Стремительное развитие онкологии ведет к увеличению выживаемости онкологических больных. В связи с этим, вопрос качества жизни после хирургического «выключения» функции яичников, либо в результате химиоили гормондепривационной терапии является крайне актуальным. Эффективная терапия тяжелых вазомоторных и психоэмоциональных симптомов, генитоуринарного менопаузального синдрома, профилактика раннего развития сердечно‑сосудистых заболеваний и остеопороза позволяет все большему количеству пациентов жить достаточно долго и качественно. В этой категории пациентов вопросы безопасности использования менопаузальной гормональной терапии (МГТ) требуют тщательного анализа.
Некорректные заключения о результатах, полученных в исследовании WHI (Инициатива женского здоровья) [1] привело к страху перед назначением МГТ, как среди населения в целом, так и среди медицинских работников. Многим врачам кажется логичным и безопасным избегать применения МГТ, считая, что такой подход точно не наносит вреда пациентке; тогда как решение о назначении эстрогенов отдельно или в сочетании с прогестинами может нести онкологический и тромбоэмболический риск и даже привести к судебным разбирательствам в случае потенциально связанного с назначением МГТ осложнения. Однако еще до публикации результатов WHI было известно, что преждевременная менопауза уменьшает ожидаемую продолжительность жизни женщин на годы из‑за негативного влияния на костную ткань и сердечно‑сосудистую систему, и этот негативный эффект коррелирует с продолжительностью периода гипоэстрогении. Следовательно, отказ от назначения МГТ также должен быть подтвержден доказательствами и определен с учетом возможных рисков, которые крайне сложно оценить.
Использование МГТ в клинической практике кардинально изменилось с 1942 г. Пик ее применения у женщин в менопаузе пришелся на 1999 г., когда в США было выписано 35 млн рецептов на препараты для МГТ [2]. Однако по результатам двух рандомизированных клинических испытаний, инициированных WHI, произошло быстрое и существенное снижение использования гормональной терапии из‑за выявленных рисков [2, 3]. В первом из исследований WHI изучались риски и преимущества конъюгированного эквин эстрогена в сочетании с медроксипрогестерона ацетатом для женщин с интактной маткой. Это исследование было остановлено в 2002 г. после среднего периода использования МГТ в течение 5,6 лет, когда пришли к выводу, что риски МГТ превышают преимущества. Риски комбинированной терапии включали рост сердечно‑сосудистых заболеваний и инвазивного рака молочной железы [2, 3].
МГТ доказала свою высокую эффективность в облегчении таких симптомов менопаузы, как приливы, ночная потливость, диспареуния, сексуальные расстройства и бессонница, а также в предотвращении развития остеопороза. Поддержание качества жизни и минимизация побочных эффектов лечения является одним из важных факторов в лечении рака; ввиду чего, применение или отказ от МГТ должны быть четко аргументированы.
У здоровых женщин назначение системной гормональной терапии на основе эстрогенов в период менопаузы для лечения климактерических симптомов и остеопороза имеет благоприятное соотношение риска и пользы для лиц в возрасте до 60 лет или до 10 лет постменопаузы [4–8]. Системная гормональная терапия в период менопаузы проводится перорально или трансдермально. Женщинам, перенесшим гистерэктомию, назначают только эстрогены. Прогестагены и селективный модулятор рецепторов эстрогена базедоксифен добавляются в схемы лечения женщин с интактной маткой, чтобы ограничить повышение риска гиперплазии эндометрия и карциномы, возникающих при приеме только эстрогенов [4, 8, 9].
Женщинам с ранней или преждевременной менопаузой рекомендуется системная гормональная терапия на основе эстрогенов, по крайней мере, до среднего возраста естественной менопаузы. Молодым женщинам могут потребоваться более высокие дозы эстрогенов для облегчения симптомов менопаузы на начальном этапе, чем женщинам более старшего возраста [4]. Для некоторых молодых женщин более приемлемым является прием комбинированных оральных контрацептивов. Для пожилых женщин рассматривается возможность применения МГТ в очень низких дозах или негормональной терапии [4]. Симптомы, вызванные вульвовагинальной атрофией, можно корригировать с помощью местного применения низких доз эстрогенов [10, 11].
Основной проблемой при использовании МГТ у женщин, перенесших рак, является потенциальная стимуляция остаточной опухоли и индукция гормонозависимого заболевания.
Рак яичников
Рак яичников (РЯ) является одним из самых агрессивных и тяжело поддающихся лечению онкологических заболеваний. В настоящее время РЯ является девятым по распространенности раком у женщин в мире, которым в 2020 г. болело 313 959 женщин во всем мире [12]. РЯ занимает восьмое место, как причина смерти от рака среди женской части населения в мире [13, 14] и имеет самый высокий уровень смертности [13, 15 –17]. РЯ составляет более 90% злокачественных новообразований яичников, в то время как неэпителиальные опухоли – герминогенные (например, незрелые тератомы) и опухоли стромы полового тяжа (например, гранулезоклеточные опухоли) составляют около 5% злокачественных опухолей яичников. Серозные аденокарциномы яичников высокой и низкой степени злокачественности составляют 75% от всего РЯ.
Средний возраст установления диагноза РЯ составляет в России 59,4 года; пик заболеваемости приходится на возраст 55–79 лет [18], в США – 63 года [17].
Пограничные опухоли яичника, как известно, опухоли с низким злокачественным потенциалом, составляют примерно 10% эпителиальных новообразований яичников. Они чаще диагностируются у молодых женщин и в периоде пременопаузы без известных факторов риска [19].
Различные морфологические варианты злокачественных новообразований яичников имеют разный потенциал злокачественности. Серозные опухоли в основном имеют высокую степень злокачественности, для которой характерно поражение обоих яичников, агрессивное течение, диагностика заболевания на поздней стадии и низкие показатели выживаемости. Другие морфологические варианты опухоли, как правило, поражают только один яичник. В настоящее время считается, что серозные опухоли с высоким потенциалом злокачественности возникают в эпителиальных клетках маточной трубы в виде микроскопических поражений, которые впоследствии мигрируют в яичники и/или брюшину. В то же время, эндометриоидные и светлоклеточные опухоли первично возникают в эндометрии, а муцинозные опухоли – в яичниках или перитонеальном переходе маточных труб [20–23]. Одним из факторов риска РЯ является предшествующее использование гормональной терапии в период менопаузы; но связь, ограничивается лишь серозным и эндометриоидным вариантами злокачественных новообразований [24].
В 1995 г.у Rodriguez C. et al. [25] проанализировали 434 случая смерти от РЯ в большом проспективном исследовании у 240 073 женщин в перии постменопаузе. Использовавшаяся когда‑либо МГТ была связана с коэффициентом вероятности РЯ 1,15 (95% ДИ, 0,94–1,42); тогда как коэффициент смертности увеличивался с увеличением продолжительности использования с 1,40 (95% ДИ, 0,92–2,11) при 6–10 годах до 1,71 (95% ДИ, 1,06–2,77) – при использовании более 11 лет. Данные свидетельствуют о том, что длительное использование МГТ может увеличить риск развития РЯ [25].
Два рандомизированных исследования, а также проспективные и ретроспективные когортные исследования и исследования случай‑контроль не показали отрицательного влияния МГТ на выживаемость у женщин, лечившихся от РЯ [26–32]. Использовались различные схемы лечения – применение только эстрогенов или в сочетании с прогестагенами или тестостероном.
В рандомизированном исследовании Guidozzi F. и Daponte A. [26], в котором 130 женщин с инвазивной эпителиальной карциномой яичников наблюдались в течение 48 месяцев, использовался непрерывный пероральный конъюгированный конский эстроген. Авторы сообщили о медиане общей выживаемости в 44 месяца (95% ДИ, 10–112 месяцев) и 34 месяца (95% ДИ 8–111 месяц) в группе гормональной терапии в период менопаузы и в контрольной группе, соответственно. Различия в безрецидивной (P=0,785) и общей выживаемости (P=0,354) между двумя группами не были статистически значимыми (таблица).
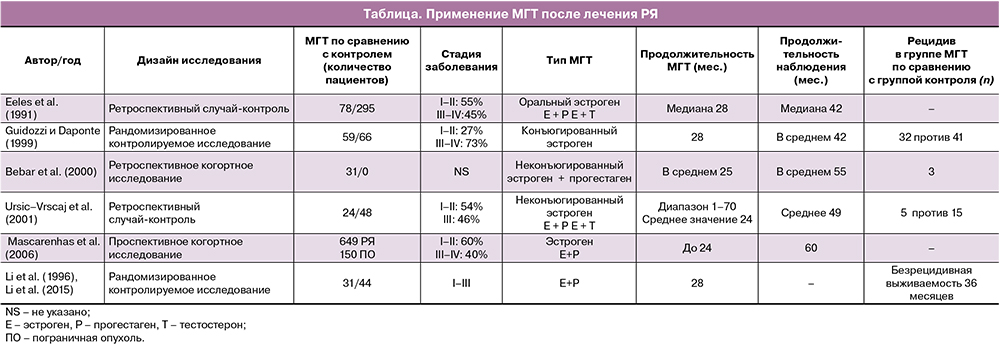
Eeles R.A. et al. [27] изучили истории болезни 150 женщин в пременопаузе и постменопаузе, у которых был диагностирован РЯ (любая стадия FIGO) девять или менее месяцев назад. Они были рандомизированы для проведения или не проведения гормональной терапии. Среднее время наблюдения за пациентами составляло 19,1 года: общая и безрецидивная выживаемость была выше в группе МГТ, чем в контрольной группе (таблица).
Ретроспективное когортное исследование с использованием Информационной сети реестра рака и программ по лекарственным препаратам Манитобы с участием 357 женщин показало, что использование гормональной терапии в период менопаузы (n=94) при несерозном эпителиальном РЯ не было связано со снижением выживаемости и развитием рецидивов [32]. Было обнаружено, что у лиц, принимающих гормональную терапию в период менопаузы в возрасте до 55 лет, выживаемость без прогрессирования была выше; не выявлено статистической разницы в общей выживаемости для этой возрастной группы. У женщин в возрасте 55 лет и старше не было обнаружено связи между использованием гормональной терапии в период менопаузы и снижением общей или безрецидивной выживаемости.
Что касается эндометриоидного РЯ, потенциально чувствительного к эстрогенам, гормональная терапия в период менопаузы не имеет побочных эффектов при ранних стадиях заболевания, но у женщин с более распространенными стадиями, применение МГТ является небезопасным [32].
Крупнейшее когортное исследование [33] показало лучшую общую выживаемость среди получавших МГТ, у женщин с серозным РЯ (HR=0,65; 95% ДИ 0,44–0,96), светлоклеточной или недифференцированной опухолью (HR=0,23, 95% ДИ 0,06–0,91). Не было обнаружено различий у женщин с муцинозными опухолями. Данные подтвердили, что применение МГТ после операции не было связано с отрицательным влиянием на общую и безрецидивную выживаемость (HR=0,68, 95% ДИ 0,54–0,86). Кроме того, в этот анализ были включены пациенты с распространенными стадиями РЯ (стадия III–IV). В большинстве исследований пациентки получали прогестерон в составе комбинированной МГТ. К сожалению, не был проведен анализ выживаемости для каждого варианта МГТ.
Как известно, МГТ эстрогенами безопасна для пациенток в менопаузе с удаленной маткой. Основываясь на выводах о том, что прогестерон подавляет стимулирующий эффект эстрогенов на нормальный и гиперпластический эндометрий, в нескольких исследованиях прогестерон был добавлен к МГТ у пациенток с начальными стадиями РЯ, у которых была сохранена матка после первичной операции. Что касается продолжительности МГТ, то исследований о влиянии длительного или кратковременного использования на прогрессирование РЯ недостаточно.
Анализируя проведенные исследования, [28] авторы сделали выводы о том, что МГТ не оказывает отрицательного влияния на прогноз пациенток при РЯ.
Guidozzi F. et al. [26] провели проспективное рандомизированное исследование у больных РЯ чтобы проанализировать влияние МГТ на выживаемость. Конъюгированные эстрогены были назначены 59 пациентам с диагнозом РЯ через 6–8 недель после операции. После периода наблюдения в течение 48 месяцев не было отмечено значительных различий выживаемости в двух группах. Исследователи пришли к выводу, что МГТ может быть назначена с целью улучшения качества жизни молодых женщин после лечения РЯ без каких‑либо неблагоприятных последствий. Таким образом, авторы отметили, что послеоперационная заместительная терапия эстрогенами не оказала отрицательного влияния на общую и безрецидивную выживаемость больных, перенесших РЯ.
В исследованиях Bebar S. et al. [29] и UrsicVrscaj M. [30] также были сделаны выводы, что МГТ не оказывает заметного влияния на общую и безрецидивную выживаемость больных РЯ (таблица).
Mascarenhas C. et al. [31] провели проспективное когортное исследование и изучили влияние МГТ на 5‑летнюю выживаемость до и после диагностики как РЯ, так и пограничных опухолей. В исследование были включены 649 больных РЯ и 150 больных пограничными опухолями яичников. В исследовании не было выявлено четких различий в выживаемости среди женщин, которые использовали любой вариант МГТ до постановки диагноза серозного РЯ, и тех, кто никогда ее не использовал. Для подгруппы эндометриоидного РЯ были обнаружены аналогичные результаты. Также в исследовании была установлена лучшая выживаемость у женщин, которые использовали МГТ после постановки диагноза, особенно среди пациенток с серозными морфологическими типами опухоли (таблица).
Как и ожидалось, влияние некоторых известных прогностических факторов, таких как остаточная опухоль после первичной циторедуктивной операции и дифференцировка опухоли на момент постановки диагноза, имеет решающую роль [30, 33].
Таким образом, назначение МГТ после лечения РЯ возможно, но необходимо тщательное обследование и консультирование пациенток для оценки преимуществ и возможных рисков назначения гормональной терапии.
Согласно литературным данным [25, 33–35] было предложено рассмотреть возможность применения комбинированной заместительной терапии эстрогенами и прогестероном у больных серозными и эндометриоидными опухолями яичников на короткий период (менее 10 лет, лучше менее 5 лет). Однако, не рекомендуется назначение МГТ таким пациенткам на период более 10 лет. Аналогичный подход предложен и в отношении локальной терапии эстрогенами.
Пограничные опухоли яичников
В настоящее время считается, что имеется взаимосвязь опухолевой прогрессии между серозными пограничными опухолями яичников и РЯ I типа, особенно серозной аденокарциномой низкой степени злокачественности, которые являются гормоночувствительными опухолями.
Необходимо с осторожностью принимать решение об использовании гормональной контрацепции или МГТ женщинам, получавшим лечение по поводу пограничных опухолей яичников с неблагоприятными факторами прогноза – инвазивными перитонеальными имплантатами, микропапиллярными структурами, стромальной микроинвазией или наличием муцинозной опухоли с интраэпителиальной карциномой, так как эти морфологические особенности связаны с более высоким риском инвазивного рецидива [36].
Варианты сохранения фертильности у женщин, ранее получивших лечение по поводу пограничных опухолей яичников, без каких‑либо гистологических критериев высокого риска (перитонеальных имплантатов, микропапиллярного варианта или наличия микроинвазии), включая стимуляцию яичников, требуют обсуждения на заседании мультидисциплинарного консилиума с участием эксперта по репродуктивной медицине для каждой женщины с двусторонними пограничными опухолями в анамнезе из‑за риска рецидива. МГТ может быть назначена женщинам, у которых в анамнезе были муцинозные пограничные опухоли или серозные пограничные опухоли без каких‑либо гистологических критериев высокого риска [36].
Редкие опухоли яичников
К редким опухолям яичников относятся опухоли стромы и полового тяжа, герминогенные опухоли и редкие эпителиальные опухоли. Вопросы, касающиеся показаний и способов сохранения фертильности, противопоказаний для гормональной контрацепции или гормональной терапии менопаузальных симптомов часто возникают в клинической практике у данной группы пациенток, ввиду того, что данные опухоли чаще диагностируются у женщин репродуктивного возраста.
Группа экспертов французской национальной сети, занимающейся редкими гинекологическими видами рака, а также эксперты в области репродуктивной медицины и гинекологии разработали рекомендации по сохранению фертильности, контрацепции и гормональной терапии в период менопаузы для женщин, проходящих лечение по поводу редких опухолей яичников. Были сформулированы следующие положения, касающиеся редких злокачественных опухолей яичников [36]:
- Перед назначением лечения, потенциально снижающего фертильность, с пациенткой необходимо обсудить возможные варианты сохранения репродуктивной функции, оценить овариальный резерв (ультразвуковое исследование органов малого таза с подсчетом антральных фолликулов и тест на антимюллеров гормон).
- При выборе типа МГТ (только эстрогены или эстрогены и прогестины) следует принимать во внимание наличие гистерэктомии в анамнезе, семейный риск рака молочной железы.
- У женщин без гистерэктомии в анамнезе прогестины необходимо назначать в сочетании с эстрогенами.
- МГТ следует проводить только после окончания адъювантного лечения.
- Противопоказания для назначения фитоэстрогенов такие же, как и для эстроген‑содержащей МГТ.
- Применение местных эстрогенов не противопоказано, за исключением назначения их женщинам, принимающим ингибиторы ароматазы. В этой ситуации их использование не рекомендуется.
- На время лечения РЯ можно оставить ранее введенную медную внутриматочную спираль.
- Применение комбинированных оральных контрацептивов не рекомендуется во время проведения лечения по поводу новообразований (особенно в период проведения химиотерапии), с целью минимизации риска развития тромбоэмболии и тромбоза.
- Противозачаточные средства, содержащие только прогестины не противопоказаны во время проведения лечения по поводу редких вариантов злокачественных опухолей яичников.
Герминогенные опухоли
При злокачественных герминогенных опухолях, диагностированных до наступления менопаузы, чаще всего проводятся органосохраняющие хирургические вмешательства. Это гормонально‑независимые опухоли, и данные об отрицательном влиянии вспомогательных репродуктивных технологий, стимуляции овуляции, гормональной контрацепции или МГТ на риск развития рецидива при данных новообразованиях отсутствуют. У женщин, ранее получавших лечение по поводу незрелой тератомы и имеющих образование контралатерального яичника, подозрительное по принадлежности к доброкачественной тератоме, сохранение фертильности с использованием стимуляции яичников можно индивидуально обсудить перед операцией.
Любые вспомогательные репродуктивные технологии, включая стимуляцию овуляции, могут использоваться при бесплодии у женщин, ранее получавших лечение по поводу герминогенных опухолей. Сохранение фертильности (включая стимуляцию яичников) следует обсудить на совещании мультидисциплинарного консилиума, включая специалиста по репродуктивной медицине, для каждой женщины с незрелой тератомой в анамнезе из‑за риска ее рецидива. После хирургического лечения герминогенных опухолей могут быть назначены все виды гормональных контрацептивов и МГТ.
Опухоли стромы и полового тяжа
Опухоли стромы и полового тяжа являются гормонозависимыми опухолями. Фолликулостимулирующий, лютеинизирующий гормон и эстрогены стимулируют пролиферацию клеток гранулезы. Агонисты гонадотропин рилизинг гормона обладают частичной эффективностью при лечении прогрессирующих опухолей из клеток гранулезы, предположительно за счет снижения уровней ЛГ и ФСГ. Высокие дозы прогестинов, действие которых может быть опосредовано через стероидные рецепторы, и их антигонадотропная активность также применяются для лечения данного вида опухолей. С другой стороны, тамоксифен и ингибиторы ароматазы также усиливают в гранулезных клетках чувствительность к эстрогенам. Таким образом, использование рекомбинантного ФСГ и гиперэстрогения после стимуляции овуляции, а также эстрогены, содержащиеся в противозачаточных средствах или МГТ, могут быть опасны при назначении женщинам, перенесшим лечение по поводу гранулезоклеточной опухоли. Клетки Сертоли и клетки Лейдига также реагируют на стимуляцию гонадотропинами.
Контрацепция у женщин, перенесших в анамнезе консервативные операции по поводу гранулезоклеточной опухоли, с использованием эстрогеносодержащих противозачаточных средств противопоказана. Могут быть назначены другие гормональные противозачаточные средства.
Эпителиальные опухоли яичников низкой степени злокачественности
В эту группу опухолей входят серозные, муцинозные, эндометриоидные и светлоклеточные аденокарциномы низкой степени злокачественности.
Муцинозная аденокарцинома является гормононезависимым новообразованием, тогда как серозные и эндометриоидные аденокарциномы низкой степени злокачественности являются потенциально гормоночувствительными опухолями с рецепторами гормонов, что подтверждается данными об эффективности антиэстрогенов. Серозная и эндометриоидная аденокарцинома низкой степени злокачественности на стадии IA или IВ лечится консервативным хирургическим вмешательством. Несмотря на отсутствие данных об использовании вспомогательных репродуктивных технологий после лечения серозной или эндометриоидной аденокарциномы низкой степени злокачественности, стимуляция овуляции противопоказана, учитывая их чувствительность к гормонам [36].
Больным серозной аденокарциномой низкой степени злокачественности в анамнезе, эндометриоидной аденокарциномой низкой степени злокачественности в анамнезе и бесплодием стимуляция яичников при лечении бесплодия противопоказана.
- После консервативного лечения серозной или эндометриоидной аденокарциномы низкой степени злокачественности использование гормональных контрацептивов не рекомендуется.
- МГТ противопоказана женщинам, ранее лечившимся по поводу серозной аденокарциномы низкой степени злокачественности стадии более IA/В стадии.
Учитывая эффективность поддерживающей гормональной терапии летрозолом, анастрозолом, тамоксифеном после первичной циторедуктивной хирургии и химиотерапии на основе платины у женщин с серозной аденокарциномой яичников или брюшины низкой степени злокачественности, в настоящее время проведение терапии эстрогенами не рекомендуется при распространенных стадиях заболевания данных гистологических подтипов [37]. Таким образом, применение системной или местной МГТ, по‑видимому, не приводит к снижению общей и безрецидивной выживаемости больных несерозными вариантами РЯ и герминогенными опухолями. Режим МГТ (эстрогены или эстрогены в сочетании с прогестагенами) зависит от того, была ли проведена гистерэктомия. Продолжительность терапии зависит от возраста женщины.
Заключение
В заключение следует отметить, что взаимосвязь между использованием гормональной терапии и риском рецидива новообразования у женщин, получавших лечение по поводу злокачественных опухолей яичников, четко установлена лишь для гранулезоклеточных опухолей яичников и серозных опухолей низкой степени злокачественности. При других морфологических вариантах злокачественных новообразований яичников назначение МГТ возможно с высокой долей осторожности. Тем не менее, перед принятием решения об использовании гормонотерапии в обязательном порядке должно быть проведено тщательное обследование и консультирование каждой пациентки на междисциплинарном консилиуме с целью индивидуализации плана проведения гормональной терапии на основе оценки потенциальных рисков и преимуществ предполагаемой терапии. Необходимы дальнейшие исследования для определенных групп пациенток и стандартизированных схем гормональной терапии для каждого морфологического варианта опухоли.
Выводы
- Лечение симптомов менопаузы, а также профилактика и лечение остеопороза у женщин, перенесших лечение злокачественного новообразования яичников, должно быть индивидуальным, с или без применения МГТ, в зависимости от гистологического варианта и стадии опухоли, а также возраста женщины.
- Решение о назначении МГТ женщинам, перенесшим злокачественное новообразование яичников, должно приниматься на междисциплинарном консилиуме.
- Женщинам, принимающим антиэстрогенные препараты, такие как ингибиторы ароматазы, терапия препаратами эстрогенов противопоказана, для коррекции менопаузальных симптомов рекомендуются негормональные варианты.
- Имеющиеся ограниченные данные свидетельствуют о том, что системная или локальная МГТ, по‑видимому, не снижает общую выживаемость или выживаемость без прогрессирования у женщин с несерозными вариантами РЯ и герминогенными опухолями.
- Необходимо соблюдать осторожность при применении как системной, так и местной гормональной терапии в период менопаузы у женщин с серозными и эндометриоидными злокачественными новообразованиями яичников; в качестве начальной терапии рекомендуются негормональные варианты.
- МГТ может быть назначена женщинам, у которых в анамнезе были муцинозные пограничные опухоли или серозные пограничные опухоли без каких‑либо гистологических критериев высокого риска прогрессирования заболевания.
- МГТ противопоказана женщинам, ранее лечившимся по поводу серозной аденокарциномы низкой степени злокачественности, а также гранулезоклеточных опухолей яичников, из‑за их гормональной зависимости.
- Оптимальное время назначения МГТ все еще остается спорным. Целесообразно назначение МГТ после окончания противоопухолевой терапии, поскольку наиболее тяжелые вазомоторные и психоэмоциональные симптомы возникают в первые месяцы после выключения функции яичников.
- У женщин без гистерэктомии в анамнезе необходимо назначать прогестины совместно с эстрогенами с целью защиты эндометрия.
- МГТ следует начинать только после окончания адъювантного лечения.
- Все пациентки репродуктивного возраста с диагнозом злокачественная или пограничная опухоль яичников перед началом специального лечения должны быть проконсультированы врачами репродуктологами для определения возможности применения вспомогательных репродуктивных технологий.



