Signaling pathway components of immune checkpoint PD-1/PD-L1 in blood plasma of patients with ovarian cancer and benign ovarian tumors: clinical and morphological correlations
Objective. To analyze the contents of soluble programmed cell death receptor and its ligand (sPD-1/sPD-L1) in blood plasma of patients with ovarian cancer taking into consideration the main clinical and morphological characteristics of the disease. Materials and methods. A total of 125 patients with ovarian neoplasms were enrolled in the study, including 94 patients with ovarian cancer, 22 patients with benign tumors and 9 patients with borderline ovarian tumors. The age of the patients was 18–78 years. The control group consisted of 34 healthy women aged 18-68 years. Before treatment all patients were performed the analysis of sPD-1 and sPD-L1 levels in blood plasma using standard ELISA kits (Affimetrix, eBioscience, USA) according to the manufacturer’s instructions. Measurements were performed with immunoassay analyzer BEP 2000 Advance (Siemens, Germany). Results. The level of plasma sPD-L1 in patients with ovarian cancer does not differ significantly from one in the control group, while this level is significantly lower in patients with benign tumors than in healthy controls and patients with ovarian cancer. The level of sPD-1 in patients with ovarian cancer is increased as compared to the healthy controls (p=0.03). There is no correlation of marker levels with the histological structure and degree of differentiation of ovarian cancer. The level of sPD-L1 rises with the increasing stage of the disease (p<0.001); it is significantly higher in patients with ascites and bilateral ovarian lesions than in patients without ascites and unilateral lesions. The level of sPD-1 does not depend on the prevalence of ovarian cancer. Conclusion. The level of plasma sPD-L1 in patients with ovarian cancer correlates with the prevalence of the process and can be considered as a marker for monitoring anti-PD1/PD-L1 therapy. The clinical significance of sPD-1 is an important issue for future research.Kushlinskii N.Е., Gershtein Е.S., Utkin D.О., Petrikova N.А., Kushlinskiy D.N., Shabanov М.А., Khulamkhanova М.М., Ashrafyan L.А., Stilidi I.S.
Keywords
Ovarian cancer is one of the most common female genital malignancies. In spite of the sufficiently specific and sensitive serological markers, the disease is still diagnosed at its advanced stages when the tumor spreads to the peritoneum. Modern standards for ovarian cancer treatment, along with active surgical intervention, include various schemes of adjuvant and neoadjuvant chemotherapy primarily based on platinum drugs which are very effective in many cases. However, the rate of recurrence and mortality remains rather high. Nowadays, many researchers and clinicians associate the possibility of further improvement of the effectiveness of its treatment not only with the rational use of existing methods of combined and complex treatment, but also with the development of fundamentally new pathogenetic approaches based on modern advances in biochemistry, molecular biology and immunology of tumors.
It should be noted that long-term attempts to use various types of hormone therapy in ovarian cancer treatment have not led to significant success; novel molecular-targeted drugs have not been widely used in the treatment of this disease yet. In recent years, particular attention has been given to the possibility of immunotherapy for ovarian cancer aimed at increasing the activity of one of the signaling pathways of the so-called immune checkpoints PD-1/PD-L1, which under physiological conditions controls the severity and duration of the autoimmune response preventing damage to its own tissues [1–3].
The main components of this signaling pathway are programmed cell death protein 1 (PD-1) and its two ligands PD-L1 and PD-L2. PD-1 is a type I membrane receptor that belongs to the CD28/CTLA-4 family of T-cell regulators and is expressed on their surface. PD-L1 is the most significant among other ligands, also known as the cluster of differentiation 274 (CD 274) or B7 homologue 1 (B7-H1). PD-L1 is usually expressed on antigen-presenting dendritic and macrophage-like cells of peripheral organs, as well as on cells of the placenta, pancreatic islets and retina. At the same time, PD-L1 mRNA is found in a much wider range of tissues, and induced PD-L1 expression can be observed in T- and B-lymphocytes, natural killer cells, macrophages, mesenchymal stem cells, and epithelial cells. Activation of the PD-1/PD-L1 pathway stimulates apoptosis of antigen-specific T-cells in the lymph nodes and simultaneously suppresses apoptosis of regulatory T-cells, which allows the tumor to escape the immune response of the organ. In this regard, monoclonal antibodies against PD-1 and PD-L1, which prevent their interaction with each other and inhibit the immunosuppressive effects of tumors, are now actively used in the treatment of many oncological diseases, primarily melanoma [4] and renal cell carcinoma [5, 6]. In recent years, quite serious attempts have been made to use this type of immunotherapy for ovarian cancer, including one that is resistant to platinum drugs [3, 7].
The expression of PD-1 and/or PD-L1 in tumors and tumor-infiltrating lymphocytes is actively studied by immunohistochemical (IHC) methods as a predictor of the effectiveness of anti-PD-1/PD-L immunotherapy [8]. These proteins are also considered as molecular markers of cancer prognosis and patient survival, and some preliminary studies have already demonstrated an adverse effect of high PD-1/PD-L pathway activity on the clinical course of a number of tumors [9–12]. Recent studies have shown a positive relationship between PD-1 and/or PD-L1 expression in ovarian cancer and the spread and degree of malignancy of the tumor [13, 14], its association with the presence of BRCA1/2 and TP53 gene mutations [15, 16] and microsatellite instability, which is one of the indicators of sensitivity to anti-PD-1/PD-L therapy [17]. The studies have also demonstrated an expression of these proteins on macrophages and lymphocytes infiltrating tumor which has an ambiguous effect on the survival of patients.
However, according to a number of large randomized studies, the relationship between IHC results of determining PD-1 and PD-L1 expression in tumors and the effectiveness of anti-PD-1 therapy is ambiguous and apparently depends on the type of malignancy [8, 18]. These contradictions are most likely to be due to the difficulties of standardizing the IHC method whose results depend on the technique of preparing samples, the applied antibodies that differ in specificity and affinity to different epitopes of the studied proteins, as well as on the criteria used in the interpretation of the obtained data. One of the most important problems of PD-1 and PD-L1 IHC testing is that these molecules are expressed not only on the cells of the tumor itself, but also on the cells of the immune system that infiltrate it. At this stage of research, it is not known which type of expression is more significant for predicting the response to immunotherapy. Another problem is the presence of non-membrane bound forms of these proteins, which can give false positive results; however, their role in the pathogenesis of tumors still remains unclear.
The study of soluble forms of PD-1 (sPD-1) and its ligand (sPD-L1) that have been found recently in peripheral blood can be helpful in solving at least some of the problems associated with IHC testing [19]. The origin of sPD-1 and sPD-L1 has not been determined yet; however, like the soluble forms of other membrane proteins, they can form either as a result of hydrolytic cleavage of the extracellular domain of a membranebound molecule, or they can develop at an earlier stage in alternative mRNA splicing of this native membrane form. Currently available data indicate that sPD-L1 forms primarily as a result of proteolytic cleavage of the extracellular part of the transmembrane protein, while sPD-1 results from alternative splicing. Experimental studies have shown the ability of sPD-1 to suppress the activity of the PD-1/PD-L1 (2) pathway and to block the binding of a ligand located on tumor cells to the membrane receptor of T-lymphocytes. sPD-L1 is also able to reduce the activity of the PD-1/PD-L1 (2) pathway by blocking the receptor; but according to some data, it can stimulate T-lymphocyte apoptosis and suppress the antitumor immunity.
The most recent publications on the roles of sPD-1 and sPD-L1 in different cancerous diseases were summarized in a fundamental review [19] and meta-analysis of the studies [20, 21], but as this area is actively developing, several studies were published after the appearance of these review articles [22–26]. Recently, we have conducted a pilot study involving 62 patients with ovarian neoplasms and showed that the level of sPD-L1, but not sPD-1, correlates with the prevalence of ovarian cancer [27]. To date, the groups of patients with cancer and benign ovarian tumors have been significantly expanded, which allowed us to carry out a more representative assessment of the clinical significance of the studied markers.
The purpose of this study is to analyze the content of soluble forms of PD–1 and PD-L1 in blood plasma of patients with malignant, borderline and benign ovarian neoplasms taking into consideration the clinical and morphological characteristics of ovarian cancer.
Materials and Methods
The study included 125 patients with ovarian neoplasms aged 18 to 78 years (median age is 54 years) who were examined and treated between March, 2017 and April, 2018 in N.N. Blokhin National Medical Research Center of Oncology (Moscow), at the Department of Oncological Gynecology of Ryazan Regional Clinical Oncology Dispensary (Ryazan), and at the Department of Innovative Oncology and Gynecology of V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology (Moscow). Among 125 examined patients, there were 22 (18%) patients with benign ovarian tumors, 9 (7%) patients with borderline tumors and 94 (75%) patients with ovarian cancer. The control group consisted of 34 healthy women aged 18–68 years (median age is 44 years).
Benign tumors were revealed in 14 of 22 patients, serous cystadenomas were detected in 1 patient (tumor with a mucinous component), endometrioid tumors were in 4 patients, papillary mucinous cystadenoma was revealed in 2 patients and serous ovarian cyst was in 2 patients. Among patients with borderline tumors, 6 (67%) of them had serous type and 3 (33%) patients had mucinous type.
Staging and histological classification of malignant ovarian tumors was performed in accordance with the recommendations of the International Federation of Obstetrics and Gynecology (FIGO), 2014. Stage IA was detected in 4 patients, IB – in 2 patients, and IC – in 18 patients; 3 patients had IIB, 6 women had IIS, 9 patients had IIIA, 6 patients had IIIB, 41 women had IIIS, and 4 patients had IV stage of the disease; 1 patient with carcinoma in situ was also examined. Due to the small size of the subgroups in the statistical analysis, patients were grouped the following way: stage I – 25 patients; stage II – 9 patients; stages IIIA-B – 15 patients, stages IIIC and IV – 45 people. According to the histological structure, serous type was revealed in 72 patients, endometrioid tumor was found in 10 women, mucinous ovarian adenocarcinomas were detected in 7 women; clear cell carcinoma was revealed in 1 patient, adenogenous tumor was in 1 patient, and undifferentiated ovarian cancer was found in 1 patient.
The study was conducted according to the requirements of local ethics committees of N.N. Blokhin National Medical Research Center of Oncology, Ryazan Regional Clinical Oncology Dispensary, and Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology.
Before specific treatment, the concentration of sPD-L1 and sPD-1 was determined in blood plasma which was obtained using a standard EDTA method with reagents for immunoassay Human PD-L1 Platinum ELISA and Human PD-1 ELISA kit (Affymetrix, eBioscience, USA) in accordance with the manufacturer’s instructions. Measurements were performed with immunoassay analyzer BEP 2000 Advance (Siemens Healthcare Diagnostics, Germany). The content of markers was expressed in picograms per 1 ml of blood plasma (pg/ mL).
Statistical Processing
Since the distribution of the studied indicators differed from the normal one, nonparametric criteria were used when comparing the indicators and analyzing their relationships with clinical and morphological factors. The Mann-Whitney U test was used when two independent groups were compared, the Kruskal – Wallis test was applied when three or more independent groups were compared; Spearman’s rank correlation test was used for measuring rank correlation (rs). The median (Me) and quartile values (Q1;Q3) are presented in the tables. Differences and correlations were considered statistically significant at a level of p<0.05. Statistical processing of the data was performed using Statistica 7.0 software package.
Results and Discussion
The concentration of sPD-L1 and sPD-1 in blood plasma of patients with different ovarian neoplasms and the control group is presented in Table 1. The level of sPD-L 1 in the plasma of ovarian cancer patients is significantly lower than in the control group (median 43.8 and 61.5 pg/mL, respectively), although the difference does not reach the level of statistical significance. The level of sPD-L1 in patients with benign tumors (median 22.7 pg/mL) is significantly reduced in comparison with the healthy controls and patients with ovarian cancer (p<0.01). The level of this marker is also significantly reduced in patients with borderline tumors (median 28.7 pg/mL), but the differences with the control group and group of ovarian cancer patients do not reach the level of statistical significance, which is most likely to be connected with a small number of observations.
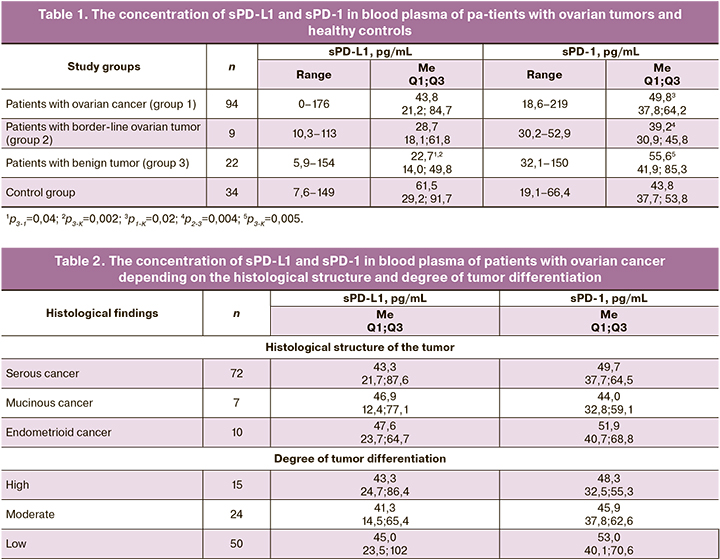
The increase in concentration of sPD-1 in the plasma of ovarian cancer patients is inconsiderable in comparison with the control group, but it is statistically significant (median 49.8 and 43.8 pg/mL, respectively; p=0.02), and it is higher than in patients with borderline tumors (median 39.2 pg/mL; p=0.004). The highest level of sPD-1 was found in blood plasma of patients with benign ovarian neoplasms (median 55.6 pg/mL), and the difference between the controls and patients with borderline tumors was statistically significant (p=0.005 and p=0.004, respectively). There was no correlation between the levels of sPD-L1 and sPD-1 in blood plasma in patients of study groups.
There was no significant correlation between plasma levels of sPD-L1 and sPD-1 with age and menopausal status in either the patients with tumors or the healthy controls, although the literature describes an increase in sPD-L1 level depending on age [22].
The majority of the patients (77%) had serous adenocarcinomas; endometrioid and mucinous cancers occurred in 11% and 7% of cases, respectively. Three other histological types (clear cell, adenogenous, and undifferentiated cancers) are represented by single observations. There were no statistically significant differences in the levels of sPD-L1 and sPD-1in blood plasma, depending on the histological structure of ovarian cancer (Table 2).
The degree of tumor differentiation was assessed in 89 patients: 53% of the tumors had a low degree of differentiation, 25% of the tumors had moderate degree of differentiation, and 22% of tumors were highly differentiated (Table 2). The levels of sPD-L1 and sPD-1 in the blood plasma of patients with ovarian cancer did not depend on the degree of tumor differentiation.
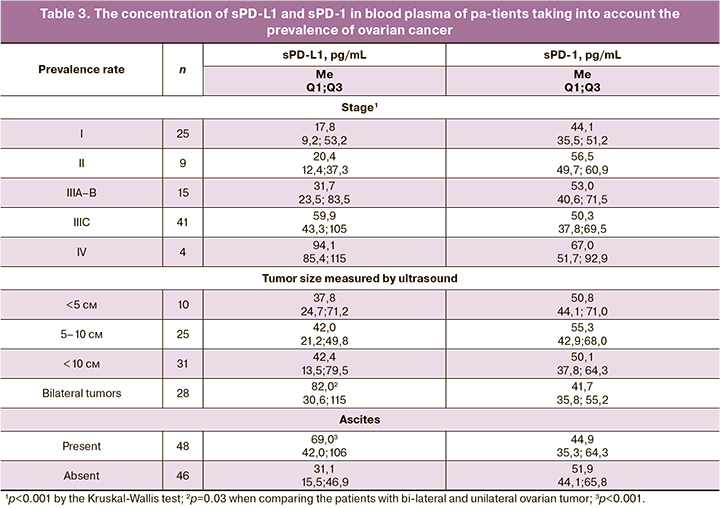
When analyzing the levels of the markers in blood plasma depending on the prevalence of ovarian cancer (Table 3), it was found that there was a statistically significant rise in sPD-L1 level with an increase in the disease stage, namely from 17.8 pg/mL at stage I to 94.1 pg/mL at stage IV (p<0.001, according to the Kruskal-Wallis test; rs=0.52; p<0.001). Thus, the decrease in the level of sPD-L1 in patients with ovarian tumors in comparison with the controls, was observed only due to the indicators of markers in patients with stages I–II, and to a lesser extent in patients with stages IIIA–B. The level of marker in patients with stage III characterized by the presence of intraperitoneal metastases occurring outside the pelvis and/or in regional lymph nodes is almost equal to the level of marker in controls, and is estimated to be 59.9 pg/mL. The concentration of sPD-1 in blood plasma does not significantly depend on the stage of ovarian cancer, although the median is 1.5 times lower at stage I than at stage IV (p=0.02, Table 3).
There were no statistically significant differences in the levels of this marker depending on the size of the primary tumor in unilateral ovarian lesions, however, in bilateral lesions, the level of sPD-L1 is twice higher than in unilateral ones (p=0.03, Table 3). The level of sPD-L1 in patients with ascites is more than twice as high as in patients without ascites (p<0.001, Table 3). The opposite trends are observed for sPD-1: it is reduced in patients with bilateral ovarian lesions and in patients with ascites (the differences do not reach the level of statistical significance).
Serum CA-125 concentration was determined in 86 ovarian cancer patients (2.7–9970; median 308 U/mL) and in 8 patients with borderline tumors (25–3109; median 455 U/mL). There was a poor but statistically significantly positive correlation of the level of sPD-L1 (but not sPD-1) with the level of serum CA-125 (rs=0.36; p<0.001). The level of tumor marker He4 was determined in 36 patients (36.8 - 8049; median 257 pmol/L), which was even more significantly positively correlated with sPD-L1 (rs=0.54; p<0.001).
Thus, the level of sPD-L1, which is the soluble form of the key ligand of the controlled cell death protein PD-1, in the blood plasma of ovarian cancer patients does not differ from the indicators of the control group, but increases with the rising prevalence of the process, and also correlates with the level of classical ovarian cancer markers CA-125 and HE4. According to the literature, an increase in the concentration of sPD-L1 in serum or plasma at advanced stages of the disease was also observed in patients with stomach cancer [28], liver [29], kidney, non-small cell lung cancer [30], and certain types of lymphomas [24]. High levels of sPD-L1 in peripheral blood have an adverse effect on patient survival in these diseases. Most of these publications, including our study of patients with renal cell carcinoma [26], also revealed an increase in the level of sPD-L1 in oncological patients, compared with the controls. However, the data presented in the literature on squamous cell carcinoma of the head and neck are contradictory; there was no statistically significant increase in the level of sPD-L1 and its correlation with clinical and morphological factors in patients with pancreatic cancer and cervical cancer [19].
In contrast to the ligand, the level of sPD-1 does not depend much on the prevalence of ovarian cancer, and its level does not correlate with the levels of CA-125 and HE4. Moreover, there is a tendency to decrease the level of sPD-1 in advanced stages in bilateral ovarian lesion and the presence of ascites. The issue of the clinical significance of sPD-1 remains understudied. There was an increase in the level of this marker after the successful treatment of non-small cell lung cancer patients with erlotinib, and correlation of this marker with the risk of developing hepatocellular cancer in patients with hepatitis C. The patients with pancreatic, cervical, head and neck cancer did not show the relationship of sPD-1 levels with the prevalence of the process and the prognosis of the disease [19].
Unfortunately, only single studies have been published (especially on sPD-1), where a variety of test systems and biological material (either serum or blood plasma) were used. All this leads to different results and it is impossible at this stage to determine clear threshold values for predictive and diagnostic goals. In particular, the researchers used a system developed in their own laboratory in the only study on sPD-L1 in ovarian neoplasms [25], whereas our study was performed in blood plasma using standardized reagents for enzyme immunoassay.
In recent years, several papers have been published on the role of the PD-1/PD-L signaling system in ovarian cancer, and their expression has been evaluated both on tumor cells and on tumor-infiltrating cells of the immune system, using not only the IHC method [13, 14], but also determining mRNAs [16]. One of these studies [13] demonstrated an increase in the expression of PD-L1 in a widespread process and in high-grade ovarian tumors, which coincides with some of the patterns we identified for sPD-L1 in blood plasma. It should be noted that J. Chatterjee et al. [25], who studied sPD-L1 in plasma, did not compare the obtained data with clinical and morphological factors and prognosis of the disease, but, in contrast to us, revealed a statistically significant increase in the level of the marker in ovarian cancer patients, compared with the healthy controls and patients with benign tumors. But this may be due to the fundamental differences between the immunoassay methods used in the investigation.
Conclusion
Comparative enzyme immunoassay of the soluble forms concentration of signaling pathway components of immune check point PD-1/PD-L1 in blood plasma of patients with cancerous, benign, borderline ovarian neoplasms and healthy women showed that the level of sPD-L1 in patients with ovarian cancer does not differ from the level of healthy controls, but it statistically significantly increases with the rising prevalence of the tumor process. The concentration of sPD-L1 is statistically significantly lower in patients with benign ovarian tumors than in patients of the control group and patients with ovarian cancer. At the same time, the level of soluble sPD-1 receptor in ovarian cancer patients is higher than in healthy women, but its indicators are not associated with the spread of the disease. According to the obtained data and published results on sPD-L1 in other tumor sites, it can be assumed that sPD-L1 circulating in the peripheral blood interacts with PD-1 on suppressor T lymphocytes and blocks their function in the same way as the membrane form of the ligand, contributing to the tumor escape from the immune response and the progression of the disease. However, the existence of such a mechanism has not been proven yet. The issue of the clinical significance of the soluble SPD-1 receptor whose level does not depend on the prevalence of ovarian cancer is also controversial. The data we have obtained suggest that the soluble PD-L1 ligand, but not its sPD-1 receptor, may be a potentially significant predictor of ovarian cancer. In addition, it should be noted that the study of the dynamics of soluble forms of sPD-L1 and sPD-1 in blood plasma of ovarian cancer patients receiving specific anti-PD-1/ PD-L therapy may be of great interest for oncologists in order to assess its effectiveness.
References
- Zhu X., Lang J. The significance and therapeutic potential of PD-1 and its ligands in ovarian cancer: a systematic review. Gynecol. Oncol. 2016; 142(1): 184-9. https://dx.doi.org/10.1016/j.ygyno.2016.04.002.
- Mandai M., Hamanishi J., Abiko K., Matsumura N., Baba T., Konishi I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int. J. Clin. Oncol. 2016; 21(3): 456-61. https://dx.doi.org/10.1007/s10147-016-0968-y.
- Inayama Y., Hamanishi J., Matsumura N., Murakami R., Abiko K., Yamaguchi K. et al. Antitumor effect of nivolumab on subsequent chemotherapy for platinum-resistant ovarian cancer. Oncologist. 2018; 23(11): 1382-4. https://dx.doi.org/10.1634/theoncologist.2018-0167.
- Yun S., Vincelette N.D., Green M.R., Wahner Hendrickson A.E., Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016; 5(7): 1481-91. https://dx.doi.org/10.1002/cam4.732.
- Massari F., Santoni M., Ciccarese C., Santini D., Alfieri S., Martignoni G. et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat. Rev. 2015; 41(2): 114-21. https://dx.doi.org/10.1016/j.ctrv.2014.12.013.
- Кушлинский Н.Е., Фридман М.В., Морозов А.А., Герштейн Е.С., Кадагидзе З.Г., Матвеев В.Б. Современные подходы к иммунотерапии рака почки. Онкоурология. 2018; 14(2): 54-67. https://dx.doi.org/10.17650/1726-9776-2018-14-2-54-67. [Kushlinskii N.E., Fridman M.V., Morozov A.A., Gershtein E.S., Kadagidze Z.G., Matveev V.B. Modern approaches to kidney cancer immunotherapy. Cancer Urology. 2018; 14(2): 54-67.(In Russian)].
- Zhu X., Xu J., Cai H., Lang, J. Carboplatin and programmed death-ligand 1 blockade synergistically produce a similar antitumor effect to carboplatin alone in murine ID8 ovarian cancer model. J. Obstet. Gynaecol. Res. 2018; 44(2): 303-11. https://dx.doi.org/10.1111/jog.13521.
- Yuasa T., Masuda H., Yamamoto S., Numao N., Yonese J. Biomarkers to predict prognosis and response to checkpoint inhibitors. Int. J. Clin. Oncol. 2017; 22(4): 629-34. https://dx.doi.org/10.1007/s10147-017-1122-1.
- Zhang Y., Kang S., Shen J., He J., Jiang L., Wang W. et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore). 2015; 94(6): e515. https://dx.doi.org/10.1097/MD.0000000000000515.
- Sacher A.G., Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016; 2(9): 1217-22. https://dx.doi.org/10.1001/jamaoncol.2016.0639.
- Kim K.S., Sekar R.R., Patil D., Dimarco M.A., Kissick H.T., Bilen M.A. et al. Evaluation of programmed cell death protein 1 (PD-1) expression as a prognostic biomarker in patients with clear cell renal cell carcinoma. Oncoimmunology. 2018; 7(4): e1413519. https://dx.doi.org/10.1080/2162402X.2017.1413519.
- Huang X., Zhang W., Zhang Z., Shi D., Wu F., Zhong B., Shao Z. Prognostic value of programmed cell death 1 ligand-1 (PD-L1) or PD-1 expression in patients with osteosarcoma: a meta-analysis. J. Cancer. 2018; 9(14): 2525-31. https://dx.doi.org/10.7150/jca.25011.
- Drakes M.L., Mehrotra S., Aldulescu M., Potkul R.K., Liu Y., Grisoli A. et al. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand-1 (PD-L1) in ovarian cancer. J. Ovarian Res. 2018; 11(1): 43. https://dx.doi.org/10.1186/s13048-018-0414-z.
- Darb-Esfahani S., Kunze C.A., Kulbe H., Sehouli J., Wienert S., Lindner J. et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016; 7(2): 1486-99. https://dx.doi.org/10.18632/oncotarget.6429.
- Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L.L., Liu J. F. et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016; 7(12): 13587-98. https://dx.doi.org/10.18632/oncotarget.7277.
- Wieser V., Gaugg I., Fleischer M., Shivalingaiah G., Wenzel S., Sprung S. et al. BRCA1/2 and TP53 mutation status associates with PD-1 and PD-L1 expression in ovarian cancer. Oncotarget. 2018; 9(25): 17501-11. https://dx.doi.org/10.18632/oncotarget.24770.
- Howitt B.E., Strickland K.C., Sholl L.M., Rodig S., Ritterhouse L.L., Chowdhury D. et al. Clear cell ovarian cancers with microsatellite instability: A unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology. 2017; 6(2): e1277308. https://dx.doi.org/10.1080/2162402X.2016.1277308.
- Topalian S.L., Taube J.M., Anders R.A., Pardoll D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer. 2016; 16(5): 275-87. https://dx.doi.org/10.1038/nrc.2016.36.
- Zhu X., Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017; 8(57): 97671-82. https://dx.doi.org/10.18632/oncotarget.18311.
- Ding Y., Sun C., Li J., Hu L., Li M., Liu J. et al. The prognostic significance of soluble programmed death ligand 1 expression in cancers: a systematic review and meta-analysis. Scand. J. Immunol. 2017; 86(5): 361-7. https://dx.doi.org/10.1111/sji.12596.
- Wei W., Xu B., Wang Y., Wu C., Jiang J., Wu C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: A meta-analysis. Medicine(Baltimore). 2018; 97(3): e9617. https://dx.doi.org/10.1097/MD.0000000000009617.
- Theodoraki M.N., Yerneni S.S., Hoffmann T.K., GoodingW.E., Whiteside T.L. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018; 24(4): 896-905. https://dx.doi.org/10.1158/1078-0432.CCR-17-2664.
- Kim H.J., Park S., Kim K.J., Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother. Oncol. 2018; 129(1): 130-5. https://dx.doi.org/10.1016/j.radonc.2017.11.027.
- Guo X., Wang J., Jin J., Chen H., Zhen Z., Jiang W. et al. High serum level of soluble programmed death ligand 1 is associated with a poor prognosis in Hodgkin lymphoma. Transl. Oncol. 2018; 11(3): 779-85. https://dx.doi.org/10.1016/j.tranon.2018.03.012.
- Chatterjee J., Dai W., Aziz N.H.A., Teo P.Y., Wahba J., Phelps D.L. et al. Clinical use of programmed cell death-1 and its ligand expression as discriminatory and predictive markers in ovarian cancer. Clin. Cancer Res. 2017; 23(13): 3453-60. https://dx.doi.org/10.1158/1078-0432.CCR-16-2366.
- Кушлинский Н.Е., Герштейн Е.С., Морозов А.А., Горячева И.О., Филипенко М.Л., Алферов А.А., Бежанова С.Д., Базаев В.В., Казанцева И. А.Растворимый лиганд рецептора контрольной точки иммунитета (sPD-L1) в сыворотке крови при почечно-клеточном раке. Бюллетень экспериментальной биологии и медицины. 2018; 166(9): 325-9. [Kushlinskii N.E., Gershtein E.S., Morozov A.A., Goryatcheva I.O., Filipenko M.L., Alferov A.A., Bezhanova S.D., Bazaev V.V., Kazantseva I.A. Soluble ligand of immune checkpoint receptor (sPD-L1) in blood serum of renal-cell carcinoma patients: clinical and pathologic correlations. Bulletin of Experimental Biology and Medicine. 2018; 166(9); 325-9 (In Russian)].
- Герштейн Е.С., Уткин Д.О., Горячева И.О., Хуламханова М.М., Петрикова Н.А., Виноградов И.И., Алферов А.А., Стилиди И.С., Кушлинский Н.Е. Растворимые формы рецептора контрольной точки иммунитета PD-1 и его лиганда PD-L1 в плазме крови больных новообразованиями яичников Альманах клинической медицины. 2018; 46(7): 690-8. [Gershtein E.S., Utkin D.O., Goryatcheva I.O., Khulamkhanova M.M., Petrikova N.A., Vinogradov I.I.,. Alferov A.A., Kushlinskii N.E. Soluble forms of immune checkpoint receptor PD-1 and its ligand PD-L1 in blood plasma of ovarian neoplasms patients. Almanac of Clinical Medicine. 2018; 46 (4): 323–329. (In Russian)]. https://dx.doi.org/10.18786/2072-0505-2018-46-4-323-329.
- Zheng Z., Bu Z., Liu X., Zhang L., Li Z., Wu A. et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin. J. Cancer Res. 2014;26(1): 104-111. https://dx.doi.org/10.3978/j.issn.1000-9604.2014.02.08.
- Finkelmeier F., Canli O., Tal A., Pleli T., Trojan J., Schmidt M. et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur. J. Cancer. 2016; 59: 152-9. https://dx.doi.org/10.1016/j.ejca.2016.03.002.
- Zhang J., Gao J., Li Y., Nie J., Dai L., Hu W. et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac. Cancer. 20156(4): 534-8. https://dx.doi.org/10.1111/1759-7714.12247.
Received 09.01.2020
Accepted 02.06.2020
About the Authors
Nikolay E. Kushlinskii, MD, Professor, Member of the Russian Academy of Sciences, Head of the Laboratory of Clinical Biochemistry, N.N. Blokhin National Medical Research Center of Oncology. E-mail: kne3108@gmail.com. 115478, Moscow, Kashirskoje shosse, 24, Russia.Elena S. Gershtein, PhD, Doctor Biol. Sci, Professor, Leading Researcher, Laboratory of Clinical Biochemistry, N.N. Blokhin National Medical Research Center of Oncology. E-mail: esgershtein@gmail.com. 115478, Moscow, Kashirskoje shosse, 24, Russia.
Dmitry O. Utkin, applicant, A.I. Yevdokimov Moscow State University of Medicine and Dentistry. E-mail: utkindo@yandex.ru. 127473, Moscow, Delegatskaja str., 20/1, Russia.
Natalya A. Petrikova, Pathologist, Department of Pathologic Anatomy with Pathomorphologic Laboratory, Ryazan State Medical University named after academician I.P.Pavlov. E-mail: petrikova.nat@yandex.ru. 390047, Ryazan, Sportivnaja str., 13, Russia.
Dmitry N. Kushlinskiy, senior researcher, Department of combined and surgical methods of treatment of gynecological diseases A.F. Tsyb Medical Radiological Research Center – Branch of the National Medical Research Center of Radiology. E-mail: drkushlinskiy@gmail.com. 117198, Moscow, Ac. Oparina str., 4, Russia.
Mikhail A. Shabanov, MD, Professor, leading researcher, Department of morphologic and molecular genetic tumor diagnostics, N.N. Blokhin National Medical Research Center of Oncology. E-mail: 0152@mail.ru. 115478, Moscow, Kashirskoje shosse, 24, Russia.
Marina M. Khulamkhanova, oncologist, N.N. Blokhin National Medical Research Center of Oncology. E-mail: marina_2705@list.ru.
115478, Moscow, Kashirskoje shosse, 24, Russia.
Lev A. Ashrafyan, MD, Professor, Member of the Russian Academy of Sciences, Director of the Institute of Oncogynecology and Mammology, Research Center for Obstetrics, Gynecology and Perinatology named after V.I.Kulakov. E-mail: Levaa@yahoo.com. 117198, Moscow, Ac. Oparina str., 4, Russia.
Ivan S. Stilidi, MD, Professor, Member of the Russian Academy of Sciences, director of the N.N. Blokhin National Medical Research Center of Oncology. E-mail: Stylidi@ronc.ru.
115478, Moscow, Kashirskoje shosse, 24, Russia.
For reference: Kushlinskii N.Е., Gershtein Е.S., Utkin D.О., Petrikova N.А., Kushlinskiy D.N., Shabanov М.А., Khulamkhanova М.М., Ashrafyan L.А., Stilidi I.S. Signaling Pathway Components of Immune Checkpoint PD-1/PD-L1 in Blood Plasma of Patients with Ovarian Cancer and Benign Ovarian Tumors: Clinical and Morphological Correlations.
Akusherstvo i Ginekologiya / Obstetrics and Gynecology. 2020; 6: 80-88 (in Russian)
https://dx.doi.org/10.18565/aig.2020.6.80-88



