Plasma hemostatic system in patients with the novel coronavirus disease 2019 (COVID-19)
Aim. To investigate plasma hemostasis in patients with different levels of COVID-19 severity. Materials and methods. The study included 46 patients with confirmed COVID-19, who were stratified into four groups based on disease severity as mild (1), moderate (2), severe (3), and critically severe (4). Laboratory investigations included APTT, Quick's prothrombin time, concentration of D-dimer, fibrinogen, ATIII, and platelet count. Results. D-dimer concentration >450 ng/ml was found in 100% of patients increasing from 973 (545–1635) ng/ml in group 1 to 13513 (7627–22512) ng/ml in group 4 (p<0.05). Patients in group 2 had the highest fibrinogen level averaging 6.1(3.9–6.4) g/L. The highest and lowest platelet counts were in groups 1 (323x109) and 4 (155×109), respectively, (p <0.05). The ATIII levels in groups 1 and 2 were within the normal range, while in groups 3 and 4, they were decreased to 76% and 64%, respectively (p <0.05). All patients enrolled in the study had normal values of APTT and Quick's prothrombin time. Unfavorable outcomes were observed at a D-dimer concentration> 3633 ng/ml (specificity 92.3%, sensitivity 100%, AUC 0.94, p <0.001, positive predictive value 99.1%, and negative predictive value 100%) and ATIII <70.8% (specificity 70%, sensitivity 82.6%, AUC 0.85, p <0.001, positive predictive value 30.5%, and negative predictive value 96.1%). Conclusion. The levels of D-dimer, fibrinogen, and ATIII were found to be associated with the severity of COVID-19.Markelov M.I., Beznoshchenko O.S., Ivanets T.Yu., Pyregov A.V., Esayan R.M., Gavrilova T.Yu., Krechetova L.V.
Keywords
The novel corona virus infection (now classified as COVID-19), first reported in December 2019 in Wuhan, China, has contributed to significant mortality in several countries. Along with respiratory failure, coagulopathy is common among patients with COVID-19, as evidenced by increased plasma levels of fibrinogen and D-dimer [1].
The major clinical challenge associated with COVID-19 is severe interstitial pneumonia. This inflammatory condition, resulting from the persistence of the virus in the lung tissue, leads to severe hemostatic abnormalities. In patients with coronavirus infection, hemostatic balance shifts toward a hypercoagulable state leading to microvascular thrombosis, pulmonary embolism, and lower limb deep vein thrombosis [2]. The severe inflammatory state secondary to the infection leads to a derangement of hemostasis that has been described as acute disseminated intravascular coagulation (DIC), based on decreased platelet count, prolonged prothrombin and activated partial thromboplastin time (aPTT), increased fibrinogen degradation products such as D dimer, as well as low fibrinogen and antithrombin III [3].
Themechanismof COVID-19-associatedcoagulopathy is multicomponent. The effect of SARS-CoV-2 infection on coagulation and fibrinolysis is regulated by proinflammatory cytokines such as IL-1-β, TNF-α, and IL-6. An increase in serum proinflammatory cytokines in patients with COVID-19 is due to the activation of innate and adaptive immunity in response to damage to many body cells, including type I alveolocytes, cardiomyocytes, liver cholangiocytes, colonocytes, esophageal keratinocytes, epithelial cells of the stomach, ileum and rectum, proximal kidney tubules, and bladder. The dysfunction of endothelial cells caused by infection leads to excessive thrombin production and suppression of fibrinolysis, which indicates a state of hypercoagulability [4]. Also, with coronavirus infection, damage to blood vessel endothelial cells leads to their activation and death. As a result, tissue factor and von Willebrand factor (VWF) are released into the blood, which leads to increased activation of prothrombinase along the external coagulation pathway by activating the coagulation factor VII. In turn, due to the increased blood level of VWF, platelet adhesion to type I and III collagen occurs more actively at the sites of endothelial damage through GP1b on the platelet membrane. In areas of thrombus formation, exocytosis of dense granules containing low molecular weight compounds such as ADP, ATP, serotonin, calcium and magnesium ions, GDP, GTP, etc., and α-granules containing VWF, fibrinogen, fibronectin, thrombospondin, clotting factors, fibrinolysis, and anticoagulants (plasminogen, protein S), proinflammatory cytokines and chemokines (platelet factor 4, β-thromboglobulin). This leads to the autoactivation of platelets and a change in the expression profile of their surface receptors. Therefore, it is possible to identify the main links affecting the development of coagulopathy: an increase in the level of fibrinogen and proinflammatory cytokines, activation of the endothelium, the release of tissue factor and VWF, and platelet activation.
International Society of Thrombosis and Hemostasis (ISTH) interim guidance on recognition and management of coagulopathy in COVID-19 suggests considering the use of prophylactic doses of low molecular weight heparin (LMWH) in all patients with COVID-19 and regular monitoring of aPTT, D-dimer, prothrombin time, platelet count, and fibrinogen [5]. However, despite research evidence indicating high rates of thromboembolic complications in patients with COVID-19 [6, 7] and the relationship of pulmonary vessel microthrombosis with the development of acute respiratory distress syndrome (ARDS) [8], no unified criteria have been developed regarding anticoagulant prophylaxis, therapy and monitoring of plasma hemostasis in such patients, as well as criteria that allow prediction of a severe course of viral pneumonia. Also, the relationship between changes in plasma hemostasis and the course of the disease has not been investigated. Therefore, the present study aimed to examine plasma hemostasis in patients with different levels of COVID-19 severity.
Materials and methods
The study included 46 patients admitted to the infectious diseases hospital at the V.I. Kulakov NMRC for OG&P of Ministry of Health of Russia from 04/23/2020 to 05/26/2020. SARS-CoV-2 was identified by RT-PCR. Patient inclusion criteria for the study were the patient's consent to participate in the study and a confirmed diagnosis of COVID-19. The exclusion criteria were pregnancy, an unconfirmed diagnosis of COVID-19, non-compliance with the protocol, and the pre-analytical stage of the study.
Patients were stratified by severity into four groups under the Interim Guidelines of the Ministry of Health of the Russian Federation for the Prevention, Diagnosis, and Treatment of New Coronavirus Infection COVID-19, Version 7 of 06/03/2020 as mild, moderate, severe, critically severe [9]. Criteria for clinical severity of a mild disease included body temperature (T) <38°C, cough, weakness, sore throat. No criteria for moderate and severe disease. Moderate disease criteria included body temperature> 38°C, respiratory rate> 22/min, shortness of breath on exertion, typical chest CT findings of COVID-19 (mild degree of lung involvement, CT 1–2), SpO2<95%, CRP>10 mg/l. Severe disease criteria included – respiratory rate> 30/min, SpO2 ≤ 93%, PaO2/FiO2 ≤ 300 mm Hg. Art., a decrease in the level of consciousness, agitation, unstable hemodynamics (systolic blood pressure less than 90 mm Hg or diastolic blood pressure less than 60 mm Hg, urine output less than 20 ml/hour), acute respiratory failure with the need for respiratory support, typical chest CT findings of COVID-19 (significant or subtotal lung involvement; CT 3–4), arterial blood lactate> 2 mmol/L, qSOFA> 2 points. Critically severe disease criteria included acute respiratory failure with the need for respiratory support, septic shock, multiple organ failure, typical chest CT findings of COVID-19 (significant or subtotal lung involvement, CT 4), or ARDS manifestations.
Clinical laboratory monitoring was conducted daily. Fasting blood samples were taken in the morning from a peripheral vein using the S-Monovette® blood collection system (Sarstedt, Germany) with the anticoagulant K3EDTA to determine platelet count (PLT) and with 3.2% sodium citrate in a ratio of 1:9 for coagulation tests. Platelet-poor citrate plasma was obtained by centrifugation for 15 min at 3000 rpm. In the resulting plasma, the analyzed parameters included aPTT, Quick's prothrombin time, the concentration of D-dimer, fibrinogen, and ATIII. Hemostatic parameters were analyzed on ACL Top 700 Hemostasis Testing System (Instrumentation Laboratory, Werfen, USA), platelet count – on an SYSMEX XT-4000 hematology analyzer (Sysmex, Japan).
Statistical analysis
The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test. Quantitative variables were expressed as the median (Me) and interquartile range (25–75%). The statistical significance of between-group differences for continuous variables was tested with the Mann–Whitney test using the IBM SPSS Statistics software. The critical level of significance when interpreting the results of statistical analysis was considered at p <0.05. ROC-curves were constructed using the MedCalc software to identify the threshold values of D-dimer and antithrombin III for predicting unfavorable outcome.
Results and discussion
The study comprised 46 patients, including 28 men (60.9%) and 18 women (39.1%). The mean age of men and women was 61.5 (53.5–67.5) and 64.5 (57–73) years, respectively. Four patients (8.7%) reported a smoking habit. Twenty-two (48%), 2 (4.3%), and 6 (13%) patients had a history of hypertension, type 1 diabetes mellitus, and type 2 diabetes mellitus, respectively.
Normal (BMI 20–24.9), underweight (BMI <20), and overweight (BMI 25–29.9) (kg/m2) body weight was found in 8 (17.4%), 1 (2.2%), and 21 (45.6%) patients, respectively. Obesity was observed in 16 patients (34.7%), of which 10 patients (21.7%) had BMI from 30 to 34.99, 5 patients(10.9%) had BMI from 35 to 39.99, and 1 patient (2.2%) had BMI> 40. All patients lived in Moscow or the Moscow region.
The study showed no differences in the aPTT and Quick’s prothrombin time in patients with coronavirus infection from normal values (p> 0.05), which were 30.3 (27.4–35.2) sec. for aPTT and 75 (70.2–82.5)% for Quick’s prothrombin time. Even in patients with critically severe COVID-19 who developed disseminated intravascular coagulation (DIC), there was no prolongation of aPTT and no decrease in Quick’s prothrombin time. These results are consistent with the literature data regarding characteristic features of DIC in patients with COVID-19. According to them, the prolongation of APT and decrease in Quick’s prothrombin time are extremely rare in the second and third phases of DIC [10, 5].
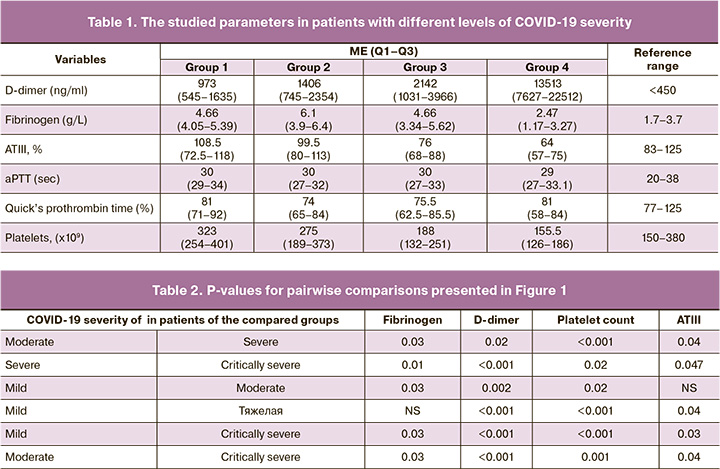
All patients with a confirmed diagnosis of COVID-19 had serum D-dimer concentrations >450 ng/ml (Fig. 1A, Table 1). Moreover, serum D-dimer concentration increased with increasing in COVID-19 severity. This suggests that deterioration of the patient's condition is associated with the development of a hypercoagulative state. Hypercoagulative state results in intravascular coagulation, activation of the fibrinolytic system, and breaking down fibrin clots to the products of fibrin degradation, including D-dimers. Patients with critically severe COVID-19 had D-dimer concentrations above 13 000 (7627–22 512) ng/ml conferring a high risk of developing PE [11]. In this study, six patients were in critical condition, 5 of whom died, and one patient recovered. This observation indicates the need for regular monitoring of the D-dimer concentration in patients in severe and extremely serious conditions.
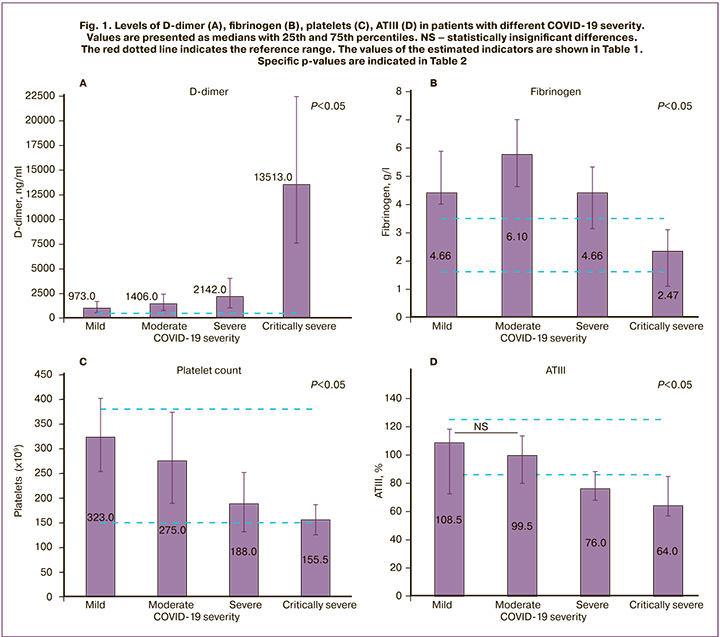
In patients with an unfavorable outcome, on days 5–10 of observation, D-dimer concentration increased to 6500–7000 ng/ml (Fig. 2). Unfavorable outcomes were observed at a D-dimer concentration> 3633 ng/ml (specificity 92.3%, sensitivity 100%, AUC 0.94, p <0.001, positive predictive value 99.1%, and negative predictive value 100%). These findings are consistent with results reported by other authors [12].
 Our results are consistent with previously published studies reporting a 2–3-fold increase in D-dimer in intensive care patients [13], and suggesting that an increased level of D-dimer is associated with the risk of ARDS [14]. A multicenter retrospective study in China showed that the concentration of D-dimer was significantly different in groups of deceased and surviving patients. An increase in D-dimer concentration >1000 ng/ml was statistically significantly associated with an unfavorable outcome (p <0.01) [2]. These results are compatible with our data on significant differences in D-dimer levels between groups with different severity of COVID-19.
Our results are consistent with previously published studies reporting a 2–3-fold increase in D-dimer in intensive care patients [13], and suggesting that an increased level of D-dimer is associated with the risk of ARDS [14]. A multicenter retrospective study in China showed that the concentration of D-dimer was significantly different in groups of deceased and surviving patients. An increase in D-dimer concentration >1000 ng/ml was statistically significantly associated with an unfavorable outcome (p <0.01) [2]. These results are compatible with our data on significant differences in D-dimer levels between groups with different severity of COVID-19.
The fibrinogen levels in patients with different levels of COVID-19 severity are shown in Fig. 1B, and Table 1. Fibrinogen is a marker of the acute phase of inflammation. All groups, except for the critically severe disease, had hyperfibrinogenemia. At the onset of the disease, there was an increase in the plasma level of fibrinogen and its accumulation; it reached the highest levels in patients with moderate COVID-19 and amounted to 6.1(3.9–6.4) g/l. If a patient progressed to severe disease, the «consumption» phase began, and the accumulated fibrinogen was spent on the formation of fibrin clots. As a result, plasma fibrinogen decreased.
Similar dynamics can be observed in the results of monitoring the platelet count (Fig. 1C, Table 1). A decrease in platelets is an indicator of the severity of COVID-19 [15]. The decline in platelet count is proportional to the increase in the severity of the disease. As with fibrinogen, when a patient progressed to moderate disease, platelet consumption began.
All 46 patients received prophylactic doses of low molecular weight heparin (LMWH). According to the literature, coronavirus infection is associated with a decrease in the ATIII level [16], which blocks the adequate effect of LMWH, thus complicating the selection of LMWH dosages. This necessitates not only regular monitoring of the ATIII level but also its timely replenishment. The monitoring of ATIII levels is shown in Figure 1D and Table 1. In patients with mild to moderate COVID-19, the ATIII level was normal: 108.5% (72.5–118%) and 99.5% (80–113%), respectively. In patients with severe and critically severe disease, ATIII levels were below normal and amounted to 76% (68–88%) and 64% (57–75%), respectively. Unfavorable outcomes were observed at ATIII <70.8% (specificity 70%, sensitivity 82.6%, AUC 0.85, p <0.001, positive predictive value 30.5%, and negative predictive value 96.1%). Current literature is lacking studies investigating the concentration of ATIII in patients with COVID19. Both decreased [8] and near-normal ATIII levels have been reported in COVID19 patients [17]. However, most researchers report a significant difference in the levels of natural anticoagulants in the group of deceased and surviving patients.
Conclusion
The present study demonstrated the relationship between the parameters of plasma hemostasis (D-dimer, fibrinogen, antithrombin III), and platelet count and the severity of coronavirus infection COVID-19. In patients with severe and critically severe disease, daily monitoring of these indicators is necessary due to their dynamic changes. The monitoring of antithrombin III levels is crucial for successful LMWH prophylaxis. D-dimer and antithrombin III are also shown to have a predictive value for disease outcome.
References
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Thromb. Haemost. 2020; 18(5): 1094-9. https://dx.doi.org/ 10.1111/jth.14817.
- Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol. Cardiothorac. Imaging. 2020; 2(2): e200067. https://dx.doi.org/10.1148/ryct.2020200067.
- Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. et al. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020; 18(7): 1738-42. https://dx.doi.org/ 10.1111/jth.14850.
- Schmitt F.C.F., Manolov V., Morgenstern J., Fleming T., Heitmeier S., Uhle F. et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann. Intensive Care. 2019; 9(1): 1-15. https://dx.doi.org/10.1186/s13613-019-0499-6.
- Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020; 18(5): 1023-6. https://dx.doi.org/10.1111/jth.14810.
- Klok F.A, Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020; 191: 145-7. https://dx.doi.org/ 10.1016/j.thromres.2020.04.013.
- Llitjos J.-F., Leclerc M., Chochois C., Monsallier J.-M., Ramakers M., Auvray M. et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020; 18(7): 1743-6. https://dx.doi.org/ 10.1111/jth.14869.
- Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020; 18(7): 1747-51. https://dx.doi.org/ 10.1111/jth.14854.
- Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Министерствo здравоохранения Российской Федерации. Версия 7(03.06.2020): 10-1. [Temporary guidelines of the Ministry of Health of the Russian Federation for the prevention, diagnosis and treatment of new coronavirus infection COVID-19. 03.06.2020. Version 7. 10-11 p. (in Russian)].
- Макацария А.Д., Григорьева К.Н., Мингалимов М.А., Бицадзе В.О.,Хизроева Д.Х., Третьякова М.В., Элалами И., Шкода А.С., Немировский В.Б., Блинов Д.В., Митрюк Д.В. Коронавирусная инфекция (COVID-19) и синдром диссеминированного внутрисосудистого свертывания. Акушерство, гинекология и репродукция. 2020; 14(2): 123-31. https://doi.org/10.17749/2313-7347.132. [Makatsariya A.D., Grigoreva K.N., Mingalimov M.A., Bitsadze V.O., Khizroeva J.K., Tretyakova M.V., Elalamy I., Shkoda A.S., Nemirovskiy V.B., Blinov D.V., Mitryuk D.V. Coronavirus disease (COVID-19) and disseminated intravascular coagulation syndrome. Obstet. Gynecol. Reprod. 2020; 14(2): 123-31. https://doi.org/10.17749/2313-7347.132.(in Russian)]
- Crawford F., Andras A., Welch K., Sheares K., Keeling D., Chappell F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst. Rev. 2016; 2016(8): CD010864. https://dx.doi.org/ 10.1002/14651858.CD010864.pub2.
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11): 1061-9. https://dx.doi.org/ 10.1001/jama.2020.1585.
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395 (10223): 497-506. https://dx.doi.org/10.1016/S0140-6736(20)30183-5.
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020; 180(7): 1-11. https://dx.doi.org/10.1001/jamainternmed.2020.0994.
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 2020; 506: 145-8. https://dx.doi.org/10.1016/j.cca.2020.03.022.
- Han H., Yang L., Liu R., Liu F., Wu K.L., Li J. et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020; . 58(7): 1116-20. https://dx.doi.org/10.1515/cclm-2020-0188.
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020; 18(4): 844-7. https://dx.doi.org/10.1111/jth.14768.
Received 21.08.2020
Accepted 28.08.2020
About the Authors
Mikhail I. Markelov, Clinical Pathologist, Junior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.E-mail: m.markelov1994@mail.ru. 177997, Russia, Moscow, Academician Oparin str., 4.
Olga S. Beznoshchenko, Clinical Pathologist, Junior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
E-mail: o_beznoshchenko@oparina4.ru. 177997, Russia, Moscow, Academician Oparin str., 4.
Tatiana Yu. Ivanets, Dr. Med. Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG & P of Minzdrav of Russia. Tel.: +7(910)404-26-69.
Е-mail: t_ivanets@oparina4.ru. 177997, Russia, Moscow, Academician Oparin str., 4.
Aleksey V. Pyregov, Dr. Med. Sci., Professor, Director of the Institute of Anesthesiology, Reanimatology and Transfusiology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. E-mail: a_pyregov@oparina4.ru. 177997, Russia, Moscow, Academician Oparin str., 4.
Rosa M. Esayan, Ph.D., Endocrinologist, Head of the Department of Internal Medicine, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. E-mail: r_esayan@oparina4.ru.
177997, Russia, Moscow, Academician Oparin str., 4.
Tatyana Yu. Gavrilova, Dr. Med. Sci., Obstetrician-Gynecologist at the Department of Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
E-mail: t_qavrilova@oparina4.ru. 177997, Russia, Moscow, Academician Oparin str., 4.
Liubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia. Tel: +7(495)438-11-83.
E-mail: l_krechetova@oparina4.ru. 177997, Russia, Moscow, Academician Oparin str., 4.
For citation: Markelov M.I., Beznoshchenko O.S., Ivanets T.Yu., Pyregov A.V., Esayan R.M., Gavrilova T.Yu., Krechetova L.V. Plasma hemostatic system in patients with the novel coronavirus disease 2019 (COVID-19).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 9: 138-144 (in Russian)
https://dx.doi.org/10.18565/aig.2020.9.138-144



