Cerebral blood flow in severe pre-eclampsia and eclampsia
Aim. To investigate the characteristics of cerebral blood flow in patients with preeclampsia and eclampsia and evaluate the feasibility of predicting and early diagnosis of cerebrovascular disordersSidorova I.S., Nikitina N.A., Tardov M.V., Stulin I.D.
Materials and methods. The present study included 174 women aged 17–44 with preeclampsia (n=117), pregnancy with chronic arterial hypertension (n=13), healthy pregnancy (n=34), and 10 non-pregnant women. Clinical evaluation included measurement of blood pressure, Doppler ultrasound of blood flow in major neck and brain arteries, as well as the venous trunks and meningeal venous sinuses. The blood flow assessment involved measurements of velocity and vascular resistance, arterial reactivity in both major territories, and combined cerebral blood flow and intracranial pressure indicators.
Results. During preeclampsia, cerebral blood flow changes in the vertebra-basilar and carotid systems increased in a wavelike and non-synchronous manner. Moderate preeclampsia was characterized by large artery spasm and small-caliber artery dilation. In severe preeclampsia, there was a marked dilation of cerebral arteries with total cerebral hyper-perfusion and a progressive decrease in the cerebral vascular autoregulation reserve, which was more pronounced in vertebrobasilar arteries. After eclamptic seizures, the degree of vascular disorders reached a maximum, manifested by a sharp drop in cerebral blood flow, the development of cerebral edema, and an increase in intracranial pressure.
Conclusion. Assessment of cerebral blood flow using transcranial Doppler ultrasound provides essential information about the condition of a patient with increasing preeclampsia severity.
Keywords
Preeclampsia (PE) is a multisystem pregnancy-specific hypertensive disease that affects 2–8% of pregnancies and is the second leading cause of maternal morbidity and mortality worldwide. [1]. Among causes of maternal mortality from PE, cerebrovascular complications account for a significant proportion [2–5].
Cerebrovascularcomplicationsof PEincludesyndrome of posterior reversible encephalopathy, reversible cerebral vasoconstriction syndrome, hemorrhagic and ischemic stroke, as well as long-term consequences in the patient's later life after severe PE and eclampsia such as cognitive impairment and vascular dementia [6, 7]. Pre-eclampsia has been associated with white matter lesions confirmed by magnetic resonance imaging 5–15 years after PE [7, 8].
The risk of developing acute cerebrovascular complications in pregnant women with PE is approximately 1 in 500 births, while the overall risk of such complications during pregnancy is about 30 cases per 100,000 births [9].
The cause and pathogenesis of cerebral complications of PE and eclampsia are not fully understood. One explanation for the etiology of cerebrovascular complications associated with PE is that it is a form of posterior reversible encephalopathy syndrome, a variant of hypertensive encephalopathy. They resulted from impaired autoregulation of cerebral blood flow. They increased blood-brain barrier (BBB) permeability with the development of vasogenic and cytotoxic brain edema, mainly in the parietooccipital regions of the brain.
Computed and magnetic resonance imaging in women after PE showed that some of them had lesions in the subcortical zones of the white matter and adjacent areas of the cerebral cortex [10], which may cause damage to the brain parenchyma with subsequent residual neurological sequelae.
Cerebral autoregulation is essential to maintain adequate cerebral perfusion in response to blood pressure changes (BP). Cerebral blood perfusion is maintained relatively constant between mean arterial pressures of about 60 and 160 mmHg but rises linearly with blood pressures below or above that range [11]. Overall, studies reporting the effect of preeclampsia on cerebral blood flow are contradictory. Many of them have revealed a significant increase in cerebral perfusion pressure (CPP) in women with PE than in healthy pregnant women [12]. Other authors observed decreased CPP at early gestational ages (sudden fainting, impairment of memory, attention, and performance).
Belfort M.A. et al. suggested that the increased CPP promotes the development of vasogenic edema and posterior reversible encephalopathy syndrome in PE and eclampsia [13]. It was reported that cerebral perfusion pressure in women with PE was elevated even after treatment of elevated blood pressure [14]. The same authors also observed a direct correlation between high blood pressure and CPP in pregnant women with preeclampsia, which indicates the absence of adequate compensatory brain response to changes in blood pressure and, in fact, an impaired cerebral blood flow autoregulation [14].
During eclampsia, acute elevations in cerebral blood flow lead to maximum CPP in the phase of eclamptic seizures; however, in contrast to PE, there is no compensatory increase in vascular resistance [15].
Impaired autoregulation of cerebral blood flow and increased BBB permeability can result in cerebral edema and convulsive syndrome [16]. The actual cause of increased BBB permeability in PE has not been reliably studied. There is a hypothesis that factors released from the ischemic placenta in PE might sensitize the cerebral vasculature to blood pressure changes, which might enhance BBB permeability [17].
Based on experimental data, two diametrically opposite hypotheses of the onset of cerebral edema have been proposed. The first to appear chronologically was the vasospastic concept, according to which a sharp rise in blood pressure leads to prolonged spasm of cerebral vessels and disruption of autoregulation [18]. These changes lead to hypoxia, endothelial dysfunction, and further vasogenic and cytotoxic cerebral edema [19].
The later concept of vasodilation postulates that abrupt or significant increases in blood pressure, on the contrary, induce intense cerebral vasodilation with the development of cerebral hyper-perfusion. The latter results from disrupted vasoconstrictor mechanisms and is the cause of an increase in cerebral blood flow, increased pressure on the vessel wall, damage to the BBB, and the development of vasogenic cerebral edema [11, 20]. The most severe disorders are known to occur in the posterior parts of the brain in the vertebrobasilar system involved in supplying blood to cortical areas of the visual analyzer, which is explained by the properties of arterial innervation in this area [21].
Given the contradictory data on the pathophysiological mechanisms of cerebrovascular complications in PE, the lack of evidence for its predicting and correction, this study aimed to investigate the characteristics of cerebral blood flow in patients with preeclampsia and eclampsia and evaluate the feasibility of predicting and early diagnosis of cerebrovascular disorders.
Materials and methods
The present study included 174 women aged 17–44. Of these, 117 (the study group) were at 33-41 weeks gestation and were hospitalized in Moscow maternity hospitals with PE (98 and 16 had moderate and severe PE, and 3 had eclampsia). The remaining patients were divided into three groups, including women with a healthy pregnancy (control group, n=34), chronic arterial hypertension (n=13), and ten non-pregnant women. The groups were comparable in terms of age, parity, somatic, and gynecological history.
Thanks to the developed algorithm for an emergency call of a Doppler ultrasound specialist to the appropriate medical institutions, maternal cerebral blood flow was examined before the treatment initiation, except for eclampsia cases. Patients who had eclamptic seizures were examined together with the consultative mobile neurodiagnostic team of the Moscow Coordination Center of Organ Donation immediately after an emergency delivery.
Before Doppler imaging of blood flow in the neck and brain major arteries, blood pressure was measured. Doppler measurements were performed according to the standard technique, with a 2-4-MHz probe on a Sonomed 325 ultrasound scanner (Spectromed, Moscow).
The blood flow velocity parameters (linear blood flow velocity - LBFV) and peripheral bed resistance in the carotid and vertebrobasilar regions' arteries were evaluated. The asymmetry of LBFV in the paired arteries of the brain base was recorded. Venous trunks and sinuses of the meninges were also examined where they were accessible for ultrasound scanning.
The second stage included the determination of the indicators of the carotid and vertebrobasilar arterial reactivity and the combined indices of cerebral blood flow, taking into account the parameters of systemic hemodynamics (CPP, hydrodynamic resistance index (HRI), and cerebral flow index (CFI).
Intracranial pressure was assessed using the Klingelhofer index, calculated based on blood pressure values and LBF in the middle cerebral artery (MCA).
The vessels' scanning took 15–20 minutes and was performed simultaneously with the obstetric examination.
Statistical analysis
Statistical analysis was performed using the Statistica v.8.0 software for Windows. It was found that numerical variables were not normally distributed as indicated by the Lilliefors and Kolmogorov-Smirnov tests, and groups were compared using nonparametric tests. Quantitative variables were expressed as the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format. Kruskal–Wallis and the median test were used for comparing numerical data between groups, followed by pairwise comparison using the Mann–Whitney U-test with the Bonferroni correction. Differences were considered statistically significant at p<0.05 and p<0.0125 when comparing four groups.
Results
Compared with non-pregnant women, women with healthy pregnancies had up to 24% lower mean, terminal diastolic, and peak LBFV in the carotid system's extracranial segments (in both carotid arteries, supratrochlear artery (STA)). In the vertebral arteries, LBFV was increased by only 3–10%.
In patients with PE, the common and internal carotid arteries (CCA, ICA) showed a gradual 15–37% decrease in LBFV against the background of a corresponding reduction in peripheral resistance. The most pronounced changes were observed in severe PE (Table 1). In severe PE, the most pronounced decrease in LBFV was also noted in the vertebral artery (VA) (by 21–28%).
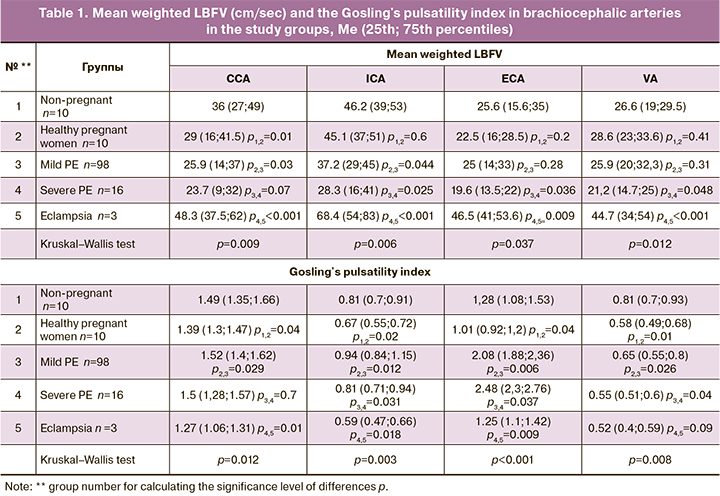
The severity of PE increased in parallel with an increase in LBFV asymmetry in the paired arteries of the base of the brain.
After eclamptic seizures, the findings changed dramatically. There was a significant decrease in peripheral resistance concurrent with a twofold increase in LBFV, which indicates vasodilation and the formation of the phenomenon of cerebral hyper-perfusion. It was accompanied by up to 170% increase in LBFV relative to the same parameters of severe PE.
In patients with moderate PE, blood flow in intracranial arteries showed several differently directed changes. LBFV was reduced in all arteries of the brain base with a concurrent increase in MCA's peripheral resistance and a decrease in peripheral resistance in the arteries of the vertebrobasilar system (Table 2). With an increase in PE severity, LBFV showed asynchronous changes with a total increase of 10–15% and a decrease in peripheral resistance by 15–20%.
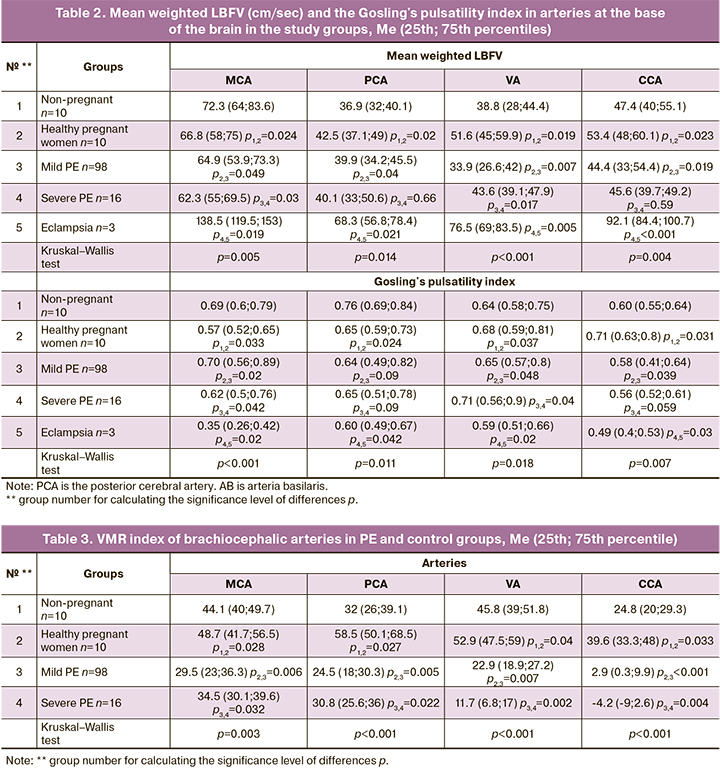
Thus, our data indicate multidirectional changes in peripheral resistance in extra- and intracranial VA segments and unidirectional changes in the same segments of the carotid arteries, partly explaining the predominant lesions of the posterior parts of the brain in PE. After eclamptic seizures, there was a sharp 60–100% acceleration of LBFV in all intracranial arteries and a decrease in peripheral resistance, which, combined with a Lindegaard index not exceeding 2.0, indicates pronounced vasodilation and cerebral hyperperfusion, similar to changes in the flow in extracranial segments of cerebral vessels.
The brachiocephalic and intracranial blood flow patterns in women in the final phase of eclampsia were reverberant, i.e., having two simultaneously directed phases. We registered a retrograde flow in the STA during eclamptic seizures, corresponding to an extreme increase in intracranial pressure (ICP) with noticeable signs of obstruction of venous outflow.
We also noted quite significant changes in blood flow resistance in the arteries of the Circle of Willis in PE, compared with healthy pregnant women and non-pregnant women. The most pronounced differences were revealed when assessing the Gosling's pulsatility index (Tables 1, 2).
Assessment of cerebral vessel reactivity during apnea and hyperventilation test in patients with PE showed a sharp decrease in vasoconstrictor reserve with a simultaneous increase in dilatation one in parallel with increasing severity of this pregnancy complication. The most significant changes were registered in the posterior cerebral arteries.
In severe PE, there was also an increase in the vasomotor reactivity index (VMR index) in the carotid system's arteries concurrently with its sharp decrease (up to negative values) in the arteries of the vertebrobasilar system. In this category of patients, these VMR index changes, characterizing the cerebral hemodynamic reserve, indicate the development of paradoxical cerebrovascular reactions to respiratory tests (Table 3).
In patients with PE, cerebral blood flow changes occur in waves and asynchronously in the vertebrobasilar and carotid systems. Moderate PE is characterized by spasms of large arteries and dilatation of small arteries. Further changes lead to paralytic dilatation of cerebral arteries with the phenomenon of total cerebral hyper-perfusion. The asymmetry of LBFV in paired arterial trunks also increases. There was a progressive decrease in cerebral vascular autoregulation reserve in parallel with an increase in PE severity, which was most pronounced in posterior cerebral arteries.
After eclamptic seizures, vascular disorders reach a maximum, manifested by a sharp drop in cerebral blood flow, the development of cerebral edema, and a maximum increase in ICP, up to cerebral tamponade with an outcome in cerebral death.
Ultrasound examination of cerebral vessels was carried out in the venous vertebral plexuses and orbital veins, middle and posterior cerebral veins, Galen's vein, sinus rectus, and cavernous sinus. As the severity of PE increased, there was an increase in the number of scanned venous trunks and a general rise in LBFV in the localized vessels. In severe PE, a characteristic finding was retrograde blood flow in the orbital veins. These changes are evidence of a pronounced impairment of venous outflow from the cranial cavity, caused by an increase in ICP.
Evaluation of ICP also revealed impaired CSF dynamics. In moderate PE, there was a relative decrease in ICP, while it increased in severe PE, especially shortly before eclamptic seizures. ICP sharply decreased after seizures, but then, in patients with post-eclamptic coma or cerebral edema, ICP again increases to critical values. The calculation of ICP was carried out using Klingelhofer's index (1988), which is based on the mean LBFV in MCA and mean blood pressure.
We did not find statistically significant differences in the Klingelhofer's index between pregnant women in the control group and non-pregnant women [1.08 (Q1; Q3 – 1.02; 1.14)] and [1.03 (Q1; Q3 – 0.9; 1.08)], respectively. In patients with moderate PE, the Klingelhofer's index increased by almost 35% up to 1.38 (Q1; Q3 – 1.3; 1.49) and reached maximum in severe PE [1.67 (Q1; Q3 – 1.58; 1.79)], increasing by 62.1%. Within 30–40 minutes after eclampsia, Klingelhofer's index reduced to values typical for the control group [1.06 (Q1; Q3 – 0.99; 1.15)]. However, this index was more than twice as high in patients with post-eclamptic coma than in pregnant women with severe PE and 3.5 times higher than in healthy pregnant women [3.71 (Q1; Q3 – 2.97; 4.53)].
Therefore, changes in ICP in PE are quite dynamic. With increasing severity of PE and aggravation of cerebrovascular disorders, ICP gradually increases, reaching maximum immediately before eclamptic seizures. After resolution of eclampsia in patients with favorable outcomes, ICP drops sharply to almost normal levels. In patients with a complicated PE course, it increases to critical levels equivalent to severe cerebral edema.
A qualitative assessment of cerebral blood flow (Table 4) should be carried out, taking into account central hemodynamics' characteristics and the magnitude of systemic blood pressure. For this purpose, at the final stage of our study, the CPP, GCI, and ICC were assessed (the indices were calculated using a modification of the formula derived by Aaslid and modified by Belfort (1994, 2001).
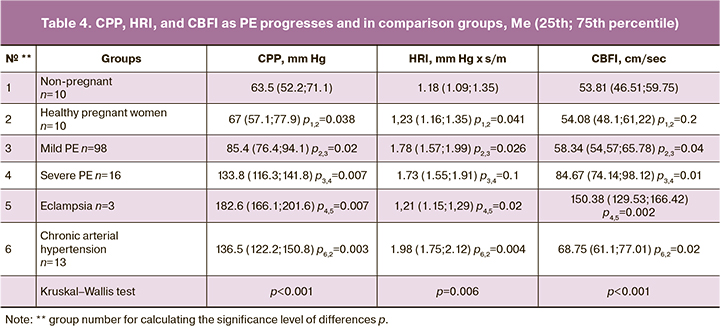
With the development of PE, we noted an increase in CPP and HRI, especially pronounced in its severe form.
CBFI is a generalized measure of cerebral blood flow, which is relatively constant and reflects the cerebral blood supply's adequacy. During physiological pregnancy, no significant changes in CBFI occur. At the onset of first signs of moderate PE, CBFI was reduced by 25% compared to healthy pregnant women. With an increase in the severity of PE, there is a significant rise to 110%. Such high CBFI values are already accompanied by neurological symptoms (headache, blurred vision, consciousness, etc.).
After eclamptic seizures, CPP increased 1.5 times, vascular resistance decreased by 50%, and CBFI increased by 75% (3 times higher than in healthy pregnant women). CPP dropped to critical values in patients with brain tamponade, HRI raised by 3.5 times, CBFI dropped to almost zero, which was the equivalent to brain death.
Among pregnant women with chronic arterial hypertension, CPP, HRI, and CBFI did not differ statistically significantly from these indicators in the group with severe PE. In this group, there was an increase in peripheral resistance, moderate cerebral vasospasm, and a unidirectional decrease in vascular reactivity in the vertebrobasilar and carotid systems, radically different from the physiological rearrangement of cerebral blood flow during pregnancy.
The following conclusions can be drawn from the present study:
1. During a healthy pregnancy, cerebral vessels undergo moderate dilatation. Simultaneously, shifts in blood flow indices in the extra- and intracranial segments of the arteries of the carotid and vertebrobasilar systems are multidirectional (a compensatory mechanism). In the vertebrobasilar system, blood flow indicators remain similar to those in non-pregnant women.
2. The development of PE is characterized by simultaneous cerebral vasodilation of some cerebral vessels and vasoconstriction of others, which are asynchronous in the carotid and vertebrobasilar systems. These changes result in a special type of blood flow - a combination of vasoconstriction of large arteries and paralytic dilation of smaller arteries.
3. The cerebral autoregulation reserve in healthy pregnancy increases mainly due to an increase in cerebral vessels' ability to dilate. With the development and progression of PE, there is a decrease in the autoregulation reserve, especially pronounced in the vertebrobasilar basin vessels.
4. In pregnant women with chronic arterial hypertension, cerebral blood flow indicators reflect persistent angiospasm in all arteries of the base of the brain with a relative decrease in cerebral autoregulation reserve.
5. As PE develops and progresses, venous outflow abnormalities from the cranial cavity increase, reaching critically high values in eclampsia.
6. Eclamptic seizures lead to an aggravation of cerebral blood flow abnormalities. In severe cases, they are accompanied by a critical increase in ICP with concurrent cerebral edema, terminally low cerebral blood flow, which indicates brain death.
Discussion
The brain blood supply system consists of the vessels of the vertebrobasilar and carotid basins with numerous anastomoses. Our data on the dynamics of cerebral perfusion in uncomplicated pregnancy indicate a relative decrease in blood flow intensity in the carotid system and its slight increase in the vertebrobasilar basin. Perhaps the reduction in LBFV in the carotid system's arteries is associated with a change in the hormonal background inherent in pregnancy. In turn, the increase in LBF in PA is due primarily to the difference in the neurogenic regulation of blood flow in the vertebrobasilar and carotid basins, including the different ratio of sympathetic and parasympathetic effects on arterial walls of the "posterior basin," a different receptor apparatus of PA.
The multidirectional vascular tone changes in the extra- and intracranial segments of the arteries of the vertebrobasilar and carotid systems, which we first identified, raise many questions. Both in healthy pregnant women and during the development of PE, a significant decrease in vascular tone is recorded in the PA's extracranial segments, and an increase in intracranial ones (in contrast to the internal carotid arteries, where the tone in both segments changes synchronously). Such an imbalance of hemodynamic parameters in severe PE and eclampsia indicates a decrease in the compensatory capabilities of blood flow regulation in the vertebrobasilar system's vessels.
In pregnant women with PE or eclampsia undergoing timely delivery in the absence of severe complications, blood flow indices restore within 2–3 days postpartum. In the case of progressive deterioration of severe PE and eclampsia, ineffective blood flow in the ICA and PA decrease in the diastolic and then the systolic component.
The study findings can be explained by the development of vasoconstriction in large arteries and dilatation in smaller ones against the background of general impairment of their reactivity. The dynamics of cerebral blood flow parameters during PE development and progression are determined by the deterioration of all cerebral blood flow velocity and resistive characteristics. They are characterized by vasoconstriction and vasodilation of the large and small trunks of the cerebral arteries and a reduction in cerebral autoregulation reserve.
Cerebral blood flow abnormalities in PE are also accompanied by venous outflow deterioration, as evidenced by an increase in BFV in the venous trunks, which reaches critical levels in eclampsia. Under conditions of cerebral hyperperfusion, the inability to rapidly restruct venous blood flow leads to an imbalance between blood pressure and ICP, which further increases CPP and creates conditions for vasogenic cerebral edema. Eclamptic seizures are characterized by a sharp increase in CPP, HRI, and CBFI, triggering a whole cascade of pathological processes that ultimately lead to damage (sometimes irreversible) of the medulla.
Arterial hypertension is an important clinical symptom of PE. However, blood pressure does not always reflect the severity of pathophysiological changes. This fact is supported by a study of cerebral blood flow in pregnant women with chronic arterial hypertension. These patients had signs of cerebral angiospasm with a relative decrease in cerebral autoregulation reserve, similar to the indices of vascular reactivity found in PE. The intensity of cerebral blood flow in patients with chronic arterial hypertension (in the absence of PE), although it was significantly higher than that in women with a healthy pregnancy, did not reach the same levels as in pregnant women with PE.
Therefore, the cardinal difference between cerebral vessels' arterial wall tone in chronic arterial hypertension and PE is vasoconstriction in the first case and vasodilation in the second. In patients with eclampsia, the effect of alternating both of these processes is recorded simultaneously, but in different segments of the same arteries (the so-called "sausage spasm").
Besides, pregnant women with chronic arterial hypertension, in contrast to patients with PE, have a less pronounced decrease in the hemodynamic reserve in the vessels of the vertebrobasilar system and a smaller difference in the reactivity of the vertebrobasilar and carotid systems. Such cerebral blood flow features in chronic arterial hypertension indicate the preservation of compensatory vascular reactions at a more physiological level, which allows maintaining cerebral perfusion during a rapid or sharp rise in blood pressure.
CPP, HRI, and CBFI in pregnant women with chronic arterial hypertension are similar to those in women with PE, but they have no severe brain damage characteristic of the latter. The time factor is probably important in this situation, i.e., the prolonged existence of chronic arterial hypertension (even before pregnancy) leads to a gradual and more stable adaptation of cerebral blood flow. Besides, systemic endotheliosis characteristic of PE determines fundamentally different reactivity and tone of cerebral vessels, especially in response to a sharp rise in blood pressure [22, 23].
From our perspective, the primary underlying factor in PE pathogenesis is damage to cerebral vessel endothelium, predominantly of small and medium caliber, the loss of its phenotypic properties, which entails an increase in BBB permeability, vasogenic edema, ischemic brain damage, and cytotoxic edema.
Further research is needed to address a differentiated approach to the prescription of certain drugs (with a predominant vasoconstrictor or vasodilating effect), maintaining an adequate CPP, and preserving compensatory mechanisms. A randomized trial of Sonneveld M.J. et al. reported that therapy with nimodipine was associated with more frequent eclamptic seizures in comparison with magnesium sulfate [14]. These findings may be explained by the different effects of these drugs on CPP.
The effect of calcium antagonists on cerebral perfusion is not always unambiguous. The earliest studies have shown that these drugs increase blood flow in healthy parts of the brain while causing steal syndrome of ischemic foci [24]. However, a recent large study by Zhang J. et al. did not confirm the negative effect of these drugs on ischemic stroke. There was no increase in the incidence of adverse outcomes and death (except for the use of sufficiently high doses of nifedipine, which worsened the outcomes) [25].
It is believed that a primary mechanism of cell death occurring during ischemia in many central nervous system cell types, including neurons, glia, and vascular endothelial cells, is oncotic cell death (oncosis) [26].
In severe cerebral ischemia, ATP depletion results in ion pump inhibition and breakdown of ionic gradients across cell membranes. The imbalance between the influx of Na + and Cl- ions into the cells and loss of K + ions creates a transmembrane osmotic gradient followed by water movement into the cells, which leads to cell swelling and oncosis. Simultaneously, a massive influx of calcium into cells is a common mechanism of their death through the activation of proteases, phospholipases, and mitochondrial destruction. ATP depletion also leads to the release of glutamate from neurons and astrocytes, which depolarizes the neuronal membrane and activates NMDA receptors. This leads to a sharp increase in calcium and sodium in neurons [27]. It has been suggested that calcium antagonist therapy may have some neuroprotective effect by blocking calcium flow into cells. Indeed, some studies have shown that calcium antagonists reduce mortality and disability, for example, in subarachnoid bleeding [28], by improving cerebral perfusion in the ischemic penumbra (secondary ischemic zone).
Blocking uncontrolled ion currents represents a new strategy in neuroprotection in ischemic brain damage.
It is possible that more rigorous and well-controlled antihypertensive therapy aimed at lowering CPP could lead to a decrease in the incidence of cerebral complications in these women, but further research is needed in this direction.
Conclusion
Development of PE is associated with a spasm in the cerebral arteries in some areas and paralytic dilatation in others, leading to uneven cerebral perfusion. Increased blood flow in the cranial cavity contributes to the disruption of venous outflow. Together, these phenomena cause increased ICP and cerebral edema.
Our study findings suggest that the onset of neurological symptoms in patients with PE is associated with pathological changes in the cerebral hemodynamics occurring in the vertebrobasilar arterial system. Assessment of cerebral blood flow using transcranial Doppler ultrasound provides essential information in pregnant women with PE. Further studies are needed to determine the optimal blood flow threshold in the vertebrobasilar arterial system to predict neurological symptoms associated with cerebral autoregulation dysfunction.
References
- Lee Y.J., Lee S., Jo H.N., Kim J.M., Kwon B.S., Joo J.K. et al. Alterations in transcranial Doppler indices of pregnant women with complicated preeclampsia. Pregnancy Hypertens. 2019; 15: 189-94. https://dx.doi.org/10.1016/j.preghy.2019.01.009.
- Ghulmiyyah L., Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012; 36(1): 56-9. https://dx.doi.org 10.1053/j.semperi.2011.09.011.
- Филиппов О.С., Гусева Е.В., Сидорова И.С., Никитина Н.А. Результаты конфиденциального аудита материнской смертности в Российской Федерации в 2014 году. Методическое письмо Министерства здравоохранения Российской Федерации № 15-4/10/2-5993 от 09.10.2015. 50с. [Filippov O.S., Guseva E.V., Sidorova I.S., Nikitina N.A. Results of a confidential audit of maternal mortality in the Russian Federation in 2014. Methodological letter of the Ministry of Health of the Russian Federation No 15-4/10/2-5993 from 09.10.2015. 50 p. (in Russian)].
- Филиппов О.С., Гусева Е.В., Сидорова И.С., Никитина Н.А. Результаты конфиденциального аудита материнской смертности в Российской Федерации в 2015 году. Методическое письмо Министерства здравоохранения Российской Федерации № 15-4/10/2-7955 от 14.12.2016. 61с. [Filippov O.S., Guseva E.V., Sidorova I.S., Nikitina N.A. Results of a confidential audit of maternal mortality in the Russian Federation in 2015. Methodological letter of the Ministry of Health of the Russian Federation No 15-4/10/2-7955 from 14.12.2016. 61 p. (in Russian)].
- Сидорова И.С., Никитина Н.А., Гусева Е.В. Результаты конфиденциального аудита материнской смертности от преэклампсии и эклампсии в России в 2017-2018 гг. Акушерство и гинекология. 2020; 1: 119-27. https://dx.doi.org/10.18565/aig.2020.l.119-127. [Sidorova I.S., Nikitina N.A., Guseva E.V. Results of a confidential audit of maternal mortality from preeclampsia and eclampsia in Russia in 2017-2018. Obstetrics and gynecology. 2020; 1: 119-27 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.119-127.
- Basit S., Wohlfahrt J., Boyd H.A. Pre-eclampsia and risk of dementia later in life: nationwide cohort study. BMJ. 2018; 363: k4109. https://dx.doi.org/10.1136/bmj.k4109.
- Siepmann T., Boardman H., Bilderbeck A., Griffanti L., Kenworthy Y., Zwager C. et al. Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 2017; 88(13): 1256-64. https://dx.doi.org/10.1212/WNL.0000000000003765.
- Ijomone O.K., Shallie P., Naicker T. Changes in the structure and function of the brain years after pre-eclampsia. Ageing Res. Rev. 2018; 47: 49-54. https://dx.doi.org/10.1016/j.arr.2018.06.006.
- Miller E.C., Gatollari H.J., Too G., Boehme A.K., Leffert L., Marshall R.S. et al. Risk factors for pregnancy-associated stroke in women with preeclampsia. Stroke. 2017; 48(7): 1752-9. https://dx.doi.org/10.1161/STROKEAHA.117.017374.
- Kutlesič M.S., Kutlesič R.M., Koratevič G.P. Posterior reversible encephalopathy syndrome in eclamptic patients: neuroradiological manifestation, pathogenesis and management. Med. Pregl. 2015; 68(1-2): 53-8. https://dx.doi.org/10.2298/mpns1502053k.
- Jones-Muhammad M., Warrington J.P. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci. 2019; 9(9): 224. https://dx.doi.org/10.3390/brainsci9090224.
- van Veen T.R., Panerai R.B., Haeri S., Griffioen A.C., Zeeman G.G., Belfort M.A. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet. Gynecol. 2013; 122(5): 1064-9. https://dx.doi.org/10.1097/AOG.0b013e3182a93fb5.
- Belfort M.A., Varner M.W., Dizon-Townson D.S., Grunewald C., Nisell H. Cerebral perfusion pressure, and not cerebral blood flow, may be the critical determinant of intracranial injury in preeclampsia: a new hypothesis. Am. J. Obstet. Gynecol. 2002; 187(3): 626-34. https://dx.doi.org/10.1067/mob.2002.125241.
- Sonneveld M.J., Brussé I.A., Duvekot J.J., Steegers E.A., Grune F., Visser G.H. Cerebral perfusion pressure in women with preeclampsia is elevated even after treatment of elevated blood pressure. Acta Obstet. Gynecol. Scand. 2014; 93(5): 508-11. https://dx.doi.org/10.1111/aogs.12358.
- Williams K., Galerneau F. Maternal transcranial Doppler in pre-eclampsia and eclampsia. Ultrasound Obstet. Gynecol. 2003; 21(5): 507-13. https://dx.doi.org /10.1002/uog.83.
- Hammer E.S., Cipolla M.J. Cerebrovascular dysfunction in preeclamptic pregnancies. Curr. Hypertens. Rep. 2015; 17(8): 64. https://dx.doi.org/10.1007/s11906-015-0575-8.
- Warrington J.P., Fan F., Murphy S.R., Roman R.J., Drummond H.A.,Granger J.P. et al. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol. Rep. 2014; 2(8): e12134. https://dx.doi.org/10.14814/phy2.12134.
- Williams K., Wilson S. Maternal middle cerebral artery blood flow velocity variation with gestational age. Obstet. Gynecol. 1994; 84(3): 445-8.
- Trommer B.L., Homer D., Mikhael M.A. Cerebral vasospasm and eclampsia. Stroke. 1988; 19(3): 326-9. https://dx.doi.org/10.1161/01.str.19.3.326.
- Bergman L., Torres-Vergara P., Penny J., Wikström J., Nelander M., Leon J. et al. Investigating maternal brain alterations in preeclampsia: the need for a multidisciplinary effort. Curr. Hypertens. Rep. 2019; 21(9): 72. https://dx.doi.org/10.1007/s11906-019-0977-0.
- Fischer M., Schmutzhard E. Posterior reversible encephalopathy syndrome. J. Neurol. 2017; 264(8): 1608-16. https://dx.doi.org/10.1007/s00415-016-8377-8.
- Сидорова И.С. Преэклампсия. М.: МИА; 2016. 528с. [Sidorova I.S. Preeclampsia. M.: MIA; 2016. 528 p. (in Russian)].
- Сидорова И.С., Никитина Н.А. Преэклампсия как гестационный иммунокомплексный комплементопосредованный эндотелиоз. Российский вестник акушера-гинеколога. 2019; 19(1): 5-11. [Sidorova I.S., Nikitina N.A. Preeclampsia as gestational immunocomplex endotheliosis. Russian Bulletin of obstetrician-gynecologist. 2019; 19(1): 5-11. (in Russian)]. https://dx.doi.org/10.17116/rosakush2019190115.
- Vorstrup S., Andersen A., Blegvad N., Paulson O.B. Calcium antagonist (PY 108-068) treatment may further decrease flow in ischemic areas in acute stroke. J. Cereb. Blood Flow Metab. 1986; 6(2): 222-9. https://dx.doi.org/10.1038/jcbfm.1986.35.
- Zhang J., Liu J., Li D., Zhang C., Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst. Rev. 2019; 2(2): CD001928. https://dx.doi.org/10.1002/14651858.CD001928.pub3.
- Loh K.Y., Wang Z., Liao P. Oncotic cell death in stroke. Rev. Physiol. Biochem. Pharmacol. 2019; 176: 37-64. https://dx.doi.org/10.1007/112_2018_13.
- Fricker M., Tolkovsky A.M., Borutaite V., Coleman M., Brown G.C. Neuronal cell death. Physiol. Rev. 2018; 98(2): 813-80. https://dx.doi.org/10.1152/physrev.00011.2017.
- Dorhout Mees S.M., Rinkel G.J.E., Feigin V.L., Algra A., van den Bergh W.M., Vermeulen M. et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007; (3): CD000277. https://dx.doi.org/10.1002/14651858.CD000277.pub3.
Received 03.09.2020
Accepted 13.10.2020
About the Authors
Iraida S. Sidorova, Dr.Med.Sci., Professor, Academician of the RAS, Merited Scholar of the Russian Federation, Merited Doctor of the Russian Federation, Professor at the Department of Obstetrics and Gynecology № 1, Medical Faculty, Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). E -mail: sidorovais@yandex.ru. ORCID: 0000-0003-2209-8662. 119991, Russia, Moscow, Trubetskaya str., 8-2.Natalya A. Nikitina, Dr.Med.Sci., Professor at the Department of Obstetrics and Gynecology № 1, Medical Faculty, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University). E-mail: natnikitina@list.ru. ORCID: 0000-0001-8659-9963. 119991, Russia, Moscow, Trubetskaya str., 8-2.
Mikhail V. Tardov, Dr.Med.Sci., Leading Researcher at the Research Department of the Inner Ear Audiology and Pathology, L.I. Sverzhevsky Research and Clinical Institute of Otorhinolaryngology. E-mail: mvtardov@rambler.ru. 117152, Russia, Moscow, Zagorodnoye shosse, 18A-2.
Igor D. Stulin, Dr.Med.Sci., Professor, Head of the Neurology department, Faculty of Medicine, A.I. Evdokimov Moscow State University of Medicine and Dentistry,
Ministry of Health of Russia. 127473, Russia, Moscow, Delegatskaya str., 20-1.
For citation: Sidorova I.S., Nikitina N.A., Tardov M.V., Stulin I.D. Cerebral blood flow in severe pre-eclampsia and eclampsia.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 90-99 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.90-99



