko L.I., Tareeva T.G., Barinova I.V., Titchenko Yu.P., Barykina O.P. New ultrasound technologies in the diagnosis of erased forms of postpartum endometritis. Rossijskij vestnik akushera-ginekologa/ Russian Bulletin of the Obstetrician-Gynecologist. 2006; 6(4): 23-6. (in Russian)].
- Горин В.С., Серов В.Н., Бирюкова Л.А., Сагинор М.Е. Диагностика и лечение послеродового эндометрита. Вопросы гинекологии, акушерства и перинатологии. 2007; 6(4): 72-83. [Gorin V.S., Serov V.N., Biryukova L.A., Saginor M.E. Diagnosis and treatment of postpartum endometritis. Voprosy ginekologii, akusherstva i perinatologii/Questions of gynecology, obstetrics and perinatology. 2007; 6(4): 72-83. (in Russian)].
- Gonik B., Shannon R.L., Shawar R., Costner M., Seibel M. Why patients fail antibiotic prophylaxis at cesarean delivery: histologic evidence for incipient infection. Obstet Gynecol. 1992; 79(2): 179-84.
- Carbillon L., Lemaistre A.I., Manoux A., Cedrin-Durnerin I., Tepper M., Hugues J.N. et. al. Immunohistochemical characterization of the inflammatory infiltrate in the human endometrium prior to in vitro fertilization and embryo transfer. Pathol. Biol. (Paris). 1998; 46(1): 21-8.
- Атилла С., Степанькова Е.А., Сичинава Л.Г. Допплерометрия маточного кровотока в диагностике послеродового эндометрита. Вопросы гинекологии, акушерства и перинатологии. 2002; 1(2): 32-5. [Atilla S., Stepan'kova E.A., Sichinava L.G. Dopplerometry of uterine blood flow in the diagnosis of postpartum endometritis. Voprosy ginekologii, akusherstva i perinatologii/ Questions of gynecology, obstetrics and perinatology. 2002; 1(2): 32-5. (in Russian)].
- Farhana M., Tamura N., Mukai M., Ikuma K., Koumura Yu., Furuta N. et al. Histological characteristics of the myometrium in the postpartum hemorrhage of unknown etiology: a possible involvement of local immune reactions. J. Reprod. Immunol. 2015; 110: 74-80. https://dx.doi.org/10.1016/j.jri.2015.04.004.
- Kim J.S., Romero R., Cushenberry E., Kim Y.M., Erez O., Nien J.K. et al. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta. 2007; 28(5-6): 571-6. https://dx.doi.org/10.1016/j.placenta.2006.07.007.
- Hecht J.L., Karumanchi S.A., Shainker S.A. Immune cell infiltrate at the utero-placental interface is altered in placenta accreta spectrum disorders. Arch. Gynecol. Obstet. 2020; 301(2): 499-507. https://dx.doi.org/10.1007/s00404-020-05453-1.
- Robinson D.P., Klein S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis/ Horm. Behav. 2012; 62(3): 263-71. https://dx.doi.org/10.1016/j.yhbeh.2012.02.023.
- Silasi M., Cardenas I., Kwon J.Y., Racicot K., Aldo P., Mor G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015; 73(3): 199-213. https://dx.doi.org/10.1111/aji.12355.
- Racicot K., Mor G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017; 127(5): 1591-9. https://dx.doi.org/10.1172/JCI87490.
- Woodd S.L., Montoya A., Barreix M., Pi L., Calvert C., Rehman A.M. et al. Incidence of maternal peripartum infection: A systematic review and meta-analysis. PLoS Med. 2019; 16(12): e1002984. https://dx.doi.org/10.1371/journal.pmed.1002984.
- Тирская Ю.И., Баринов С.В., Долгих Т.И., Пьянова Л.Г., Чернышев А.К., Ковалева Ю.А., Корнеев Д.В., Шамина И.В. Прогнозирование инфекционного риска и способ профилактики послеродового эндометрита у родильниц инфекционного риска. Акушерство и гинекология. 2014; 5: 37-42. [Tirskaya Yu.I., Barinov S.V., Dolgih T.I., P'yanova L.G., Chernyshev A.K., Kovaleva Yu.A. et. al. Prediction of infectious risk and a method for the prevention of postpartum endometritis in puerperas of infectious ris. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2014; 5: 37-42. (in Russian)].
- Долгих О.В., Агафонов Ю.В., Зашихин А.Л. Гладкая мышечная ткань матки в период раннего пуэрперия механизмы инволюции. Экология человека. 2012; 12: 31-5. [Dolgih O.V., Agafonov Yu.V., Zashihin A.L. Smooth muscle tissue of the uterus during the early period of puerperium involution mechanisms. Ekologiya cheloveka/Human ecology. 2012; 12: 31-5. (in Russian)].
- Ivanisevic M., Segerer S., Rieger L., Kapp M., Dietl J., Kämmerer U. Antigen-presenting cells in pregnant and non-pregnant human myometrium. Am. J. Reprod. Immunol, 2010; 64(3): 188-96. https://dx.doi.org/10.1111/j.1600-0897.2010.00858.x.
Received 10.04.2020
Accepted 21.04.2020
About the Authors
Sergey V. Barinov, Dr.Med.Sci., Professor, Head of the Department of Obstetrics and Gynecology №2, Omsk State Medical University.Tel.: +7(913)633-80-48. E-mail: barinov_omsk@mail.ru. ORCID: 0000-0002-0357-7097. 644099, Russia, Omsk, Lenin’s str., 12.
Yuliya I. Tirskaya, Dr.Med.Sci., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University.
Tel.: +7(3812)26-24-58. E-mail: yulia.tirskaya@yandex.ru. ORCID: 0000-0001-5365-7119. 644099, Russia, Omsk, Lenin’s str., 12.
Tat’yana V. Kadtsyna, Ph.D., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University.
Tel.: +7(3812)24-06-58. E-mail: tatianavlad@list.ru. ORCID: 0000-0002-0348-5985. 644099, Russia, Omsk, Lenin’s str., 12.
Oksana V. Lazareva, Ph.D., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University.
Tel.: +7(3812)24-06-58. E-mail: lazow@mail.ru. ORCID: 0000-0002-0895-4066. 644099, Russia, Omsk, Lenin’s str., 12.
Irina V. Medyannikova, Dr.Med.Sci., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University.
Tel.: +7(3812)24-06-58. E-mail: mediren@mail.ru. ORCID: 0000-0001-6892-2800.
Yurij I. Chulovsky, Ph.D., Associate Professor, Department of Obstetrics and Gynecology №2 Omsk State Medical University.
Tel.: +7(3812)24-06-58. Е-mail: akusheromsk@rambler.ru. 644099, Russia, Omsk, Lenin’s str., 12.
For citation: Barinov S.V., Tirskaya Yu.I., Kadtsyna T.V., Lazareva O.V., Medyannikova I.V., Chulovsky Yu.I. Structural and functional state of the lower uterine segment myometrium in postpartum women at risk for infection.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 8: 96-104 (in Russian)
https://dx.doi.org/10.18565/aig.2020.8.96-104



