Microbiota of preterm infants born to mothers with endocrine disorders
Aim. The study aimed to investigate differences in the microbiota composition of newborn infants from mothers with and without endocrine disorders.Priputnevich T.V., Nikolaeva A.V., Shabanova N.E., Fedorov D.E., Manolov A.I., Pavlenko A.V., Konanov D.N., Krivonos D.V., Klimina K.M., Veselovskii V.A., Zubkov V.V., Il’ina E.N.
Materials and methods. We collected 143 stool samples from preterm infants born to mothers with or without endocrine disorders. Samples were taken from the first stool passed by the newborn (meconium) one week and two weeks after birth. DNA isolated from the samples underwent sequencing of the V3–V4 region of the 16S rRNA gene. Taxonomic profiles were assessed using the DADA2. The search for differentially represented bacterial genera was carried out using the DESeq2 package.
Results. Newborn infants from mothers with and without endocrine disorders had a significantly different composition of the gut microbiota. Differences started to emerge one week after birth. Infants from mothers with endocrine disorders had increased relative abundance of opportunistic microorganisms and reduced relative abundance of genera Bifidobacterium and Lactobacillus.
Conclusion. Our findings of differences in preterm infant gut microbiota composition suggest that the maternal endocrine system’s state can influence the infant gut microbiome’s formation during the early stages of its colonization.
Keywords
Studying the microbiota remains highly relevant because microbial communities influence many areas of human health, including the functioning of the gastrointestinal tract and the immune system [1]. This question is also relevant to neonatal microbiota. Human babies are born with virtually sterile intestines. Bacterial colonization occurs gradually, and by the age of 2–3, the baby's microbiota stabilizes and becomes similar to the adult microbiota [2]. The first months of a baby's life are believed to be the critical period for the proper formation of the gut microbiota. Several researchers have shown that abnormal colonization of the gut microbiota may have multiple late sequelae, particularly the immune system's impaired formation.
The initially developing microbiota is influenced mainly by tactile contact with the mother and microbes in breast milk [2]. Several factors can interfere with the normal colonization of infant gut microbiota with maternal microbial communities. Of the most common are cesarean delivery, antibiotics in the first days of a child's life, and replacing breastfeeding with formula feeding [2].
Maternal microbiota changes during pregnancy. It has been suggested that these changes are adaptive and aimed at compensating for the increase in maternal and fetal energy consumption [3]. According to [4], children exposed to antibiotics in the second or third trimester had an 84% higher risk of obesity than children whose mothers did not take antibiotics.
Impact of maternal health on her newborn's health
A parturient woman's health makes a significant contribution to the formation of the newborn gut microbiota [5]. The association between maternal disease and child health depends on many causes and includes genetic, epigenetic, and environmental factors. The impact of fetal exposure to maternal metabolic syndrome on the child's health has been extensively studied. Experiments involving rodents and monkeys and human studies have shown the association between obesity or a high-fat diet during pregnancy and newborn immune system and microbiota [6]. The observed changes increased the child's predisposition to obesity.
Maternal type 1 or 2 increases the risk of adverse outcomes, including the need for surgery, preterm birth, preeclampsia, macrosomia, and newborn congenital defects [7].
Maternal thyroid disease can exert adverse effects on childhood cognitive function since thyroid hormones play an essential role in nervous system development [8,9]. Fetal hypothyroidism (long-term lack of thyroid hormones) resulting from maternal iodine deficiency can lead to childhood pathologies, including cretinism with severe mental retardation, hearing loss, and gait ataxia. Moreover, maternal hyperthyroidism multiplies the obstetric risk, contributes to low birth weight, and in the worst case, can lead to fetal death or preterm birth [10,11]. Also, some genetic thyroid diseases can cause hormone receptor abnormalities, which, in turn, can lead to increased sensitivity to human chorionic gonadotropin and resistance to thyroid hormones.
Maternal adrenal gland diseases can slow down fetal development and contribute to some negative consequences. It has been shown that maternal hyperaldosteronism (increased secretion of aldosterone) is associated with increased blood pressure in children, which can also provoke hypertension [12]. It is also known that increased synthesis of androgens can contribute to the development of preeclampsia [13], preterm birth, and extremely low birth weight delivery [14].
As mentioned earlier, various diseases that have arisen in the maternal endocrine system can also lead to preterm birth and extreme prematurity. Many factors increase this risk, including age over 40, poor diet, low body weight, cervical injuries, severe stress, smoking, strenuous work, a short inter-pregnancy interval, etc. Prematurity is a risk factor for childhood morbidity and mortality and is associated with a high risk of bacterial inflammatory diseases such as sepsis and necrotizing enterocolitis (NEC). Preterm infants have an immature intestine with underdeveloped peristalsis, barrier function, and immunity, making the intestine a potential source of infections and inflammation [15]. In comparison with those born on time, premature babies often have an unstable and less diverse intestinal microbiota composition [16]. This can contribute to bacterial infections in healthcare settings, including nosocomial opportunistic and pathogenic strains. Several studies on small groups of children have shown that both NEC and late sepsis are associated with microbiota changes [17], and microbiota composition correlates with the clinical picture [18].
Preterm neonates born via cesarean section do not directly contact the maternal vaginal microbial population, which prevents them from obtaining the necessary microorganisms for the normal development of their microflora. It is not uncommon for them to be born with very low birth weight, necessitating feeding special sterile nutritional mixtures instead of maternal breast milk. Besides, preterm babies are initially kept in special wards, where the risk of developing dysbiosis is much higher [19].
Preterm infants often receive antibiotic therapy for suspected neonatal infections, but in some cases, this is not always warranted but serves only as a preventive measure. Studies have shown that antibiotic use has significant effects on the neonatal metabolome [20].
Besides maternal diseases, the process of postpartum recovery also affects the formation of a healthy infant's microflora. Adherence to particular postpartum practices can impact an infant's gut microbiota [21]. The same study reported that the invasion of pathogenic microorganisms into the microbiome and excessive use of probiotics during the first month after delivery results in a significant dominance of opportunistic pathogens. However, rational modification of the microbiota with probiotics or breast milk can reduce NEC's risk in preterm infants [22]. Maternal probiotic administration has a similar positive effect [23].
Therefore, combining all those factors impedes the formation of a healthy infant microbiota and contributes to the development of pathogenic microorganisms. Metagenomic analyses showed that preterm infant fecal samples lacked beneficial Bifidobacterium spp. and were dominated by Enterobacteriaceae, Enterococcus, and Staphylococcus organisms [24].
The current literature lacks information related to the impact of maternal health on the establishment of the infant microbiome. This information is of particular interest regarding preterm babies due to the distinctive features of microbiome colonization. Considering the above facts, this study aimed to investigate differences in the microbiota composition of newborn infants from mothers with and without endocrine disorders.
Materials and methods
The study design
The study was conducted at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia from 2019 to 2020. It included 59 preterm (25–36 weeks) and full-term (37–39 weeks) infants born to mothers with (thyroid disease or diabetes mellitus) or without (control group) endocrine disorders. The study did not include children born to mothers with concomitant chronic inflammation or cancer or receiving antibiotic therapy during pregnancy. In accordance with the existing clinical guidelines, all children underwent the same management and nursing, regardless of the presence or absence of maternal endocrine disorders. Premature infants with extremely low, very low, and low body weight and full-term infants with respiratory conditions that required respiratory support were observed in the neonatal intensive care unit. According to the indications, the children were administered infusion, antibacterial, symptomatic, supportive therapy, parenteral, and enteral nutrition. Preterm babies received modern neonatal nursing, including a protective regime and early breastfeeding. The minimal enteral nutrition with expressed breast milk was started from the first day of life. The protective regime included maintaining the temperature regime, minimizing painful procedures and reducing noise exposure, direct light rays, giving the child a comfortable position in the incubator/crib, and mandatory maternal care and compliance with the sanitary and epidemiological regime. After stabilizing newborns’ condition, they were transferred to the department of pathology of newborns and preterm babies for the 2nd stage of nursing. Some of the children received probiotic therapy.
All mothers signed informed consent for the collection of clinical material.
Stool samples were taken from preterm infants at four-time points: first stool passed by the newborn (meconium), one and two weeks postpartum, and one month postpatum.
Sample collection
Stool samples from preterm infants were collected in plastic containers and stored in a low-temperature freezer at -80°C. The samples were transported to the laboratory on dry ice.
DNA isolation
DNA was extracted from samples using the QIAamp Fast DNA Stool Mini kit (Qiagen) according to the manufacturer's instructions. DNA was dissolved in 50 μl of buffer and stored at –18°С.
Library preparation and sequencing
For the bacterial communities' metagenomic analysis, the V3–V4 region of the 16S rRNA gene was used. Sequencing library preparation was performed according to the protocol (16S Metagenomic Sequencing Library preparation) for MiSeq, Illumina. The resulting library was sequenced on a MiSeq instrument (Illumina) using a set of paired reads 2×250 b with 8% Phix as a control.
Statistical analysis
The taxonomic analysis was performed using the DADA2 program [25]. R language was used for further analysis. The Shannon alpha diversity index was calculated using the diversity function from the vegan package. Wald test was used when comparing differential gene expression as implemented in the DESeq2 package. [26]. P values < 0.05 were considered statistically significant after adjusting for multiple comparisons. The significance was assessed for comparing the bacterial populations in children born to mothers with or without endocrine diseases. The calculation of distances for multidimensional scaling was conducted based on the Bray–Curtis metric using the phyloseq package in R [27].
Results
The study included a total of 143 stool samples that underwent metagenomic analysis. The analysis included 85 samples comprising over 2,000 sequencing reads.
Alpha diversity analysis
Figure 1 shows rarefaction curves reflecting the dependence of the Shannon alpha diversity index on the number of reads when choosing a part of the full set of reads for each sample. The number of reads above 2,000 practically reached a plateau. Samples with less than 1,000 reads were excluded from the analysis.
Subsequent analysis aimed to identify differences in gut microbiota composition of infants from mothers with endocrine diseases from that in the control group.
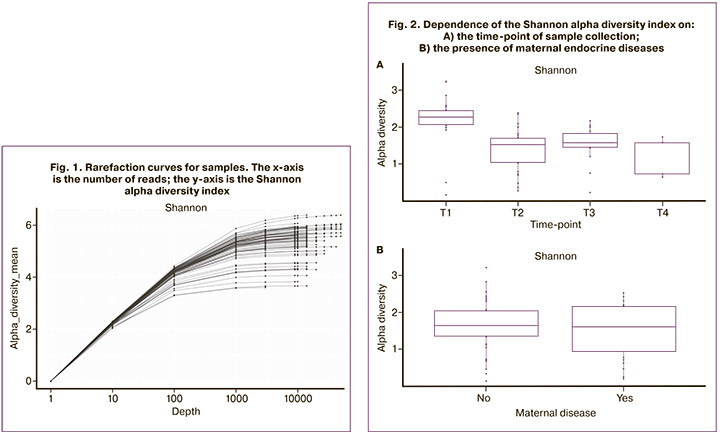
Analysis of alpha diversity of communities
The diversity of communities (Shannon index) was highest in stool samples taken from the first stool passed by the newborn (meconium) and lowest at the second time-point (one week postpartum) (Fig. 2A). We found no significant differences in alpha diversity in children from mothers with or without endocrine diseases (Fig. 2B).
Analysis of the taxonomic composition of samples
Figure 3 shows the distribution of taxonomic profiles of accessions for the first two principal components. Samples collected at the first time-point (meconium) differ significantly from those taken at the subsequent time-points.
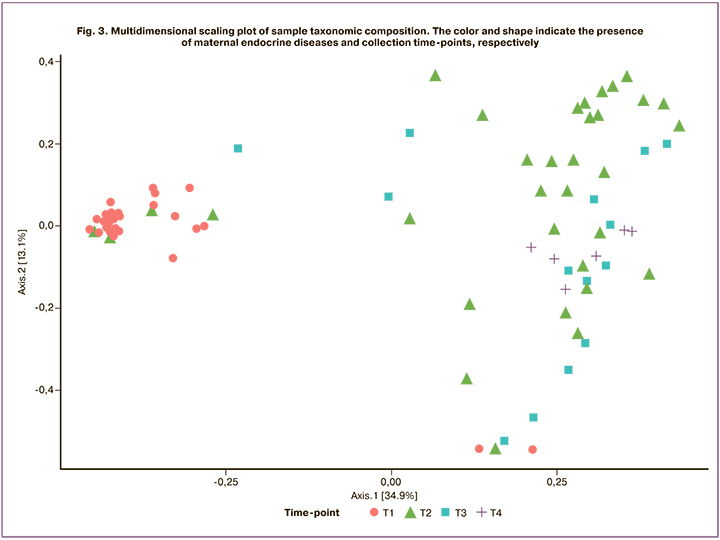
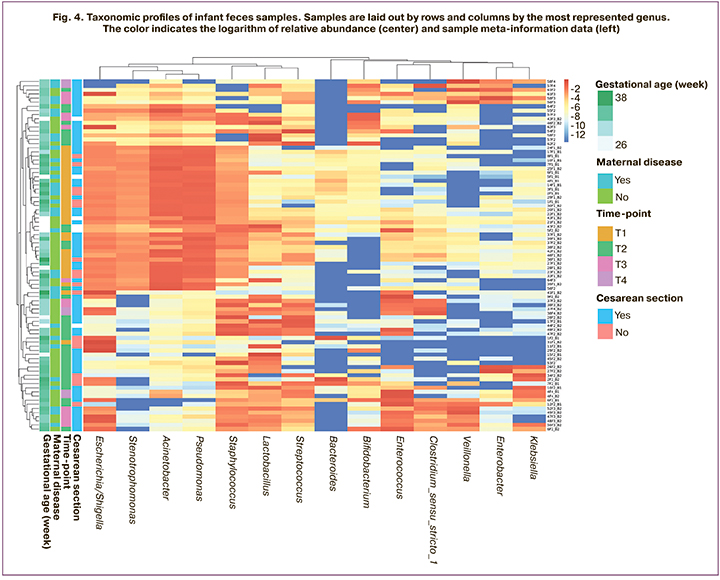
Figure 4 shows a heat map of the collected samples' taxonomic profiles, indicating maternal endocrine diseases, gestational age, and operative delivery.
Analysis of the influence of maternal diseases on the taxonomic profile of samples
To search for bacterial genera associated with maternal endocrine disorders, we used the Deseq2 package for the R. We submitted a set of taxonomic profiles, and the following meta-information as input: 1) sequencing start number (1–3); 2) gestational age (25–39 week); 3) whether a cesarean section was performed (yes/no); 4) the presence of maternal endocrine disorders (yes/no).
First time-point
For the first time point (meconium), no statistically significant associations with maternal endocrine disorders were found.
Second time-point
For the second time point (the first week postpartum, Table 1), there was a differential abundance of genera including Lactobacillus, Streptococcus, Bifidobacterium, Clostridium_sensu_stricto_1, Klebsiella, Veillonella, Sphingomonas, Enterobacter, Bradyrhizobium was observed (listed in decreasing order of occurrence, the proportion of samples where they were detected).
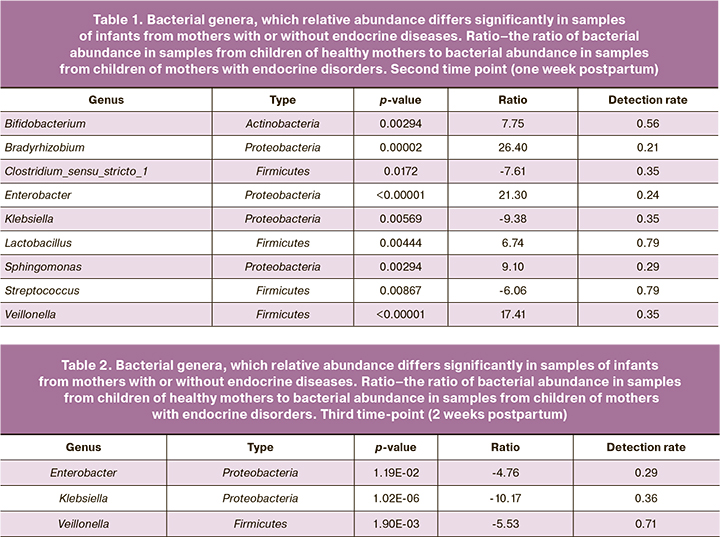
Three genera that were statistically significant and found in more than half of the samples are Lactobacillus, Streptococcus, Bifidobacterium. The abundance of genera Lactobacillus and Bifidobacterium was higher in children born to healthy mothers, and the abundance of the genus Streptococcus in children born to mothers with endocrine diseases.
Third time-point
There were fewer samples collected at the third time-point than at the second time-point, making it impossible to include all metadata in the analysis. Below we provide a list of genera that are significantly associated with maternal disease when only two factors were taken into account: sequencing start number (1–3) and maternal endocrine disorders (yes/no). We observed a large abundance of genera Enterobacter, Klebsiella, and Veillonellа (Table 2).
Discussion
This study is based on the assumption that maternal endocrine diseases may affect newborn gut microbiota composition both at the time of delivery and later. We focused on puerperas with thyroid pathology (hypothyroidism and thyrotoxicosis) or gestational diabetes mellitus as the most common endocrine diseases in this age group (4.6% – hypothyroidism, 7% – gestational diabetes).
At the first time-point (meconium), no differences in gut microbiota composition were observed between groups of children. This may be due to mothers' compensated state, lack of influence of the fetus's immune and endocrine systems, or low content of bacterial DNA in this type of samples and, consequently, with a high level of contamination.
At the second time-point (one week postpartum), the infant's immune and endocrine systems are activated. Also, at this time-point, prolonged hospital stay as the risk factor is not too significant. We observed substantial individual variability in gut microbiota composition and differences in individual bacterial abundance in infants born to mothers with pathology or healthy mothers. In infants born to mothers with endocrine diseases, the colonization by conditionally pathogenic flora (streptococci) was observed, which is typical for cases of decreased immune competence [28]. In infants born to healthy mothers, the colonization by bifidobacteria and lactobacilli occurs to a greater extent, which is characteristic of the normal development of newborns' intestinal microbiota [29].
At the third time-point (2 weeks postpartum), differences in gut microbiota composition between the groups were also observed. In the group of infants born to mothers with endocrine diseases, there was an increased abundance of the causative agents of healthcare-associated infections, including genera Klebsiella and Enterobacter. The same trends were observed at the second time-point.
An increase in the relative abundance of the genus Enterobacter may also be associated with prolonged exposure of preterm infants to antibiotic therapy [30]. This bacterial genus is associated with NEC in prematurity. The increased abundance of the Enterobacter genus in children born to mothers with endocrine disorders may be because the mother's body cannot adequately produce protective antibodies, particularly secretory immunoglobulin A (SIgA) antibodies. In infants born at term, the production of secretory SIgA begins 7–30 days after birth. In preterm babies, this process starts later. In a study [31], intrinsic IgAs in preterm formula-fed infants' feces were detected only 40 days after birth. Well-designed and adequately powered clinical trials are needed to investigate further changes in newborn microbiota in women with endocrine diseases. This implies the possibility of finding more associations, and secondly, dividing the effects depending on the specific maternal pathology. We believe that further studies involving methods for assessing the immune and endocrine status of newborns are warranted.
Conclusion
Our findings of differences in preterm infant gut microbiota composition suggest that the maternal endocrine system's state can influence the infant gut microbiome's formation during the early stages of its colonization. In turn, impaired formation and development of gut microbiota in childhood are linked to later life diseases.
References
- Tanaka M., Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017; 66(4): 515-22. https://dx.doi.org/10.1016/j.alit.2017.07.010.
- Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Reddy D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015; 21(29): 8787-803. https://dx.doi.org/10.3748/wjg.v21.i29.8787.
- Koren O., Goodrich J.K., Cullender T.C., Spor A., Laitinen K., Bäckhed H.K. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012; 150(3): 470-80. https://dx.doi.org/10.1016/j.cell.2012.07.008.
- Mueller N.T., Whyatt R., Hoepner L., Oberfield S., Dominguez-Bello M.G., Widen E.M. et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. (Lond). 2015; 39(4): 665-70. https://dx.doi.org/10.1038/ijo.2014.180.
- Mueller N.T., Bakacs E., Combellick J., Grigoryan Z., Dominguez-Bello M.G. The infant microbiome development: mom matters. Trends Mol. Med. 2015; 21(2): 109-17. https://dx.doi.org/10.1016/j.molmed.2014.12.002.
- Mulligan C.M., Friedman J.E. Maternal modifiers of the infant gut microbiota: metabolic consequences. J. Endocrinol. 2017; 235(1): R1-12. https://dx.doi.org/10.1530/JOE-17-0303.
- Ringholm L., Damm P., Mathiesen E.R. Improving pregnancy outcomes in women with diabetes mellitus: modern management. Nat. Rev. Endocrinol. 2019; 15(7): 406-16. https://dx.doi.org/10.1038/s41574-019-0197-3.
- Huget-Penner S., Feig D.S. Maternal thyroid disease and its effects on the fetus and perinatal outcomes. Prenat. Diagn. 2020; 40(9): 1077-84. https://dx.doi.org/10.1002/pd.5684.
- Zhou M., Wang M., Li J., Luo X., Lei M. Effects of thyroid diseases on pregnancy outcomes. Exp. Ther. Med. 2019; 18(3): 1807-15. https://dx.doi.org/10.3892/etm.2019.7739.
- Delitala A.P., Capobianco G., Cherchi P.L., Dessole S., Delitala G. Thyroid function and thyroid disorders during pregnancy: a review and care pathway. Arch. Gynecol. Obstet. 2019; 299(2): 327-38. https://dx.doi.org/10.1007/s00404-018-5018-8.
- Korevaar T.I.M., Derakhshan A., Taylor P.N., Meima M., Chen L., Bliddal S. et al. Consortium on Thyroid and Pregnancy - Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019; 322(7): 632-41. https://dx.doi.org/10.1001/jama.2019.10931.
- Dodic M., Peers A., Coghlan J.P., Wintour M. Can excess glucocorticoid, in utero, predispose to cardiovascular and metabolic disease in middle age? Trends Endocrinol. Metab. 1999; 10(3): 86-91. https://dx.doi.org/10.1016/s1043-2760(98)00125-8.
- Berkane N., Liere P., Oudinet J.P., Hertig A., Lefèvre G., Pluchino N. et al. From pregnancy to preeclampsia: a key role for estrogens. Endocr. Rev. 2017; 38(2): 123-44. https://dx.doi.org/ 10.1210/er.2016-1065.
- Amaral L.M., Wallace K., Owens M., LaMarca B. Pathophysiology and current clinical management of preeclampsia. Curr. Hypertens. Rep. 2017; 19(8): 61. https://dx.doi.org/10.1007/s11906-017-0757-7.
- Korpela K., Blakstad E.W., Moltu S.J., Strømmen K., Nakstad B., Rønnestad A.E. et al. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018; 8(1): 2453. https://dx.doi.org/10.1038/s41598-018-20827-x.
- Younge N.E., Newgard C.B., Cotten C.M., Goldberg R.N., Muehlbauer M.J., Bain J.R. et al. Disrupted maturation of the microbiota and metabolome among extremely preterm infants with postnatal growth failure. Sci. Rep. 2019; 9(1): 8167. https://dx.doi.org/10.1038/s41598-019-44547-y.
- Stewart C.J., Marrs E.C.L., Magorrian S., Nelson A., Lanyon C., Perry J.D. et al. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012; 101(11): 1121-7. https://dx.doi.org/10.1111/j.1651-2227.2012.02801.x.
- Pärtty A., Luoto R., Kalliomäki M., Salminen S., Isolauri E. Effects of early prebiotic and probiotic supplementation on development of gut microbiota and fussing and crying in preterm infants: a randomized, double-blind, placebo-controlled trial. J. Pediatr. 2013; 163(5): 1272-7. e1-2. https://dx.doi.org/10.1016/j.jpeds.2013.05.035.
- Kumbhare S.V., Patangia D.V.V., Patil R.H., Shouche Y.S., Patil N.P. Factors influencing the gut microbiome in children: from infancy to childhood. J. Biosci. 2019; 44(2): 49.
- Patton L., Li N., Garrett T.J., Ruoss J.L., Russell J.T., de la Cruz D. et al. Antibiotics effects on the fecal metabolome in preterm infants. Metabolites. 2020; 10(8): 331. https://dx.doi.org/10.3390/metabo10080331.
- Wang Y., Liu Y., Bai J., Chen X. The effect of maternal postpartum practices on infant gut microbiota: a Chinese cohort study. Microorganisms. 2019; 7(11): 511. https://dx.doi.org/10.3390/microorganisms7110511.
- Deshpande G., Rao S., Patole S., Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010; 125(5): 921-30. https://dx.doi.org/ 10.1542/peds.2009-1301.
- Grev J., Berg M., Soll R. Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2018; (12): CD012519. https://dx.doi.org/10.1002/14651858.CD012519.pub2.
- Wandro S., Osborne S., Enriquez C., Bixby C., Arrieta A., Whiteson K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. mSphere. 2018; 3(3): e00104-18. https://dx.doi.org/10.1128/mSphere.00104-18.
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016; 13(7): 581-3. https://dx.doi.org/10.1038/nmeth.3869.
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014; 15(12): 550. https://dx.doi.org/10.1186/s13059-014-0550-8.
- McMurdie P.J., Holme S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One. 2013; 8(4): e61217. https://dx.doi.org/10.1371/journal.pone.0061217.
- Korir M.L., Manning S.D., Davies H.D. Intrinsic maturational neonatal immune deficiencies and susceptibility to group B Streptococcus infection. Clin. Microbiol. Rev. 2017; 30(4): 973-89. https://dx.doi.org/10.1128/CMR.00019-17.
- Wall R., Ross R.P., Ryan C.A., Hussey S., Murphy B., Fitzgerald G.F. et al. Role of gut microbiota in early infant development. Clin. Med. Pediatr. 2009; 3: 45-54. https://dx.doi.org/10.4137/cmped.s2008.
- Greenwood C., Morrow A.L., Lagomarcino A.J., Altaye M., Taft D.H., Yu Z. et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J. Pediatr. 2014; 165(1): 23-9. https://dx.doi.org/10.1016/j.jpeds.2014.01.010.
- Dunne-Castagna V.P., Taft D.H. Mother’s touch: milk IgA and protection from necrotizing enterocolitis. Cell Host Microbe. 2019; 26(2):147-8. https://dx.doi.org/10.1016/j.chom.2019.07.013.
Received 09.12.2020
Accepted 19.01.2021
About the Authors
Tatiana V. Priputnevich, Dr. Med. Sci., Director of the Institute of Microbiology, Antimicrobial Therapy and Epidemiology, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia. Tel.: +7(910)414-56-16. E-mail: priput1@gmail.com. ORCID: 0000-0002-4126-9730. 4, Oparin str., Moscow, 117997, Russia.
Anastasia V. Nikolaeva, Ph.D., Chief physician, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(916)653-43-09. E-mail: a_nikolaeva@oparina4.ru.
4, Oparin str., Moscow, 117997, Russia.
Natalia E. Shabanova, Ph.D., Associate Professor, Researcher at the Unit of Clinical Pharmacology, Institute of microbiology, Antimicrobial Therapy and Epidemiology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Tel.: +7(985)097-58-27. E-mail: n_shabanova@oparina4.ru. ORCID: 0000-0001-6838-3616. 4 Oparin str., Moscow, 117997, Russia.
Dmitry E. Fedorov, Research Laboratory Assistant at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(985)115-48-96. E-mail: fedorov.de@gmail.com. ORCID: 0000-0001-8468-7011. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Alexander I. Manolov, Junior Researcher at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(985)417-73-08.
E-mail: paraslonic@gmail.com. ORCID: 0000-0003-3912-429X. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Alexander V. Pavlenko, Researcher at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(926)111-72-82.
E-mail: pavav@mail.ru. ORCID: 0000-0002-9549-0289. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Dmitry N. Konanov, Laboratory Research at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(915)499-74-62.
E-mail: konanovdmitriy@gmail.com. ORCID: 0000-0002-1217-9234. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Danil V. Krivonos, Student at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(905)771-66-82.
E-mail: Danil01060106@gmail.com. ORCID: 0000-0002-3851-5873. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Ksenia M. Klimina, Senior Researcher at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(916)133-60-90.
E-mail: ppp843@yandex.ru. ORCID: 0000-0002-5563-644X. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Vladimir A. Veselovskiy, Junior Researcher at the Laboratory of Genome Research and Computational Biology, SRI PCM of FMBA of Russia. Tel.: +7(916)415-78-79.
E-mail: djdf26@gmail.com. ORCID: 0000-0002-4336-9452. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
Viktor V. Zubkov, Dr. Med. Sci., Professor, Director of the Institute of Neonatology and Pediatrics, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Head of the Department of Neonatology, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia; Professor of the Department of Neonatology, Institute of Children’s Health, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). E-mail: victor.zubkov@mail.ru. ORCID: 0000-0002-9697-9596. 4 Oparin str., Moscow, 117997, Russia.
Elena N. Il’ina, Corr. Member of the RAS, Dr. Bio. Sci., Deputy Director for Science, Head of the Department of Molecular Biology and Genetics, SRI PCM of FMBA of Russia. Tel.: +7(499) 245-04-71. E-mail: ilinaen@gmail.com. ORCID: 0000-0003-0130-5079. 1A Malaya Pirogovskaya str., Moscow, 119435, Russia.
For citation: Priputnevich T.V., Nikolaeva A.V., Shabanova N.E., Fedorov D.E., Manolov A.I., Pavlenko A.V., Konanov D.N., Krivonos D.V., Klimina K.M., Veselovskii V.A., Zubkov V.V., Il’ina E.N. Microbiota of preterm infants born to mothers with endocrine disorders.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 2: 96-104 (in Russian)
https://dx.doi.org/10.18565/aig.2021.2.96-104



