Infertility treatment outcomes in women over 40 years treated by assisted reproductive technologies using their own oocytes cultured in low lactate embryo culture medium
Khachatryan L.V., Smolnikova V.Yu., Makarova N.P., Sysoeva A.P., Kulakova E.V., Kalinina E.A.
Relevance: Advanced maternal age is one of the most significant non-modifiable factors affecting fertility and success of assisted reproductive technology (ART) programs. Currently, researchers are focusing on optimizing the embryonic stage to enhance embryo viability and implantation potential.
Objective: To study pregnancy outcomes in women over 40 years of age following infertility treatment using ART with oocytes cultured in low-lactate embryo culture medium.
Materials and methods: This study included 520 infertile couples with women of advanced reproductive age (>40 years). They were divided into two groups based on the embryo culture medium used: group 1 (n=264), which used low-lactate CSCM-NX medium, and group 2 (n=256), which used one-stage classical G-TL medium.
Results: Embryo culture in low-lactate medium (group 1) resulted in a statistically significant increase in the embryo blastulation rate (39.0% compared to 31.1% in group 2, p=0.05) and blastocyst rate (p=0.04). Additionally, embryo culture in low-lactate medium led to a statistically significant decrease in the number of cycles with canceled embryo transfers because of unsatisfactory quality (14.7% in group 1 compared to 24.2% in group 2; RR=0.61, 95% CI: 0.43–0.88, p=0.007), and an increase in the pregnancy rate (14.4% in group 1 compared to 8.2% in group 2; RR=1.8, 95% CI: 1.0–3.3, p=0.02). There was no statistically significant difference in birth rate.
Conclusion: For women of advanced reproductive age, the use of a low-lactate embryo culture medium is a promising approach to increase blastocyst rates, which may reduce the number of ovarian stimulations and improve the chances of obtaining euploid embryos and live births.
Authors' contributions: Khachatryan L.V. – data collection and analysis, review and analysis of relevant literature, drafting of the manuscript; Smolnikova V.Yu., Kalinina E.A., Kulakova E.V. – drafting of the manuscript, critical analysis; Makarova N.P., Sysoeva A.P. – conception and design of the study, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Khachatryan L.V., Smolnikova V.Yu., Makarova N.P., Sysoeva A.P., Kulakova E.V., Kalinina E.A. Infertility treatment outcomes in women over 40 years treated by assisted reproductive technologies
using their own oocytes cultured in low lactate embryo culture medium.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (4): 71-78 (in Russian)
https://dx.doi.org/10.18565/aig.2025.46
Keywords
The use of assisted reproductive technologies (ART) for infertility treatment is on the rise worldwide. Concurrently, more couples of advanced reproductive age seek treatment, necessitating the integration of new approaches to enhance treatment efficacy in this patient cohort. It is well established that a woman's age is a non-modifiable factor that significantly affects the success of in vitro fertilization (IVF) [1]. Additionally, in clinical practice, age is a crucial aspect in assessing male fertility, as it contributes to abnormalities in embryonic development and adverse pregnancy outcomes [2, 3]. However, a woman's age plays a more critical role in the outcomes of infertility treatment; as age increases, both the quantity and quality of oocytes obtained during ovarian stimulation decrease [4]. The primary factors contributing to the failure of infertility treatment programs utilizing ART in women of advanced reproductive age include not only a reduced number of oocytes but also a diminished potential for fertilization [5, 6]. The number of oocytes is recognized as an independent factor that influences the live birth rate. A systematic review and meta-analysis by Sermondade N. et al. (2023) identified that a threshold of 15 oocytes is optimal for achieving live birth [7]. For patients under 35 years of age, a higher number of oocytes does not significantly affect live birth rates; however, for women over 35 years of age, a greater number of oocytes correlates with an increased cumulative live birth rate. Specifically, in groups of women aged 35–37, 38–40, 41–42, and over 42 years, the required number of oocytes to obtain one euploid embryo is 5, 7, 10, and 20, respectively [8].
Beyond age, the cumulative live birth rate is influenced by various other factors, including somatic, gynecological, and obstetric history, ovarian reserve, the choice of ovarian stimulation protocol, embryonic development characteristics, and endometrial receptivity. Currently, researchers in the field of ART focus on optimizing the embryological stage by improving the embryo culture media to enhance viability and implantation potential. Optimizing culture protocols is a modifiable factor in ART that can improve treatment effectiveness. Media selection is based on two distinct approaches. The first, grounded in the “back to nature” principle, employs a sequential culture system in which the culture medium changes at different stages of embryonic development, creating conditions akin to those observed during natural fertilization. The second approach, termed “let the embryo choose,” utilizes continuous culture, providing a variety of conditions and essential nutrients, allowing the embryo to interact with its environment and select optimal developmental conditions [9]. One promising area based on this concept involves the use of culture media with reduced lactate, which, according to experimental studies, affects embryo viability and improves ART outcomes in women of advanced reproductive age [10].
Consequently, investigating various embryological approaches, particularly modified culture media, in patients of advanced reproductive age is a significant task for reproductive medicine and clinical embryology specialists. Addressing this challenge will help optimize programs and enhance the outcomes of infertility treatments using ART.
This study aimed to investigate pregnancy outcomes in women over 40 years of age following infertility treatment using ART with oocytes cultured in a low-lactate embryo culture medium.
Materials and methods
The study included 520 married couples, with women aged 40–42 years who were undergoing infertility treatment using ART at the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, V.I. Kulakov NMRC for OG&P of the Ministry of Health of Russia. The inclusion criteria were as follows: women aged 40–42 years, using only their own oocytes for fertilization, and obtaining more than one oocyte-cumulus complex (OCC) during transvaginal follicle puncture. The exclusion criteria included contraindications to infertility treatment using ART methods, as outlined in the Order of the Ministry of Health of the Russian Federation No. 803n dated July 31, 2020, "On the Procedure for Using Assisted Reproductive Technologies, Contraindications and Limitations to Their Use," pronounced pathozoospermia in the form of azoospermia, and abnormalities in the karyotypes of the spouses. All married couples included in the study provided informed voluntary consent.
Based on the culture medium used, the women were divided into two groups: group 1 (n=264), which utilized a medium with reduced lactate CSCM-NX ("Fujifilm Irvine Scientific, Inc.," USA), and group 2 (n=256), where embryos were cultured in a one-stage classical G-TL medium ("Vitrolife," Sweden). The selection of these culture media was based on the mass spectrometric analysis conducted and published by Morbeck D.E. et al. [11]. According to this publication, the lactate concentration in the G-TL medium was 10.01 mmol/ml, while in the CSCM-NX medium, it was 5.71 mmol/ml [11]. Manufacturers do not disclose the specific composition of culture media in their official documentation.
Standard protocols for ovarian stimulation with gonadotropin-releasing hormone antagonists were employed and exogenous gonadotropins were used for folliculogenesis. The choice of drugs, their dosages, and stimulation protocol were determined individually based on the hormonal profile and medical history. When follicles reached a diameter ≥18 mm, an ovulation trigger was administered to facilitate the final maturation of oocytes. After 35–37 h, transvaginal puncture of the ovaries was performed under anesthesia. All oocyte-cumulus complexes obtained were collected in a washing medium containing HEPES buffer (GametBuffer, Vitrolife, Sweden), washed to remove blood cells and follicular fluid, and placed in the appropriate culture medium. depending on the group. Fertilization was conducted according to the standard operating procedures of the laboratory. Depending on the ejaculate parameters, either classical IVF or intracytoplasmic sperm injection (ICSI) was performed. After 16–18 hours post-fertilization, the presence of two pronuclei and two polar bodies (2PN2PB) was interpreted as normal fertilization. Morphological assessment of the embryos was carried out after 120 h, following the Gardner classification [12]. The onset of pregnancy was evaluated through laboratory and instrumental examinations, including determination of the beta subunit of human chorionic gonadotropin level 14 days after embryo transfer (a result of more than 20 IU/l was considered positive) and ultrasound examination of the pelvic organs. Clinical pregnancy was confirmed upon the detection of the ovum in the uterine cavity.
The study endpoints included clinical indicators, such as the pregnancy rate per ART cycle (%), early pregnancy loss rate per pregnancy (%), and live birth rate per ART cycle (%).
Statistical analysis
Statistical analysis was conducted using Microsoft Excel and IBM SPSS Statistics v. 27.0.1.0 software. The distribution of continuous variables was assessed for normality using the Shapiro–Wilk test. Counts (n) and percentages (%) were used to describe categorical binary data. For non-normal distributions, non-parametric methods were employed to report the median (Me), along with the upper and lower quartiles (25th and 75th percentiles). The nonparametric Mann–Whitney U test was used to compare continuous variables, and the Pearson χ² test was used for categorical data. To evaluate the strength of the association between binary data, the risk ratio (RR) and 95% confidence interval (95% CI) were calculated. The significance threshold for the p-value was set at 0.05.
Results
At the first stage of the study, the clinical and anamnestic characteristics of the women included in the study were assessed. The median age of the women in both groups was 41 [40, 42] years. The incidence of primary infertility was 38.1%, and that of secondary infertility was 61.9% in the entire study cohort. When analyzing the reproductive history, it was found that married couples had a median of 2 [1; 3] unsuccessful IVF attempts before inclusion in this study. In the structure of infertility, male sex (46.7%), tubal-peritoneal (21.7%), and endocrine (5%) factors predominated, as well as their combination in 15.4% of couples. A detailed between-group analysis of the clinical and anamnestic data of the women in groups 1 (low-lactate culture medium) and 2 (classical culture medium) was performed. The results are presented in Table 1.
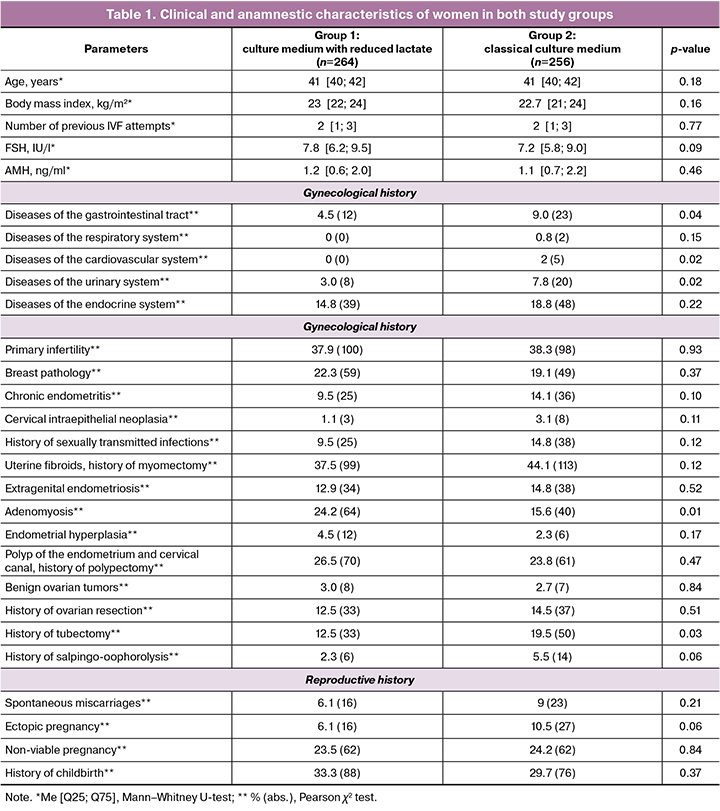
When analyzing the basic parameters of the hormonal tests, the level of follicle-stimulating hormone (FSH) in group 1 was 7.8 [6.2; 9.5] IU/L in group 2 – 7.2 [5.8; 9.0] IU/L (p=0.09). The anti-Müllerian hormone (AMH) levels were also comparable between the two groups. The patients did not differ in body mass index; in both groups, it was within the normal range: in group 1 – 23 [22; 24] kg/m2 in group 2 – 22.7 [21; 24] kg/m2 (p=0.16). When analyzing somatic history, statistically significant differences were obtained only in the frequency of diseases of the gastrointestinal tract, cardiovascular system, and urinary system. Thus, in group 1, no diseases of the circulatory system were noted, whereas in group 2, 5/256 (2%) patients had a history of compensated varicose veins of the lower extremities. In women from group 2 (classical culture medium), chronic cystitis was diagnosed significantly more often (7.8% vs. 3.0%, p=0.02). However, the contribution of this factor to the effectiveness of ART does not seem to be significant, since, according to the recommendations of a specialist, no exacerbations before joining the program and no contraindications were identified. In group 2, the increase in the frequency of gastrointestinal diseases was due to chronic gastritis, which at the time of joining the ART program was in a stage of stable remission and did not require treatment, according to the conclusion of a general practitioner.
When analyzing gynecological status, attention is drawn to the even distribution between groups of diseases such as breast pathology, cervical intraepithelial neoplasia, chronic endometritis, sexually transmitted infections, and uterine fibroids. Adenomyosis was significantly more common in women in group 1 (low lactate culture medium) (24.2 %), while in group 2 (classical culture medium), it was diagnosed in only 15.6% of cases (p=0.01). The frequencies of benign ovarian tumors, ovarian resections, and salpingo-ovariolysis were comparable in both groups. The frequency of tubectomy was significantly higher in group 2 (19.5%) than in group 1 (12.5%; p=0.03). When assessing the reproductive status of the recruited patients, no significant differences were found in the frequency of spontaneous miscarriages, ectopic and nonviable pregnancies, or previous deliveries (Table 1).
Thus, in women from group 1, where a low-lactate medium was used for embryo culture, adenomyosis was more common, while in patients from group 2, with the use of a one-stage classical culture medium, the anamnesis was complicated by tubectomy and somatic diseases such as diseases of the gastrointestinal tract, cardiovascular, and urinary systems.
The data obtained from the analysis of clinical and anamnestic parameters allowed us to compare the parameters of infertility treatment programs using ART. The results of ovarian stimulation and embryological stage were assessed in both groups and did not show any statistically significant differences in the number of OCCs, mature oocytes, or zygotes with two pronuclei 2PN (Table 2). In group 1, an average of 3 oocytes were obtained at the MII stage (3 [2; 6]), and in group 2, also 3 (3 [2; 6]). The fertilization rate was comparable between the two groups, and no statistically significant difference was found. However, when assessing the blastulation rate, significant differences were found among women in the selected groups. When cultured in a medium with reduced lactate (group 1), the blastulation percentage was 39.0%, whereas with traditional culture, it was 31.1% (p=0.05). The number of blastocysts obtained in both the groups also differed. Group 1 yielded a larger number of blastocysts that were cryopreserved and subsequently used for transfer into the uterine cavity in ART (Table 2). Cryopreservation of blastocysts on the 5th day of development was routinely performed in 35.2% (74/264) of the patients in group 1 and 17.0% (36/256) in group 2.
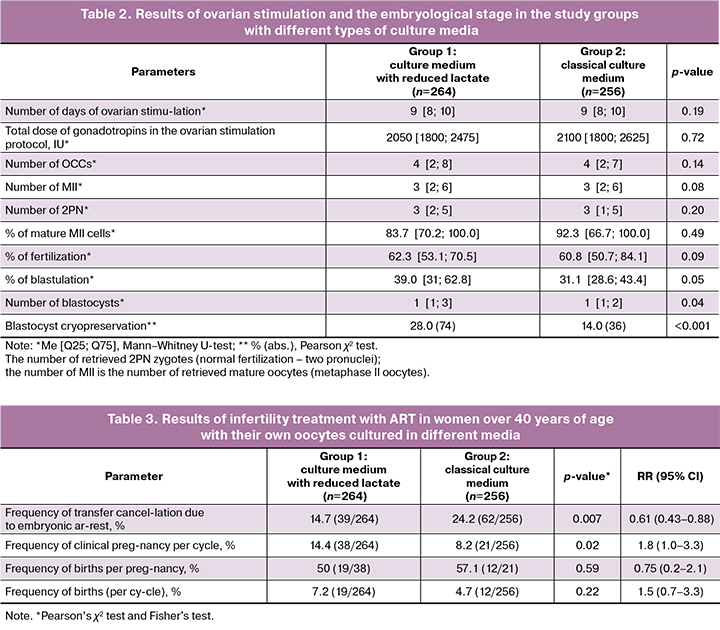
A total of 312 embryo transfers into the uterine cavity were performed in 520 selected couples, of which 136 were transferred to group 1, where embryo culture was performed in a low-lactate medium, and 176 to group 2 using a classical culture medium.
When assessing the endpoints of the study, a significant decrease in the number of cycles with the cancellation of embryo transfer due to unsatisfactory quality was noted in group 1 (culture medium with reduced lactate). The results are presented in Table 3. The cancellation rate in group 1 was 14.7% compared with 24.2% in group 2 (p=0.007, RR=0.61, 95% CI: 0.43–0.88). Culturing embryos of women of advanced reproductive age in a medium with reduced lactate levels allows for an increase in the blastulation rate and a decrease in the number of ART cycles, with cancellation of transfer.
When calculating the pregnancy rate, attention was drawn to the significant increase in the indicator in group 1 (culture medium with reduced lactate), 14.4% (38/264), compared to group 2, 8.2% (21/256) (p=0.02; RR=1.8; 95% CI: 1.0–3.3).
The obtained results indicate that in women of advanced reproductive age, culturing embryos in a medium with reduced lactate content increases the probability of clinical pregnancy by 1.8 times in infertility treatment programs using ART.
It is important to note that the birth rate per ART cycle in both groups did not differ significantly. In group 1 (low-lactate culture medium), 19/264 ART programs resulted in births, which was 7.1%; in group 2 (classical culture medium), 12/256 (4.6%) (p=0.22). This is an important clinical result since a low birth rate is associated with the advanced reproductive age of women.
The results once again indicate the need for a married couple with infertility to have timely contact specialists to implement reproductive function. Optimization of the embryological stage, as shown by the results of this analysis, can lead to an increase in the number of blastocysts and an increase in the frequency of clinical pregnancies, but does not affect the number of births.
Discussion
The embryological stage of the program for treating male and female infertility using ART is crucial, as the assessment of oocyte quality, fertilization, and in vitro cultivation to the blastocyst stage largely determine success. A key aspect of the embryological stage is the selection of appropriate culture media and conditions that closely resemble the natural environment for preimplantation embryo development. Despite ongoing advancements in optimizing this stage, there is currently no consensus on the ideal composition of culture medium [13]. A promising approach to improve ART outcomes in patients of advanced reproductive age involves using culture media with reduced lactate content, which may minimize metabolic stress on the embryo and consequently enhance its implantation potential. As the embryo develops, its glucose requirements steadily increase, potentially leading to higher lactate production in the nutrient medium owing to enhanced aerobic glycolysis [14, 15]. Elevated lactate concentrations can adversely affect embryo metabolism and disrupt embryonic development. Kobanawa M. et al. demonstrated that utilizing a low-lactate medium enhances the energy metabolism of oocytes and cumulus cells, resulting in improved blastocyst quality [16]. Such a culture medium may be particularly beneficial for patients of advanced reproductive age who experience higher levels of metabolic stress.
In the present study, the use of a low-lactate culture medium resulted in an increase in the rate of blastocyst formation and clinical pregnancy rates per ART cycle, rising from 8.2% to 14.4% compared to the classical medium (p=0.02). However, it did not affect the live birth rate. Among women over 40 years old using their own oocytes, the birth rates in both groups remained low, not exceeding 58% (50% in group 1 and 57.1% in group 2), which is critically low. More than half of the pregnancies in women of advanced reproductive age ended in miscarriages or nonviable pregnancies at various gestational ages.
In 2020, a comparative study involving 1,673 infertility treatment cycles with culture media containing both normal and six-fold reduced lactate concentrations was conducted across five IVF clinics in New Zealand [17]. This study indicated that lower lactate concentrations in single-stage media significantly benefited blastocyst development, particularly in women of advanced reproductive age. The authors achieved pregnancy rates comparable to those of younger women when using culture media with reduced lactate content, attributing the success to decreased metabolic stress during cultivation. Notably, the authors did not provide data on pregnancy outcomes in older reproductive age patients [17]. The findings on enhancing the embryological stage align with those of our study; however, this did not translate into increased live birth rates. The increase in the number of high-quality blastocysts obtained from culturing embryos in low-lactate media among women of advanced reproductive age is promising, especially when coupled with preimplantation genetic testing, which enhances the chances of obtaining euploid embryos and ultimately delivering healthy children.
Thus, integration of culture media with reduced lactate content in clinical practice appears to be a promising direction in reproductive medicine. This approach may enhance the number of blastocysts obtained, in conjunction with preimplantation genetic testing, potentially reducing the number of ovarian stimulation cycles and facilitating the acquisition of euploid embryos suitable for transfer.
Conclusion
Despite the unfavorable prognosis for achieving pregnancy and live birth in women of advanced reproductive age in ART programs, efforts to optimize treatment methods are ongoing. The primary challenge in this patient cohort is the biological aging of the ovaries, leading to decreased quality and quantity of retrieved oocytes. One promising strategy to improve infertility treatment outcomes in these women is the modification of embryo culture conditions using media with low lactate content, which may enhance embryonic morphokinetics.
References
- Сухих Г.Т., Назаренко Т.А., ред. Бесплодие в позднем репродуктивном возрасте: реалии и перспективы. М.: Издательство «Медком-Про»; 2024. 560 с. [Sukhikh G.T., Nazarenko T.A., ed. Infertility in late reproductive age: realities and prospects. Moscow: Medkom-Pro Publishing House; 2024. 560 p. (in Russian)].
- Драпкина Ю.С., Макарова Н.П., Васильев Р.А., Амелин В.В., Калинина Е.А. Сравнение прогностических моделей, построенных с помощью разных методов машинного обучения, на примере прогнозирования результатов лечения бесплодия методом вспомогательных репродуктивных технологий. Акушерство и гинекология. 2024; 2: 97-105. [Drapkina Yu.S., Makarova N.P., Vasiliev R.A., Amelin V.V., Kalinina E.A. Comparison of predictive models built with different machine learning techniques using the example of predicting the outcome of assisted reproductive technologie. Obstetrics and Gynegology. 2024; (2): 97-105. (in Russian)]. https://dx.doi.org/10.18565/aig.2024.124.
- Datta A.K., Campbell S., Diaz-Fernandez R., Nargund G. Livebirth rates are influenced by an interaction between male and female partners' age: analysis of 59 951 fresh IVF/ICSI cycles with and without male infertility. Hum. Reprod. 2024; 39(11): 2491-500. https://dx.doi.org/10.1093/humrep/deae198.
- Mazzilli R., Cimadomo D., Innocenti F., Taggi M., Cermisoni G.C., Ginesi S. et al. A WHO 2021-based comprehensive scheme outlining sperm parameters' associations with IVF outcomes in PGT-A cycles. Andrology. 2024; Nov 28. https://dx.doi.org/10.1111/andr.13811.
- Seifer D.B., Feinberg E.C., Hsu A.L. Ovarian aging and fertility. JAMA. 2024; 332(20): 1750-51. https://dx.doi.org/10.1001/jama.2024.18207.
- Wang X., Tian P.Z., Zhao Y.J., Lu J., Dong C.Y., Zhang C.L. The association between female age and pregnancy outcomes in patients receiving first elective single embryo transfer cycle: a retrospective cohort study. Sci. Rep. 2024; 14((1): 19216. https://dx.doi.org/10.1038/s41598-024-70249-1.
- Sermondade N., Sonigo C., Pasquier M., Ahdad-Yata N., Fraison E., Grynberg M. Searching for the optimal number of oocytes to reach a live birth after in vitro fertilization: a systematic review with meta-analysis. F&S Reviews. 2023; 4(2): 101-15.
- Neves A.R., Montoya-Botero P., Sachs-Guedj N., Polyzos N.P. Association between the number of oocytes and cumulative live birth rate: A systematic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2023; 87: 102307. https://dx.doi.org/10.1016/j.bpobgyn.2022.102307.
- Zagers M.S., Laverde M., Goddijn M., de Groot J.J., Schrauwen F.A.P., Vaz F.M., Mastenbroek S. The composition of commercially available human embryo culture media. Hum. Reprod. 2025; 40(1): 30-40. https://dx.doi.org/10.1093/humrep/deae248.
- Gardner D.K. Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? Bioessays. 2015; 37(4): 364-71. https://dx.doi.org/10.1002/bies.201400155.
- Morbeck D.E., Baumann N.A., Oglesbee D. Composition of single-step media used for human embryo culture. Fertil. Steril. 2017; 107(4): 1055-1060.e1. https://dx.doi.org/10.1016/j.fertnstert.2017.01.007.
- Gardner D.K., Schoolcraft W.B. In vitro culture of human blastocysts. In: Jansen R., Mortimer D. eds. Towards Reproductive Certainty: Infertility and Genetics Beyond 1999: The Plenary Proceedings of the 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics. Parthenon Press, Pearl River; 1999: 378-88.
- Gurner K.H., Evans J., Hutchison J.C., Harvey A.J., Gardner D.K. A microenvironment of high lactate and low pH created by the blastocyst promotes endometrial receptivity and implantation. Reprod. Biomed. Online. 2022; 44(1): 14-26. https://dx.doi.org/10.1016/j.rbmo.2021.09.012.
- Rinaudo P. Understanding embryo metabolism to improve embryo selection. RBMO. 2023; 47(Suppl.): 103425.
- Gardner D.K. Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? Bioessays. 2015; 37(4): 364-71. https://dx.doi.org/10.1002/bies.201400155.
- Kobanawa M. Fertilization, embryo culture, and clinical results using low lactate embryo culture medium for pre-culture, insemination, and beyond. Reprod. Med. Biol. 2022; 21(1): e12458. https://dx.doi.org/10.1002/rmb2.12458.
- Hammond E.R., Morbeck D. Reducing the stress of culture? Low lactate embryo culture medium increases usable blastocyst rate for woman of advanced material age. Fertil. Steril. 2020; 114(3): e7. https://dx.doi.org/10.1016/j.fertnstert.2020.08.045.
Received 25.02.2025
Accepted 31.03.2025
About the Authors
Lia V. Khachatryan, PhD student at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, Faculty of Postgraduate Professional Training of Physicians, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8-2, +7(963)977-88-94, leahkhachatryan@gmail.com, https://orcid.org/0000-0003-4867-500XVeronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher at the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, v_smolnikova@oparina4.ru
Natalya P. Makarova, Dr. Med. Sci., Leading Researcher at the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, np_makarova@oparina4.ru,
https://orcid.org/0000-0003-1396-7272
Anastasia P. Sysoeva, PhD, Clinical Embryologist, Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, sysoeva.a.p@gmail.com, https://orcid.org/0000-0002-6502-4498
Elena V. Kulakova, Dr. Med. Sci., Senior Researcher at the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kulakova@oparina4.ru,
https://orcid.org/0000-0002-4433-4163
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kalinina@oparina4.ru,
https://orcid.org/0000-0002-8922-2878



