Pregnancy complications and outcomes in women with COVID-19
Aim: Analysis of pregnancy complications and outcomes in women with COVID-19.Dobrokhotova Yu.E., Gumenyuk L.N., Puchkina G.A., Mikhailichenko V.Yu.
Materials and methods: The retrospective study included 34pregnant women aged 16—40years, who underwent treatment for COVID-19 in hospital. The diagnosis of COVID-19 infection was confirmed by positive PCR test results for SARS-CoV-2 RNA detection in allpregnant women.
Results: Most pregnant women (52.9%) had mild symptoms, 20.7% had moderate symptoms and 17.6% had severe symptoms. Pneumonia was diagnosed in 67.1% of pregnant women. Obesity was predominant in the structure of extragenital disorders. It was in 44.1% of women. With COVID-19, iron deficiency anemia (44.2%), preeclampsia (38.2%), risk of preterm birth (35.3%) were predominant in the structure of pregnancy complications. Preterm birth occurred in 36.8% of cases. Cesarean section was performed in 73.3% of cases. The incidence of complications in newborns was 11.6%. 8.8% of newborns were referred to the neonatal intensive care unit.
Conclusion: It was found that mostpregnant women had mild cases of COVID-19. Pregnant women with COVID-19 had high incidence of preeclampsia, preterm birth and cesarean section versus pregnant women without COVID-19. Intrauterine vertical transmission of infection was not detected. The incidence of complications in newborns was in compliance with general population indicators.
Keywords
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is a new strain of coronaviruses identified at the end of 2019 that induces a dangerous infectious disease – Corona Virus Disease 2019 (COVID-19). Starting with a single case of the disease in a seafood market in Wuhan (PRC), the infection rapidly spread around the world, covering almost all countries. Following the global spread of SARS-CoV-2, WHO has declared the outbreak of COVID-19 a public health emergency of international concern. With the development of the infectious process around the world, the experience in management of patients of different age and social groups is being accumulated and systematized. Specific features of the clinical course of the disease in pregnant women remain the most relevant and controversial issue in the context of COVID-19 pandemic.
Pregnant women with chronic diseases are more susceptible to viral and bacterial infections largely due to impaired maternal physiological tolerance to fetal alloantigens [1]. Despite continuing COVID-19 pandemic, the evidence regarding the greater exposure of pregnant women to COVID-19 versus general population is controversial. According to a number of domestic and foreign researchers [2], the clinical characteristics of the course of the disease detected in pregnant women with confirmed SARS-CoV-2 infection are similar to those in the general population. According to other publications, pregnant women are significantly more likely to have a severe course of the disease [3].
The WHO, RCOG and RANZCOG do not define pregnant women as a high-risk group of SARS-CoV-2 infection [4–6]. At the same time, adaptive changes specific for pregnancy, such as immunological restructuring of the body, increased circulating blood volume and an increase in oxygen demand can contribute to high susceptibility to infections and increased risk of complications. Currently, greater exposure of pregnant women to COVID-19 is a conflicting evidence [3, 7–9]. Also, some authors describe that lightning-fast development of critical condition in pregnant women with COVID-19 is possible on the background of a fairly stable course of the disease. At the same time, the highest risk occurs in pregnant women with concomitant comorbid pathology [10]. A number of previous studies [11–13] demonstrated the absence of vertical transmission of SARS-CoV-2 infection from mother to fetus.
The experience of previous SARS and MERS pandemics has demonstrated that the infectious process in pregnant women increases the risks of maternal and perinatal mortality, intrauterine growth retardation and preterm birth [14, 15]. Considering previously obtained data, it is consequential to raise a question of possible adverse effect of SARS-CoV-2 infection on pregnancy course and outcomes [16].
Currently, there is no clear evidence of vertical transmission of SARS-CoV-2 [11, 12], although some researchers reported that the virus in breast milk was identified with polymerase chain reaction (PCR) [17].
The aim of this study was to analyze pregnancy complications and outcomes in women with COVID-19.
Materials and methods
A retrospective analysis of 34 COVID-19 cases in pregnant women (average age was 30.1 [26.5; 32.1] years), who underwent treatment in hospitals belonging to the system of mandatory medical insurance in Simperopol, and matched the inclusion criteria.
Inclusion criteria for pregnant women under study were women aged 16–40 years, PCR positive test result for the presence of SARS-CoV-2 RNA; gestation term at the time of infection – the third trimester; and written consent of pregnant women for participation in the study.
16 women (48.4%) were primiparous, and 18 women (51.6%) were multiparous. 9 women (27.5%) had abortions in medical history, and 3 women (9.8%) had miscarriages.
The diagnosis of COVID-19 was confirmed in all women by PCR test for detection of SARS-CoV-2 infection; the material was collected using nasopharyngeal and oropharyngeal swabs. The diagnosis and severity of COVID-19, the prevalence of pneumonia by the results of computed tomography (CT) were assessed according to Temporary Guidelines of the Ministry of Health of the Russian Federation for prevention, diagnosis and treatment of a new coronavirus infection COVID-19 (versions 6–9).
Statistical analysis
Statistical data processing was performed using software packages STATISTICA 8.0 (StatSoft.Inc., USA). Qualitative parameters were described with absolute and relative values of parameters (in %). The Kolmogorov–Smirnov test was used to assess, if distribution of variables followed normal distribution. Since most of the quantitative variables did not conform to the normal distribution, they were described using the median (Me) and quartiles [25%; 75%]. Mann–Whitney U-test was used to assess the quantitative characteristics, and χ2 test was used to compare the qualitative characteristics. The differences were considered significant at р<0.05. The Spearman's rank correlation test was used to measure the degree of association between the variables.
Results
The characteristics of pregnant women with COVID-19 participating in the study are shown in Table 1. The average gestation age at the time of SARS-CoV-2 infection was 33.4 [29.8; 35.2] weeks. In 3/34 (8.8%) pregnant women COVID-19 disease was asymptomatic. Most patients – 18/34 (52,9%) had mild symptoms, 7/34 (20.7%) patients had moderate and 6/34 (17.6%) patients had severe symptoms. The most common symptoms of the disease were: fever (temperature range was 37.3–39.1°C) in 27/34 (79.4%) cases, asthenia – in 25/34 (73.5%) cases, sore throat – in 21/34 (61.8%) cases, cough in 19/34 (55.9%) cases; shortness of breath was in 13/34 (38.2%) cases. The level of C-reactive protein (CRP) was 2.8 times higher than standard values at normal D-dimer level in the blood. Pneumonia was diagnosed in 23/34 (67.1%) pregnant women. All pregnant women with COVID-19 received standard therapy, 2/34 (5.9%) patients required treatment in intensive care unit, among them 1/34 (2.9%) woman required invasive mechanical ventilation (IMV).
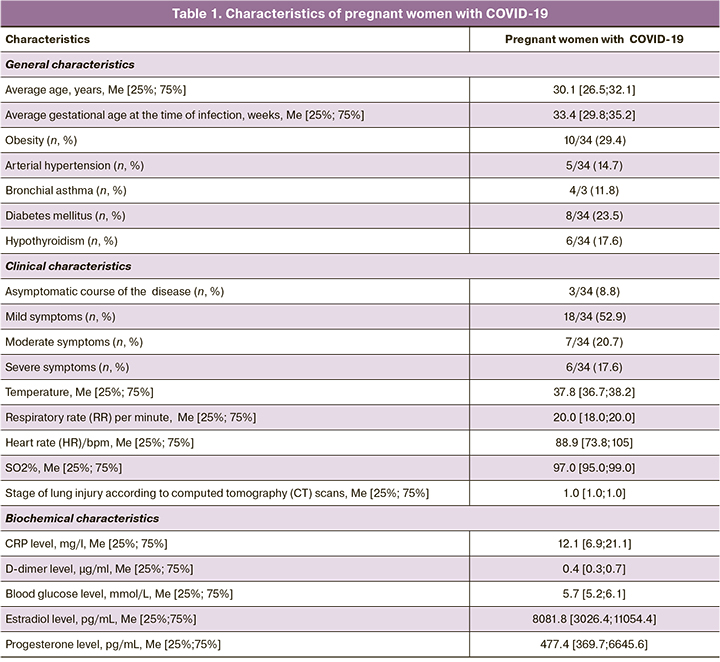
26/34 (76.5%) pregnant women with COVID-19 had extragenital pathologies in history, among them, obesity was most common – in 15/34 (44.1%) patients (average BMI 34.2 [30.3; 38.1] kg/m2). Class 1 obesity was in 5/34 (23.5%) women, class 2 obesity was in 3/34 (11.8%) women, and class 3 obesity was in 2/34 (8.8%) women. At the same time, among pregnant women with severe course of COVID-19, 6/10 (60.0%) women had obesity. A significant correlation was found between the development of severe course of COVID-19 and varying degrees of obesity before pregnancy (r=0.55; p=0.001, 95% CI 0.26–0.81).
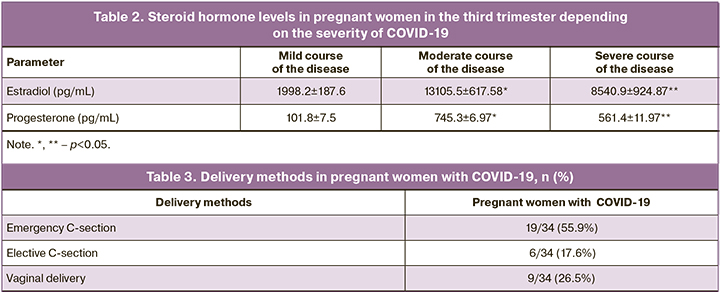
Assessment of plasma hormone levels in pregnant women with COVID-19 showed that all pregnant women had normal ranges of estradiol and progesterone levels. However, in pregnant women with severe course of the disease, the levels of estradiol and progesterone were significantly lower versus women with mild and moderate course of the disease – 1998.2 [1880.9; 3047.2] ph/mL, 101.8 [92.7; 106.2] pg/mL versus 13105.5 [11806.1; 13866.9] pg/mL (p=0.001), 745.3 [739.1; 763.2] pg/mL (p<0.001) and 8540.9 [7990.6; 9678.2] pg/mL (p=0.004), 561.4 [549.2; 578.9] pg/mL (p=0.034), respectively (Table 2). Statistically significant inverse correlation between the levels of estradiol, progesterone and the severity of COVID-19 was determined (r=-0,58; p=0.012, 95% CI 0.32–0.77 and r=-0.51; p=0.001, 95% CI 0.27–0.82, respectively).
Complications of pregnancy were in 26 (76,5%) women with COVID-19. The most common complication of pregnancy was mild or moderate iron-deficiency anemia in 15 (44.2%) cases, the mean hemoglobin level was 105.5 [86.7; 124 3] g/L. At the same time, in pregnant women with severe course of COVID-19, hemoglobin concentrations in the blood were significantly lower than in pregnant women with a mild course of the disease – 81.2 [74.5; 84.7] g/L versus 109.4 [96.7; 116.1] g/L (p=0.034). The relationship between hemoglobin levels and the severity of COVID-19 was detected (r=-0.44; p=0.006, CI 0.42–0.88). Pregnancy complication, such as preeclampsia was in 13/34 (38.2%) women. Moderate preeclampsia was diagnosed in 9/34 (26.8%) women, severe preeclampsia was in 4/34 (11.8%) women. Moreover, in 3/34 (8.8%) cases, preeclamsia complications, such as HELLP syndrome was in 2/34 (5.9%) women, and premature placental abruption was in 1/34 (2.9%) woman. The risk of preterm labor was in 12/34 (35.3%) cases. In 8/34 (23.5%) cases, pregnancy was complicated by preterm premature rupture of membranes. Cardiotocography showed worsening of the functional status of fetuses – bradycardia, meconium stained amniotic fluid was registered in 7/34 (20.5%) cases. Placental insufficiency was in 7/34 (20.5%) cases. The clinical criteria were discrepancies between ultrasound fetometric parameters and the gestational age; ultrasound and laboratory test results for chronic placental insufficiency; impairment of blood flow in uterine and umbilical arteries; integral indicator of the fetal status; and fetal cardiovascular response.
Chronic pyelonephritis was diagnosed in 7/34 (20.5%) cases. Urine analysis showed that the average urinary protein concentration was 0.05 [0.01; 0.06] g/L, daily levels of proteinuria was 0.16 [0.12; 0.30] g/L. In 5/34 (14.7%) cases, pregnancy was complicated by gestational diabetes mellitus: mild degree was in 3/34 (8.8%) women, moderate degree was in 2/34 (5.9%) women. The condition of all pregnant women improved after therapy; daily plasma glucose level was 5.7 [5.4; 6.0] mmol/L). In 2/34 (5.9%) cases, pregnancy was complicated by cholestasis.
The average term of delivery was 38.7 [36.2; 39.4] weeks. Pregnancy ended in preterm birth in 13 (36.8%) women. Term births were in 21 (63.2%) women (Table 3). In our study, emergency cesarean section (C-section) was performed in 55.9% of cases, elective cesarean section was in 17.6% of cases, and vaginal delivery was in 26.5% of cases.
Body weight of newborns varied from 2400 g to 4450 g, the average weight at birth was 3139.3 [2589.1; 3689.4] g, the average body length was 52.4 [50.8; 54.0] cm. In the structure of, moderate birth asphyxia was in 2/34 (5.9%) cases, bacterial pneumonia was in 1/34 (2.9%) case, and moderate cerebral ischemia was in 1/34 (2.9%) case. 3/34 (8.8%) newborns were transferred to the neonatal intensive care unit.
All probes for SARS-CoV-2 from nasopharyngeal and oropharyngeal swabs from newborns were negative.
Discussion
This study clarifies the impact of CОVID-19 on pregnancy complications and outcomes in the third trimester.
Our study showed that most pregnant women – 54.1% had a mild course of COVID-19 and 19.1% of women had a severe course of the disease. The obtained data correlate with the previously conducted research by Antoun L. et al. [18] and is statistically similar to the data on general population [19]. The most common symptoms of the disease were fever, asthenia, sore throat. Cough and shortness of breath were less common. Practically all pregnant women had a clear pandemic anamnesis.
Among pregnant women examined by us, pneumonia was diagnosed in 67.1% of women. It has been proven that timely diagnosis of pneumonia associated with COVID-19 is extremely important for pregnant women, since this complication can be clinically asymptomatic, but in all cases increases the risk of adverse maternal and fetal outcomes. Vallejo V. et al. [20] reported that critical conditions may suddenly develop in clinically stable pregnant women with COVID-19. According to researchers in China, 3% of pregnant women die from pneumonia caused by COVID-19. In our study, 5.9% of women were admitted to resuscitation and intensive care unit (RICU). Among them, 2.9% of women required IMV. All described cases of hospitalization in RICU resulted in improvement of patients’ condition and hospital discharge. There were no COVID-19 deaths.
A number of studies demonstrated that pregnant women with obesity are at higher risk of severe COVID-19 illness [21, 22]. It is known that with obesity the levels of leptin, interleukin-6 and tumor necrosis factor-α are increased, and adiponectin levels are reduced, and this leads to dysregulation of the immune response [23]. Our study showed that among pregnant women with severe course of COVID-19, the rate of women with obesity was 61.1%. A significant correlation between the development of a severe course of COVID-19 and obesity of varying severity before pregnancy was found. The obtained results correlated with the data in previously conducted researches. Thus, Lokken E.M. et al. [21] showed, that in 45 pregnant women with severe course of COVID-19, obesity was a leading disease among comorbid somatic disorders.
Despite the existing opinion that at late terms of pregnancy SARS-CoV-2 may affect formation of the hormonal function of the fetoplacental system in women and fetal health, until present no clear ideas were presented about the changes in steroid hormone levels in patients with various courses of COVID-19 in the third trimester of pregnancy. Despite the fact that concentrations of estradiol and progesterone were within the normal range, we have found a statistically significant decrease in steroid hormones in pregnant women with severe course of COVID-19 versus pregnant women with mild or moderate course of the disease. This suggests that steroid hormones have a protective effect against viral infection [24]. Thus, Pinna G. [25] from the University of Illinois suggested that estrogen and progesterone, as well as its metabolite allopregnanolone in women can provide anti-inflammatory functions, stimulate the production of antibodies and restore epithelial cells of the respiratory tract by suppressing ACE2 receptor with which the coronavirus interacts.
According to Koumoutsea E.V. et al. [26], COVID-19 significantly exacerbates pregnancy pathology. Alternatively, pregnancy pathology can aggravate coronavirus disease. The results of our study showed that iron-deficiency anemia was the most common disorder among pregnancy complications in women with COVID-19 (it was in 44.2% of women). Bao J. et al. [27] reported a complex bidirectional relationship between COVID-19 and anemia. At the same time, according to Poon L.C. et al. [10], a combination of pregnancy and COVID-19, especially on the background of iron-deficiency anemia leads to a more severe course of the disease (predominantly in the third trimester, when inflammatory processes are maximally activated). Indeed, in our study, concentrations of hemoglobin in the blood were statistically significantly lower in pregnant women with severe COVID-19 compared to pregnant women with a mild course of the disease.
According to Chen H. еt al. [28], intrauterine pneumonia was diagnosed in 2% of newborns, and cerebral ischemia – in 20% of newborns. On the contrary, other studies demonstrated the absence of immediate negative consequences of COVID-19 for newborns. Thus, Liu D. et al. [29] reported that all babies born at 38–41 weeks of gestation had good Apgar scores.
In a number of previous studies, it was noted that respiratory infections can have either a direct embryotoxic effect or lead to impaired uteroplacental blood flow on the background of intoxication and hyperthermia [30]. In our study 38.2% of pregnant women developed preeclampsia. Among them, severe preeclampsia was in 11.8% of women, while the risk of severe preeclampsia in general population was 1–2% [19]. Pregnancy was complicated by preterm premature rupture of membranes in 23.5% of cases. High rates (41.1%) of preterm births in women with COVID-10 reported by Di Mascio D. et al. [31] were confirmed in our study. In 35.4% of women in this study, pregnancies ended in preterm births, and this rate was much higher than in general population (4.4%, p<0.001) [19]. Moreover, the rate of cesarean section in pregnant women with COVID-19 was 2.4 times higher versus the rate of C-section in Russia (73.3% versus 30%, p=0.034) [20].
Conclusion
Currently, the discussion is conducted regarding the impact of COVID-19 on newborn babies. In our study, 5.9% of newborns were diagnosed with moderate asphyxia, 2,9% with bacterial pneumonia, and 2.9% with moderate cerebral ischemia. The cases of perinatal deaths were not registered.
The obtained data showed, that in all babies born to mothers with COVID-19, PCR tests of oropharyngeal and nasopharyngeal swabs did not detect SARS-CoV-2 RNA.
The cases of vertical transmission of SARS-CoV-2 from mother to fetus were not detected in the study. It was found that most pregnant women had a mild case of COVID-19. The incidence of preeclampsia, preterm birth, and caesarean section is higher in pregnant women with COVID-19 versus pregnant women without COVID-19. It was found, that steroid hormones in pregnant women with severe SARS-CoV-2 decreased. This was manifested by decreased levels of estradiol and progesterone depending of the severity of the course of COVID-19. In our study, emergency cesarean section was performed in 55.9% of cases, elective cesarean section in 17.6% of cases and vaginal delivery in 26.5% of cases.
References
1. Mathad J.S., Gupta A. Pulmonary infections in pregnancy. Semin. Respir. Crit. Care Med. 2017; 38(2): 174-84. https://dx.doi.org/10.1055/s-0037-1602375.
2. Gregg W.E., Brown A.A. Cognitive and physical disabilities and aging- related complications of diabetes. Clin. Diabetes. 2003; 21(3): 113-8. https://dx.doi.org/10.2337/diaclin.21.3.113.
3. Westgren M., Pettersson K., Hagberg H., Acharya G. Severe maternal morbidity and mortality associated with COVID-19: The risk should not be down-played. Acta Obstet. Gynecol. Scand. 2020; 99(7): 815-6. https://dx.doi.org/10.1111/ aogs.13900.
4. WHO Q & A on COVID-19, pregnancy, childbirth and breastfeeding. 18 March 2020. Available at: https://www.who.int/news-room/q-a-detail/q-a-on- covid- 19-pregnancychildbirth-and-breastfeeding Accessed April 20 2020.
5. Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID- 19) infectionin pregnancy. Version 8, Published 17 April 2020. Available at: https://www.nih.gov/health-information/coronavirus Accessed April 20 2020.
6. RANZCOG. A message for pregnant women and their families. Available at: https://ranzcog.edu.au/statementsguidelines/covid-19-statement/information-for-pregnant-women Accessed April 20 2020.
7. Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K. et al. Coronavirus disease 2019 among a symptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM. 2020; 2(2): 100118. https://dx.doi.org/10.1016/j.ajogmf.2020.100118.
8. Ковальчук А.С., Кучерявенко А.Н. Течение новой коронавирусной инфекции (COVID-19) у беременной (клинический случай). Журнал инфектологии. 2020; 12(3): 75-9. [Kovalchuk A.S., Kutsheriavenko A.N. new coronavirus infection (CoVID-19) in a pregnant woman (clinical case). Journal Infectology. 2020; 12(3): 75-9. (in Russian)].
9. Артымук Н.В., Белокриницкая Т.Е., Филиппов О.С., Шифман Е.М. Новая коронавирусная инфекция COVID-19 у беременных Сибири и Дальнего Востока. Вестник интенсивной терапии им. А.И. Салтанова. 2020; 2: 41-8. [Artymuk N.V., Belokrinitskaya T.E., Filippov O.S., Shifman E.M. New coronavirus infection COVID-19 in pregnant women in Siberia and the Far East. Intensive Care Bulletin named after A.I. Saltanov. 2020; 2: 41-8. (in Russian)].
10. Poon L.C., Yang H., Lee J.C.S., Copel J.A., Leung T.Y., Zhang Y. et al. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet. Gynecol. 2020; 55(5): 700-8. https://dx.doi.org/10.1002/uog.22013.
11. Припутневич Т.В., Гордеев А.Б., Любасовская Л.А., Шабанова Н.Е. Новый коронавирус SARS-COV-2 и беременность: обзор литературы. Акушерство и гинекология. 2020; 5: 6-12. [Priputnevich T.V., Gordeev A.B., Lyubasovskaya L.A., Shabanova N.E. The novel coronavirus SARS-CoV-2 and pregnancy: literature review. Obstetrics and Gynecology. 2020; 5: 6-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.5.6-12.
12. Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospectivere view of medical records. Lancet. 2020; 395(10226): 809-15. https://dx.doi.org/10.1016/S0140-673(20)30360-3.
13. Артымук Н.В., Белокриницкая Т.Е., Филиппов О.С., Марочко К.В. Особенности течения беременности, акушерская и терапевтическая тактика при новой коронавирусной инфекции COVID-19 у беременных. Акушерство и гинекология. 2020; 12: 6-13. [Artymuk N.V., Belokrinitskaya T.E., Filippov O.S., Marochko K.V. Pregnancy course, obstetric and therapeutic tactics for novel coronavirus infection (COVID-19) in pregnant women. Obstetrics and Gynecology. 2020; 12: 6-13. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.6-13.
14. Припутневич Т.В., Ачкасова Е.Н., Чубаров В.В., Гордеев А.Б. Острые респираторные заболевания и грипп в современном акушерстве: эпи-демиологические особенности и проблемы диагностики: обзор лите-ратуры. Эпидемиология и вакцинопрофилактика. 2019; 18(3): 89-97. [Priputnevich T.V., Achkasova E.N., Chubarov V.V., Gordeev A.B. Acute Respiratory Diseases and Influenza in Modern Obstetrics: Epidemiological Features and Diagnostic Problems: Literature Review. Epidemiology and Vaccinal Prevention. 2019; 18(3): 89-97. (in Russian)]. https://dx.doi.org/10.31631/2073-3046-201.
15. Malik A., El Masry K.M., Ravi M., Sayed F. Middle East respiratory syndrome coronavirus during pregnancy. Emerg. Infect. Dis. 2016; 22(3): 515-7. https://dx.doi.org/10.3201/eid2203.151049.
16. Беженарь В.Ф., Зазерская И.Е., Беттихер О.А., Нестеров И.М., Баутин А.Е. Спорные вопросы акушерской тактики при ведении беременности и родоразрешении пациенток с новой коронавирусной инфекцией COVID-19. Акушерство и гинекология. 2020; 5: 13-21. [Bezhenar V.F.,. Zazerskaya I.E, Bettikher O.A., Nesterov I.M., Bautin A.E. Controversial issues in obstetric management of women with novel coronavirus disease covid-19 during pregnancy and childbirth. Obstetrics and Gynecology. 2020; 5: 13-21. (in Russian)]. https://dx.doi.org/10.18565/ aig.2020.5.13-21.
17. Tam P.C.K., Ly K.M., Kernich M.L., Spurrier N., Lawrence D., Gordon D.L., Tucker E.C. Detectable severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 2021; 72(1): 128-30.
18. Antoun L. Taweel N.E., Ahmed I., Patni S., Honest H. Maternal COVID- 19 infection, clinical characteristics, pregnancy, and neonatal outcome: A prospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 252: 559-62. https://dx.doi.org/10.1016/j.ejogrb.2020.07.008.
19. Агеева Л.И., Александрова Г.А., Зайченко Н.М., Кириллова Г.Н., Леонов С.А. Здравоохранение в России. 2019: Стат. сб./Росстат. М.; 2019. 170с. [Ageeva L.I., Alexandrova G.A., Zaichenko N.M., Kirillova G.N., Leonov S.A. Healthcare in Russia. 2019: Statistical collection/Rosstat. M.; 2019. 170 p. (in Russian)].
20. Vallejo V., Ilagan J.G. A postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstet. Gynecol. 2020; 136(1): 52-5. https://dx.doi.org/10.1097/AOG.0000000000003950.
21. Lokken E.M., Walker C.L., Delaney S., Kachikis A,, Kretzer N,M,, Erickson A. et al. Clinical characteristics of 46 pregnant women with a SARS-CoV-2 infection in Washington State. Am. J. Obstet. Gynecol. 2020; 223(6): 911.e1-911.e14. https://dx.doi.org/10.1016/j.ajog.2020.05.031.
22. De Jong A., Chanques G., Jaber S. Mechanical ventilation in obese ICU patients: from intubation to extubation. Crit. Care. 2017; 21(1): 63. https://dx.doi.org/10.1186/s13054-017-1641-1.
23. Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokinesin inflammation and metabolic disease. Nat. Rev. Immunol. 2011; 11(2): 85-97.
24. Hall O.J., Limjunyawong N., Vermillion M.S., Robinson D.P., Wohlgemuth N., Pekosz A. et al. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016; 12(9): e1005840.
25. Pinna G. Sex and COVID-19: a protective role for reproductive steroids. Trends Endocrinol. Metab. 2021; 32(1): 3-6. https://dx.doi.org/10.1016/ j.tem.2020.11.004.
26. Koumoutsea E.V., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C. et al. COVID-19 and acute coagulopathy in pregnancy. J. Thromb. Haemost. 2020; 18(7): 1648-52. https://dx.doi.org/10.1111/jth.14856.
27. Bao J., Li C., Zhang K., Kang H., Wensen Chen W., Bing Gu B. Comparative analysis of laboratory indexes of severe and non-severe patients infected with COVID-19. Clin. Chim. Acta. 2020; 509: 180-94. https://dx.doi.org/10.1016/ j.cca.2020.06.009.
28. Liu D., Li L., Wu X., Zheng D., Wang D., Yang L., Zheng C. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR. Am. J. Roentgenol. 2020; 215(1): 127-32. https://dx.doi.org/10.2214/AJR.20.23072.
29. Давыдова, Ю.В., Лиманская А.Ю. Безопасность применения препаратов интерферона в лечении ОРВИ у беременных высокого риска. Перинатология и педиатрия. 2016; 1: 27-32. [Davydova, Yu.V., Limanskaya A.Yu. Safety of the use of interferon preparations in the treatment of ARVI in high-risk pregnant women. Perinatology and Pediatrics. 2016; 1(65): 27-32. (in Russian)].
30. Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M. et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020; 2(2): 100107. https://dx.doi.org/10.1016/j.ajogmf.2020.100107.
Received 08.06.2021
Accepted 18.02.2022
About the Authors
Yulia E. Dobrokhotova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Medical Faculty, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, pr.dobrohotova@mail.ru, https://orcid.org/0000-0002-9091-4097, 117997, Moscow, Russia, Ostrovityanova str., 1.Lesya N. Gumenyuk, Dr. Med. Sci., Professor, Department of Psychiatry, Narcology, and Psychotherapy with a Course of General and Medical Psychology, S.I. Georgievsky Medical Academy, V.I. Vernadsky Crimean Federal University. lesya_gymenyuk@mail.ru, https://orcid.org/0000-0002-0944-3591, 295051, Russia, Republic of Crimea, Simferopol, Lenina Blvd, 5/7.
Galina A. Puchkina, Assistant of the Department of Obstetrics, Gynecology and Perinatology No. 1, S.I. Georgievsky Medical Academy, V.I. Vernadsky
Crimean Federal University, puchkina.g.a@mail.ru, https://orcid.org/0000-0002-8882-8317, 295051, Russia, Republic of Crimea, Simferopol, Lenina Blvd, 5/7.
Vyacheslav Yu. Mikhaylichenko, Professor, Head of the Department of General Surgery, Anesthesiology-Resuscitation and Emergency Medicine, S.I. Georgievsky
Medical Academy, V.I. Vernadsky Crimean Federal University, puchkina.g.a@mail.ru, https://orcid.org/0000-0003-4204-5912,
295051, Russia, Republic of Crimea, Simferopol, Lenina Blvd, 5/7.
Corresponding author: Galina A. Puchkina, puchkina.g.a@mail.ru
Authors’ contributions: Dobrokhotova Yu.E., Gumenyuk L.N. - the concept and design of the study; Puchkina G.A., Mikhailichenko V.Yu. - writing the article.
Conflicts of interest: The authors declare that they have no conflict of interests.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of V.I. Vernadsky Crimean Federal University (extract from protocol No.2 of 18.02.2021).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Dobrokhotova Yu.E., Gumenyuk L.N., Puchkina G.A., Mikhailichenko V.Yu.
Pregnancy complications and outcomes in women with COVID-19.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 3: 32-38 (in Russian)
https://dx.doi.org/10.18565/aig.2022.3.32-38



