Characteristics of the organic acid profile of amniotic and cervicovaginal fluids in pregnant women at high risk for spontaneous preterm birth
Objective: To study the metabolomic profile of amniotic and cervicovaginal fluids and identify the potential predictors for spontaneous preterm birth in high-risk patients.Gorina K.A., Khodzhaeva Z.S., Chagovets V.V., Starodubtseva N.L., Frankevich V.E., Priputnevich T.V.
Materials and methods: The prospective study included 46 pregnant women at high risk for preterm birth, the informed consent was obtained from all the patients. The patients were divided into two groups: group I consisted of 12 pregnant women who had preterm birth and group II included 34 pregnant women who had full-term delivery. Amniotic fluid sampling was performed using diagnostic transabdominal amniocentesis. An Agilent 1260 high performance liquid chromatography system was used for the analysis of the samples.
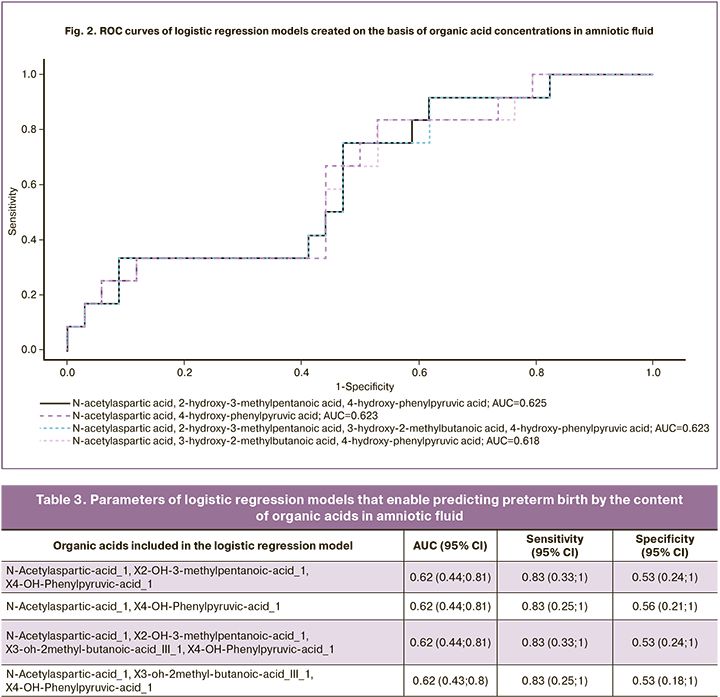
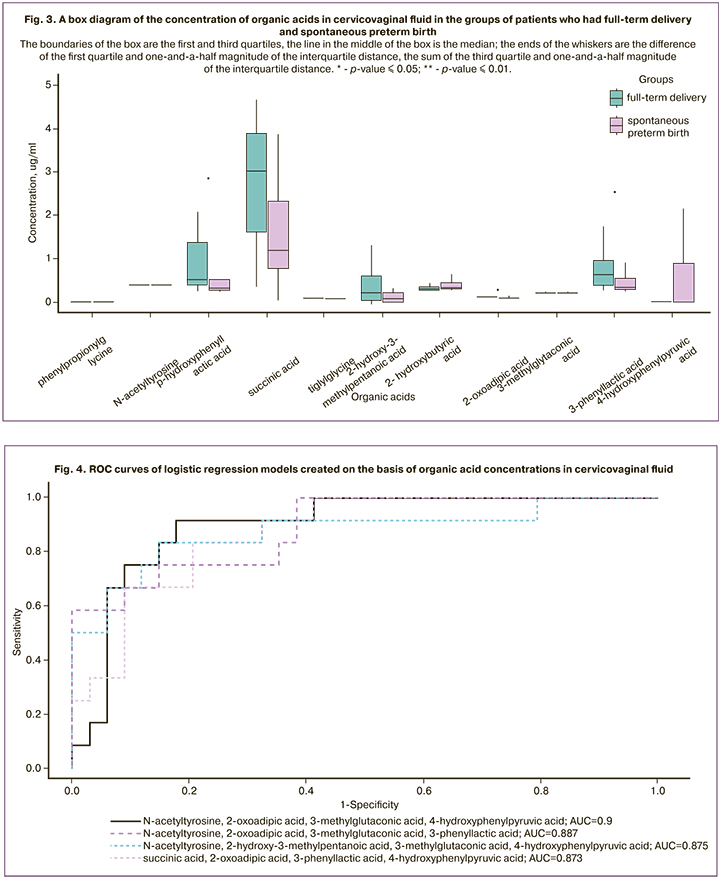
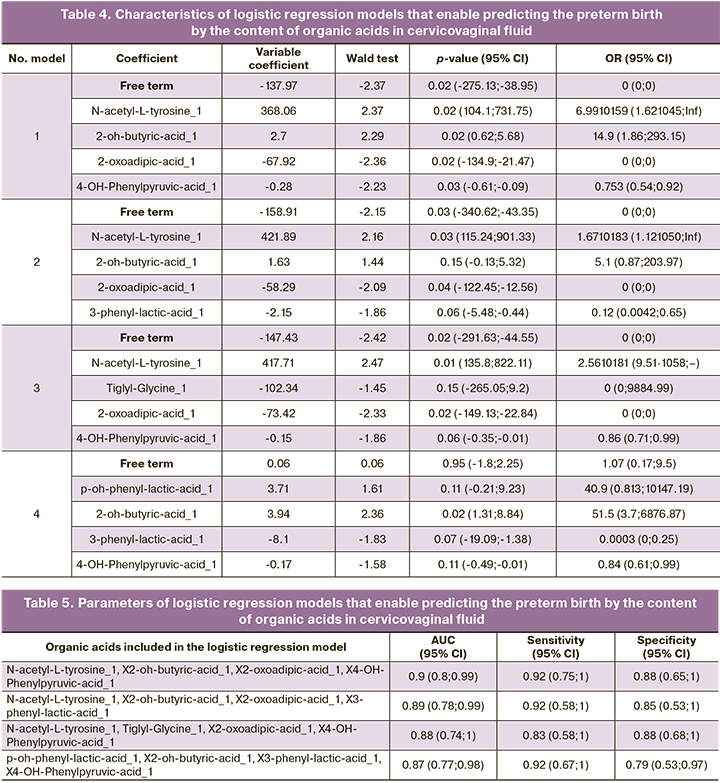
Results: The amniotic fluid of patients with spontaneous preterm birth had the panels of the following organic acids: N-acetylaspartic acid, 2-hydroxy-3-methylpentanoic acid, 4-hydroxyphenylpyruvic acid; sensitivity 92% and specificity 38%. The cervicovaginal fluid had N-acetyl-tyrosine, 2-oxoadipic acid, 3-methylglutaconic acid, 4-hydroxyphenylpyruvic acid; sensitivity 92% and specificity 82%.
Conclusion: The identification of the organic acid panel in the amniotic fluid invasively and in the cervicovaginal fluid noninvasively using high-performance liquid chromatography makes it possible to predict the likelihood of spontaneous preterm labor.
Keywords
Preterm birth is a polyetiological syndrome with different clinical phenotypes despite the common pathogenetic mechanisms. Therefore, it should be noted that spontaneous preterm birth is caused by the combination of several risk factors [1]. In this connection, the search for new predictors and the identification of accurate markers is an important research task [2, 3].
Precision medicine is a new approach in modern medicine aimed at developing strategies for the prevention and treatment of diseases using the individual variability of the molecular phenotype. A standard example of this approach is genetic research; metabolomic profiles of various pathogenetic pathways are currently actively examined in the format of these studies [4, 5]. Nowadays, the studies are aimed at identifying metabolic pathways as a method of fundamental understanding of spontaneous preterm birth pathogenesis at the level of office technologies [6]. Scientists have proposed a new term metabolotype which reflects the metabolomic profile of a person [7]. Pathological and physiological intrauterine processes are better reflected in the analysis of the metabolome than in genomic studies, since the metabolomic pathways are part of the phenotypic manifestation of the pathological process [8].
According to the modern concept, the leading cause of spontaneous preterm birth is an inflammation acting as a trigger component that initiates the production of prostaglandins which in turn trigger labor regardless of the gestation period [9, 10]. Metabolomic analysis with subsequent identification of targeted profiles of organic acids enables verifying intraamniotic inflammation and the risks of spontaneous preterm birth [8]. The analysis of metabolomic signatures of the placenta showed high levels of proteins and lipids including derivatives of aliphatic amino alcohols as well as prostaglandins and acylcarnitines in patients with preterm birth [11].
Amniotic fluid is a unique biomaterial that reflects the processes occurring simultaneously on the “territory” of the mother and fetus. Therefore, amniotic fluid can be used as a control sample in the analysis of obstetric pathology, especially in spontaneous preterm birth [12]. R. Menon from the University of Texas showed in his research that concentrations of essential fatty acids in amniotic fluid were statistically significantly higher in patients with spontaneous preterm birth [8, 13]. Higher values of unsaturated fatty acids, oxylipins and aldehydes, namely 3-methoxybenzenepropanoic acid, 4-hydroxynonenal and muconic aldehyde were noted in women who had preterm birth [14].
Along with this, the search for non-invasive predictors poses new challenges for researchers, and cervicovaginal fluid is a potential effective and available biomaterial. This fluid acts as a primary immune barrier on the way of microbial adhesion and is a collection of secretions of various small glands of the external genitalia, as well as plasma transudate and local immune cells [15]. At the same time, the advantages of its use are due to the time-consuming process associated with obtaining amniotic fluid. The analysis of works on the study of cervicovaginal fluid in pregnant women reveals to a greater extent the proteomic composition of cervicovaginal fluid (a significant increase in N-acetylneuramic and sialic acids in the third trimester was characteristic of spontaneous preterm birth) [16], whereas, the metabolomic profile of cervicovaginal fluid in spontaneous preterm birth requires further study.
The objective of the research is to study the characteristics of the metabolomic profile of amniotic and cervicovaginal fluids in patients at high risk of developing preterm birth.
Materials and methods
The prospective study which was carried out at the National Medical Research Centre for Obstetrics, Gynecology and Perinatology in Moscow included 46 pregnant women at high risk for preterm birth. The group of women at high risk was formed on the basis of anamnestic data (spontaneous preterm birth in history, late miscarriages), as well as the clinical signs and echographic markers of cervical incompetence. In order to adequately verify and assess the potential impact of the metabolic profiles in amniotic and cervicovaginal fluids, the patients were divided into two groups: the main group consisted of 12 pregnant women who had preterm birth (the average gestational age at delivery was 35.1±1.56 weeks), the control group included 34 pregnant women who had full-term delivery which occurred at 38.6±0.99 weeks.
Anamnestic and general clinical and laboratory data were analyzed for all pregnant women, microbial composition of the discharge from the lower genital tract was studied, histological examination of the afterbirth tissues was performed. Methods of functional assessment of fetal condition and fetometry were used.
There were the following inclusion criteria: singleton pregnancy which occurred without the use of assisted reproductive technologies, gestational age 280–366 weeks, compliance with the above criteria of high risk for preterm birth. The special feature of this study was the obligatory informed consent of pregnant women to the procedure of transabdominal amniocentesis under ultrasound guidance for the exfusion of amniotic fluid. The women who had a multifetal pregnancy, placenta previa, severe extragenital and obstetric pathology were not eligible for inclusion in the study.
There were the following exclusion criteria in our study: premature rupture of membranes, contamination of amniotic fluid during amniocentesis (with blood, meconium and other impurities), infectious processes in the acute phase and/or exacerbation of chronic somatic pathology, as well as the patient's unwillingness to participate in the study. The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Sampling of amniotic and cervicovaginal fluids was performed for metabolomic analysis. Amniotic fluid was obtained by transabdominal amniocentesis and cervicovaginal fluid was collected as aspirate after the treatment of the cervix and vagina with 5.0 ml of 0.9% sodium chloride solution. After the samples were subjected to centrifugations (amniotic fluid at 1300g for 10 minutes at room temperature and cervicovaginal fluid at 1500g for 10 minutes at room temperature), chromatographic–mass spectrometric analysis of the biomaterial was carried out in the laboratory [17]. The samples were examined using an Agilent 1260 high performance liquid chromatography system with mass spectrometric detection on an Agilent 6460 mass spectrometer. The indicators of chromatographic separation, transitions between parent ions and daughter fragments for monitored organic acids, their proper chromatographic retention times, concentrations of internal standards, as well as the information on the sensitivity and reproducibility of the analysis are given in the manual of JASEM for the kit LC-MS/MS ANALYSIS OF ORGANIC ACIDS produced by JASEM, Turkey. The concentration of metabolites was carried out using the program QuantAnalysis (Agilent, USA) on the basis of the calibration curve constructed for each organic acid. The reliability of the calibration curve was defined using the coefficient of determination, its minimum value was chosen as 0.95 (r≥0.95).
Statistical analysis
The statistical analysis of the obtained data was carried out using programs GraphPad Prism 8.3 and IBM SPSS Statistics 22 in accordance with the general recommendations for medical and biological research. The description of quantitative data with a normal distribution is presented as the arithmetic mean; if the distribution differs from the normal one, standard deviation is used. The data are presented in the form of medians and quartiles. In order to determine the normality of the distribution, the D’Agostino–Pearson test, Anderson–Darling test, as well as Kolmogorov–Smirnov test and the Levene’s test of equality of variances were used. The following data processing methods were used in the study: Fisher’s exact test, comparative analysis of variables using the Student’s t-test for unrelated samples. If there was no normal distribution of data, the methods of nonparametric statistics, such as the Mann–Whitney U-test, were used. Logistic regression models have been developed to assess the possibility of predicting preterm birth on the basis of organic acid concentrations in amniotic and cervicovaginal fluids. The group of the patient was a dependent variable. Four models with the largest area under the ROC curve (AUC) were selected from all the developed models. The Wald test, 95% confidence interval (CI), odds ratio (OR) and its confidence interval were identified for each model. The quality of the developed models was determined using the construction of the ROC curve, estimation of the area under the ROC curve and calculation of sensitivity and specificity.
The results of all analyses were considered statistically significant at a significance level of p<0.05.
Results
Gestational age at the time of amniocentesis, woman's age, weight and height indices and body mass index (BMI) were evaluated in 46 pregnant women at high risk for preterm birth. The clinical and anamnestic characteristics of the patients included in the study as well as the results of histological examination of the afterbirth tissues are presented in Table 1.
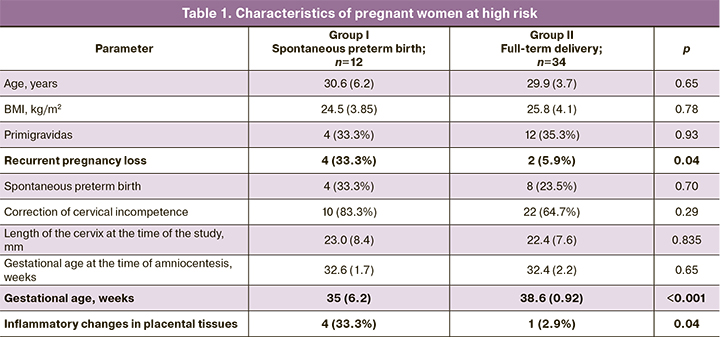
The patient’s age (p=0.65), BMI (p=0.78), and characteristics of reproductive history (spontaneous preterm birth, p=0.70, correction of cervical incompetence, p=0.29) were comparable between the groups. However, the rate of recurrent pregnancy loss (p=0.04), as well as the presence of the signs of inflammatory changes in the afterbirth tissues (diffuse and focal lymphocyte and leukocyte infiltrates) were significantly higher in the group of patients who had spontaneous preterm birth (p=0.04). It should be noted that these factors most likely indicate a high risk of latent intra-amniotic inflammation.
The analysis of the course of pregnancy showed that there were no statistically significant differences in the rate of episodes of threatened preterm labor in the I (p=0.14), II (p=0.92) and III (p=1.00) trimesters, respiratory diseases (p=0.236) and anemia of pregnant women before the study (p=0.901). Since the length of the cervix was one of the criteria for inclusion in the high-risk group, ultrasound cervicometry data were analyzed; there were no statistically significant differences in both groups.
A comparative analysis of modes of delivery, regardless of the gestation period, showed that the dominant method in both groups was vaginal delivery (8/12 (66.7%) and 25/34 (73.5%), p=0.65). Indications for cesarean section in group I were premature rupture of membranes and/or the onset of labor in pregnant women with a uterine scar and footling breech presentation. Besides characteristic typical differences in newborns, such as the Apgar score at birth (7.5 [7; 8] and 8 [8; 8] points, p=0.01) and anthropometric data (average body weight 2524 (427.7) and 3217 (460.7) g, p <0.001), there was a higher and statistically significant prevalence of infection specific for the perinatal period (2/12 (16.7%) and 0/34 (0%), p=0.02, namely, intrauterine pneumonia, infections and inflammations of the urinary tract, etc.).
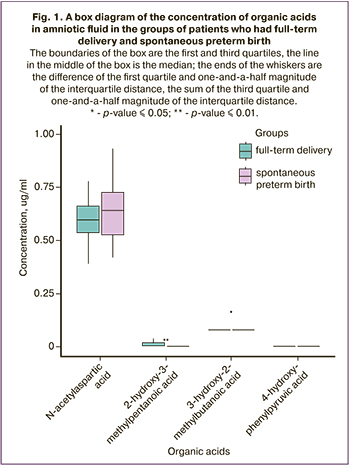
The results of the analysis of the organic acid profile in amniotic fluid are presented in Figure 1. The data indicate that the concentration of N-acetylaspartic acid which is a derivative of aspartic acid is higher in the group of patients who had spontaneous preterm birth. According to scientific data, this acid facilitates the inflammatory cascade and increases the contractile activity of smooth muscle cells [18]. The concentrations of 2-hydroxy-3-methylpentanoic and 3-hydroxy-2-methylbutanoic acids were statistically significantly higher in the group of full-term delivery; these acids participate in the exchange of isoleucine which demonstrates intense anti-inflammatory activity [19].
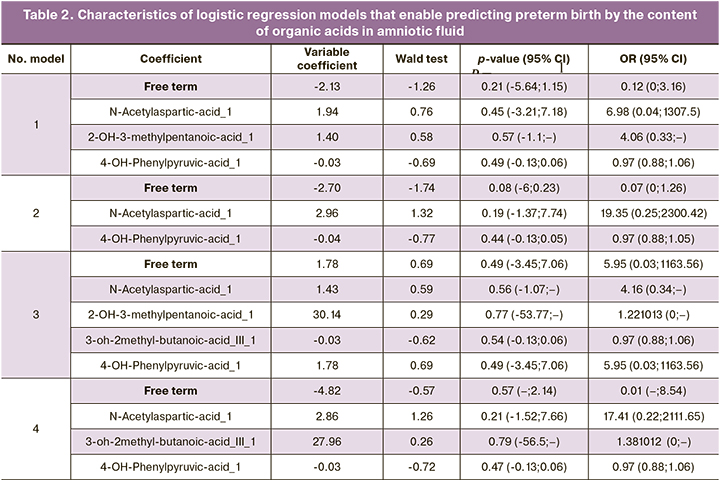
Four models of logistic regression of concentrations of organic acids in amniotic fluid (levels 2-4) with a maximum AUC value were created on the basis of the obtained data (Tables 2, 3):
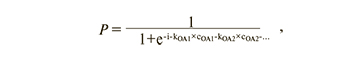
where i is a free term, kOA1, kOA2, … are coefficients for organic acid concentrations, cOA1, cOA2, … are concentrations of the proper organic acids. ROC curves of the constructed models are shown in Figure 2.
The analysis of the metabolomic profiles of cervicovaginal fluid showed that there were the higher concentrations of succinic acid. These metabolites are involved in the cycle of tricarboxylic acids and they are also metabolites of the inflammatory signal. The concentration of 2-hydroxy-3-methylpentanoic acid was as high as in amniotic fluid in patients who had full-term delivery, which confirms its protective contribution as a blocker of proinflammatory reactions. 4-hydroxyphenylpyruvic acid was detected in insignificant amounts in pregnant women who had full-term delivery, while its concentration could be 3 mcg/ml in the group of patients who had spontaneous preterm birth. 2-oxoadipic acid was statistically significantly more common in patients who had full-term delivery. The results of p-hydroxyphenylacetic and 3-phenyl-lactic acid were statistically significantly different. Lactic acid derivatives in particular n-hydroxyphenyl lactate, in higher concentrations were found in patients from the group of full-term delivery; it is known to be a natural antioxidant and reduce the production of reactive oxygen species in neutrophils. The analysis of the profile of organic acids in cervicovaginal fluid is presented as a graph in Figure 3.
Four models of logistic regression of concentrations of organic acids in cervicovaginal fluid with a maximum AUC value were created on the basis of the obtained data (Tables 4, 5). ROC curves of the constructed models are shown in Figure 4. The obtained ROC curves are characterized by a high AUC value, not less than 0.8.
Discussion
The advantage of amniotic fluid obtained by transabdominal amniocentesis is the purity of the analyzed samples which are not contaminated with blood, meconium and vaginal secretions. In general, one can conclude that the analysis of amniotic fluid is not sufficiently presented in the world literature; this is probably due to the peculiarities of the preanalytical stage associated with this biomaterial sampling. We were able to investigate potential new predictors of preterm birth with the help of modern analytical methods. The latest scientific data on the study of metabolism show promising results clarifying the pathogenetic characteristics of spontaneous preterm birth [8]. The results of the study showed that metabolites with anti-inflammatory activity in amniotic and cervicovaginal fluids were detected in statistically significantly higher concentrations in the group of patients who had full-term delivery. An increase in the amount of N-acetylaspartic acid in amniotic fluid probably activates the uterotonic activity of the myometrium as a consequence of phosphorylation of myosin light chain kinase. According to the literature data, high values of N-acetylaspartic acid are also associated with oxidative stress; it increases the level of nitric oxide and reduces the potential of antioxidants [20]. Since 3-hydroxy-2-methylbutanoic acid and 2-hydroxy-3-methylpentanoic acid are metabolites of isoleucine, they show an anti-inflammatory activity. 2-hydroxy-3-methylpentanoic acid dominated in patients who had full-term delivery due to a decrease in the expression of the inducible isoform of NO-synthase, as well as interleukin-6 and cyclooxygenase-2 [19]. On the contrary, 3-hydroxy-2-methylbutanoic acid is a product of isoleucine catabolism and fatty acid oxidation and was detected in higher concentrations in patients with preterm birth. Cervicovaginal fluid is a promising biomaterial for the search for noninvasive predictors of preterm birth. The high concentrations of succinate in the group of patients who had full-term delivery may indicate the anti-inflammatory potential of this metabolite. Nevertheless, according to the literature data, succinate demonstrates a binary activity and can be both a pro- and anti-inflammatory factor depending on the cellular environment [21]. At the same time, the influence of succinate receptor 1 (SUCNR1), which causes the formation of an anti-inflammatory phenotype in macrophages, is not excluded [22]. 2-hydroxy-3-methylpentanoic acid dominated in cervicovaginal fluid in the cohort of patients who had full-term delivery. It may be suggestive of its protective contribution as an inhibitor of proinflammatory reactions [19]. A number of the obtained metabolites, in particular, 4-hydroxyphenylpyruvic acid, represent the metabolic products of bacteria of the genus Escherichia, that reflect the microbial composition of the vagina [23]. Lactic acid derivatives were statistically significantly more common in the group of patients who had full-term delivery due to the characteristics of vaginal microbiocenosis and the protective contribution of lactobacilli.
Conclusion
The metabolomic profile of amniotic fluid in pregnant women who had spontaneous preterm birth reflects higher concentrations of metabolites that contribute to the activation of proinflammatory and contractile agents when proinflammatory factors are generally decreased. However, cervicovaginal fluid of pregnant women who had preterm birth showed a decrease in anti-inflammatory factors in comparison with the group of patients who had full-term delivery. The use of postgenomic metabolomic approaches for the selection of patients at high risk for preterm birth is the subject of an active modern scientific search which can be implemented into clinical practice after validation in large population-based studies.
References
1. Villar J., Papageorghiou A.T., Knight H.E., Gravett M.G., Iams J., Waller S.A. et al. The preterm birth syndrome: a prototype phenotypic classification. Am. J. Obstet. Gynecol. 2012; 206(2): 119-23. https://dx.doi.org/10.1016/j. ajog.2011.10.866.
2. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet. Gynecol. 2012; 120(4): 964-73. https://dx.doi.org/10.1097/AOG.0b013e3182723b1b.
3. Romero R., Espinoza J., Kusanovic J.P., Gotsch F., Hassan S., Erez O. et al. The preterm parturition syndrome. BJOG. 2006; 113(Suppl.): 17-42. https://dx.doi.org/10.1111/j.1471-0528.2006.01120.x.
4. Azad R.K., Shulaev V. Metabolomics technology and bioinformatics for precision medicine. Brief Bioinform. 2019; 20(6): 1957-71. https://dx.doi.org/10.1093/ bib/bbx170.
5. Clish C.B. Metabolomics: an emerging but powerful tool for precision medicine. Mol. Case Stud. 2015; 1(1): a000588. https://dx.doi.org/10.1101/mcs.a000588.
6. Beger R.D., Dunn W., Schmidt M.A., Gross S.S., Kirwan J.A., Cascante M. et al. Metabolomics enables precision medicine: A white paper, community perspective. Metabolomics. 2016; 12(9): 149. https://dx.doi.org/10.1007/ s11306-016-1094-6.
7. Beger R.D., Schmidt M.A., Kaddurah-Daouk R. Current concepts in pharmacometabolomics, biomarker discovery, and precision medicine. Metabolites. 2020; 10(4): 129. https://dx.doi.org/10.3390/metabo10040129.
8. Menon R., Jones J., Gunst P.R., Kacerovsky M., Fortunato S.J., Saade G.R., Basraon S. Amniotic fluid metabolomic analysis in spontaneous preterm birth. Reprod. Sci. 2014; 21(6): 791-803. https://dx.doi.org/10.1177/1933719113518987.
9. Romero R., Espinoza J., Kusanovic J.P., Gotsch F., Hassan S., Erez O. et al. The preterm parturition syndrome. BJOG. 2006; 113(Suppl. 3): 17-42. https://dx.doi.org/10.1111/j.1471-0528.2006.01120.x.
10. Горина К.А., Ходжаева З.С., Белоусов Д.М., Баранов И.И., Гохберг Я.А., Пащенко А.А. Преждевременные роды: прошлые ограничения и новые возможности. Акушерство и гинекология. 2020; 1: 12-9. [Gorina K.A., Khodzhaeva Z.S., Belousov D.M., Baranov I.I., Gokhberg Y.A., Pashchenko A.A. Premature birth: past restrictions and new opportunities. Obstetrics and Gynecology. 2020; 1: 12-19. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.12-119.
11. Elshenawy S., Pinney S.E., Stuart T., Doulias P.-T., Zura G., Parry S. et al. The metabolomic signature of the placenta in spontaneous preterm birth. Int. J. Mol. Sci. 2020; 21(3): 1043. https://dx.doi.org/10.3390/ijms21031043.
12. Ходжаева З.С., Горина К.А., Муминова К.Т., Иванец Т.Ю., Кесслер Ю.В., Припутневич Т.В., Белоусов Д.М. Особенности состава амниотической жидкости у беременных высокого риска преждевременных родов. Акушерство и гинекология. 2020; 8: 82-7. [Khodzhaeva Z.S., Gorina K.A., Muminova K.T., Ivanets T.Y., Kessler Y.V., Priputnevich T.V., Belousov D.M. Amniotic fluid composition in pregnant women at high risk of preterm birth. Obstetrics and Gynecology. 2020; 8: 82-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.8.82-87.
13. Mozurkewich E.L., Klemens C. Omega-3 fatty acids and pregnancy: current implications for practice. Curr. Opin. Obstet. Gynecol. 2012; 24(2): 72-7. https://dx.doi.org/10.1097/GCO.0b013e328350fd34.
14. Baraldi E., Giordano G., Stocchero M., Moschino L., Zaramella P., Tran M.R. et al. Untargeted metabolomic analysis of amniotic fluid in the prediction of preterm delivery and bronchopulmonary dysplasia. PLoS One. 2016; 11(10): e0164211. https://dx.doi.org/10.1371/journal.pone.0164211.
15. Zegels G., Van Raemdonck G.A., Tjalma W.A., Van Ostade X.W. Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci. 2010; 8: 63. https://dx.doi.org/10.1186/ 1477-5956-8-63.
16. Ghartey J., Bastek J.A., Brown A.G., Anglim L., Elovitz M.A. Women with preterm birth have a distinct cervicovaginal metabolome. Am. J. Obstet. Gynecol. 2015; 212(6): 776. e1-776. e12. https://dx.doi.org/10.1016/j.ajog.2015.03.052.
17. Starodubtseva N.L., Kononikhin A.S., Bugrova A.E., Chagovets V., Indeykina M., Krokhina K.N., Nikitina I.V., Kostyukevich Y.I., Popov I.A., Larina I.M., Timofeeva L.A., Frankevich V.E., Ionov O.V., Degtyarev D.N., Nikolaev E.N., Sukhikh G.T. Investigation of urine proteome of preterm newborns with respiratory pathologies. J Proteomics. 2016; 149: 31-7. https://dx.doi.org/10.1016/j.jprot.2016.06.012.
18. Surendran S. Upregulation of N-acetylaspartic acid alters inflammation, transcription and contractile associated protein levels in the stomach and smooth muscle contractility. Mol. Biol. Rep. 2009; 36(1): 201-6. https://dx.doi.org/10.1007/s11033-007-9167-2.
19. Lee J.H., Park E., Jin H.J., Lee Y., Choi S.J., Lee G.W. et al. Antiinflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food Sci. Biotechnol. 2017; 26(5): 1371-7. https://dx.doi.org/10.1007/ s10068-017-0165-4.
20. Surendran S., Bhatnagar M. Upregulation of N-acetylaspartic acid induces oxidative stress to contribute in disease pathophysiology. Int. J. Neurosci. 2011; 121(6): 305-9. https://dx.doi.org/10.3109/00207454.2011.558225.
21. Grimolizzi F., Arranz L. Multiple faces of succinate beyond metabolism in blood. Haematologica. 2018; 103(10): 1586-92. https://dx.doi.org/10.3324/ haematol.2018.196097.
22. Keiran N., Ceperuelo-Mallafre V., Calvo E., Hernandez-Alvarez M.I., Ejarque M., Nunez-Roa C. et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 2019; 20(5): 581-92. https://dx.doi.org/10.1038/s41590-019-0372-7.
23. Guo A.C., Jewison T., Wilson M., Liu Y., Knox C., Djoumbou Y. et al. ECMDB: the E. coli metabolome database. Nucleic Acids Res. 2013; 41(Database issue): D625-30. https://dx.doi.org/10.1093/nar/gks992.
Received 01.09.2021
Accepted 05.03.2022
About the Authors
Ksenia A. Gorina, Junior Researcher at the Department of Pregnancy Pathology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(926)649-77-32, k_gorina@oparina4.ru, https://orcid.org/0000-0001-6266-2067, 117997, Russia, Moscow, Ac. Oparin str., 4.Zulfiya S. Khodzhaeva, Dr. Med. Sci., Professor, Deputy Director for Research of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(916)407-75-67, zkhodjaeva@mail.ru, https:// orcid.org/0000-0001-8159-3714, 117997, Russia, Moscow, Ac. Oparin str., 4.
Vitaliy V. Chagovets, PhD, Senior Researcher at the Laboratory of Proteomics and Metabolomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(926)562-65-90, vvchagovets@gmail.com, https://orcid.org/0000-0002-5120-376X, 117997, Russia, Moscow, Ac. Oparin str., 4.
Nataliia L. Starodubtseva, PhD, Head of the Laboratory of Proteomics of Human Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, ++7(916)463-98-67, n_starodubtseva@oparina4.ru, https://orcid.org/0000-0001-6650-5915, 117997, Russia, Moscow, Ac. Oparin str., 4.
Vladimir E. Frankevich, PhD, Head of the Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(495)438-07-88, v_frankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579, 117997, Russia, Moscow, Ac. Oparin str., 4.
Tatyana V. Priputnevich, Dr. Med. Sci., Head of the Departament of Microbiology and Clinical Pharmacology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(903) 264-12-57, priputl@gmail.com, https://orcid.org/0000-0002-4126-9730, 117997, Russia, Moscow, Ac. Oparin str., 4.
Authors’ contributions: Gorina K.A., Khodzhaeva Z.S., Chagovets V.V., Starodubtseva N.L., Frankevich V.E., Priputnevich T.V - developing the design of the study, obtaining data for analysis, reviewing publications on the subject of the manuscript, their translation, statistical analysis of the data obtained, writing the text of the manuscript, editing; Gorina K.A., Khodzhaeva Z.S. - writing the text of the manuscript, editing.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without external funding.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P , Ministry of Health of Russia.
Patients’ Consent to Publication: Mothers of newborns signed an informed consent to the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Gorina K.A., Khodzhaeva Z.S., Chagovets V.V., Starodubtseva N.L., Frankevich V.E., Priputnevich T.V. Characteristics of the organic acid profile of amniotic and cervicovaginal fluids in pregnant women at high risk for spontaneous preterm birth. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 3: 39-48 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.39-48



