Quantitative and qualitative determination of human papillomavirus DNA in women with cervical lesions
Bayramova G.R., Andreev A.O., Ilyasova N.A., Badlaeva A.S., Tregubova A.V., Trofimov D.Yu.
Objective: This study aimed to investigate the relationship between quantified human papillomavirus (HPV) viral load and the severity of cervical lesions.
Materials and methods: Data from 819 HPV-positive women aged 18–81 years were analyzed. Patients were categorized into three groups based on histologically verified diagnoses. Group 1 comprised 186 patients with chronic cervicitis, Group 2 included 341 patients with low-grade squamous intraepithelial lesions (LSIL), and Group 3 included 292 patients with high-grade squamous intraepithelial lesions (HSIL). HPV DNA testing was conducted by real-time PCR to determine the number of genomic equivalents of the virus. The HPV Kvant-21 reagent kit, designed for the detection, typing, and quantification of human papillomavirus DNA with low carcinogenic risk (HPV types 6, 11, 44) and high carcinogenic risk (HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82), was used for analysis.
Results: The study found that detection of a high viral load was associated with a statistically significantly higher risk of HSIL than LSIL, regardless of HPV genotype (OR=5.26; 95% CI 3.19–8.64). A similar trend was observed with moderate viral loads (OR=2.32; 95% CI 1.78–3.03). Furthermore, LSIL was significantly more common than HSIL when a low viral load was detected (OR=3.09; 95% CI 1.91–4.99). Additionally, a statistically significant positive relationship between the viral load and the degree of cervical involvement was identified for HPV genotypes 16, 18, 31, 39, and 44.
Conclusion: This study highlights the significance of HPV load as an important diagnostic marker. This suggests that the value of HPV load is currently underrated owing to challenges in interpreting the results of quantitative HPV DNA determination and the complexity of conducting research in this domain.
Authors' contributions: Andreev A.O., Bayramova G.R. – conception and design of the study; Bayramova G.R., Tregubova A.V. – data collection and processing; Ilyasova N.A. – statistical analysis; Andreev A.O., Bayramova G.R., Badlaeva A.S. – drafting of the manuscript; Bayramova G.R., Trofimov D.Yu. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Bayramova G.R., Andreev A.O., Ilyasova N.A., Badlaeva A.S., Tregubova A.V., Trofimov D.Yu. Quantitative and qualitative determination of human papillomavirus DNA in women with cervical lesions.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (1): 102-109 (in Russian)
https://dx.doi.org/10.18565/aig.2023.260
Keywords
Cervical cancer ranks as the third most common cancer among gynecological malignancies [1, 2]. Despite the implementation of national screening programs for cervical cancer in many countries, the World Health Organization has reported a consistent increase in morbidity and mortality due to malignant cervical neoplasms [3, 4]. Human papillomavirus (HPV) is widely recognized as the primary etiological factor for the development of cervical cancer and cervical lesions. Several countries that have adopted HPV testing as the primary screening method for cervical cancer have reported a decrease in its incidence [5–8]. Notably, Australia, which initiated a screening and vaccination program against HPV in 2007, is projected to eliminate the incidence of cervical cancer by 2028 using mathematical models. Conversely, countries without national screening programs are not anticipated to achieve a similar reduction until the end of the 21st century [9].
The role of HPV in the development of cervical pathologies has been established since the 1990s. During the 20th century, studies on predictors of cervical cancer identified HPV as a strong independent factor for its occurrence [10, 11]. A deeper understanding of the HPV problem has evolved over the past decades, encompassing a range of combined indicators, such as detection of specific HPV genotypes, viral load, duration of HPV persistence, and geographical distribution of genotypes in cervical pathology [12, 13]. Nevertheless, the significance of HPV load in the development and progression of cervical pathology remains a contentious issue. Many HPV DNA test systems do not consider quantitative assessment, which potentially limits the diagnostic and prognostic efficacy of HPV testing. Several researchers have observed a lack of statistical significance when comparing HPV viral load among different cervical lesion groups [14–18]. However, other studies suggest that HPV quantification may serve as a marker for subsequent progression of squamous intraepithelial lesions [19–21].
The objective of this study was to investigate the relationship between quantified human papillomavirus (HPV) viral load and histologically confirmed severity of cervical lesions.
Materials and methods
We analyzed data from 819 HPV-positive women aged 18–81 years with a histologically confirmed diagnosis. We described a sample of patients in a previously published article [22]. HPV DNA testing was conducted using real-time PCR to determine the number of genomic equivalents of the virus. The HPV Kvant-21 reagent kit, designed for the detection, typing, and quantification of human papillomavirus DNA with low carcinogenic risk (HPV types 6, 11, and 44) and high carcinogenic risk (HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82), was used for analysis. The detection limit for HPV genotypes is 5 DNA copies per amplification tube (103 copies/ml DNA preparation). The detection limit was established by analyzing serial dilutions of the laboratory control samples. The presence of HPV DNA in the test sample was indicated as the absolute amount (the power of the decimal logarithm of the concentration corresponding to the number of HPV DNA copies per sample) of this type of virus in the sample. The ranking of the HPV viral load is as follows: low viral load, < 3 log; moderate viral load, 3–5 log; high viral load, > 5 log.
The diagnosis was histologically confirmed using cervical samples obtained during targeted biopsy, loop electrosurgical excision, or conization of the uterine cervix. The study included data from 819 histological reports, and the patients were categorized into three groups based on histologically confirmed diagnoses. Group 1 included 186 patients with chronic cervicitis, Group 2 included 341 patients with low-grade squamous intraepithelial lesions (LSIL), and Group 3 included 292 patients with high-grade squamous intraepithelial lesions (HSIL).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics Version 20. Most of the variables did not meet the assumptions of normality; therefore, the continuous variables were expressed as median (Me) with interquartile range (Q1; Q3). To assess the relationship between the severity of the detected cervical pathology and the HPV viral load, the odds ratio (OR) was used, as the risk factor and the expected outcome were binary variables; that is, they had only two possible values, and the compared groups were independent. A 95% confidence interval (95% CI) was determined for the calculated OR. Differences between independent groups were assessed using the chi-square test for categorical variables and Mann–Whitney U test for continuous variables. The strength of the relationship was assessed using the Cramer's V test, and its values were interpreted according to the recommendations of Rea&Parker. The relationship between the following categorical variables was assessed: a histologically confirmed conclusion, presented in three gradations: chronic cervicitis, LSIL, HSIL, and viral load, coded in three categories: low, moderate, and high. Viral load levels between 0 and 3.0 log were considered low; between 3.1 and 5 log were considered moderate; 5.1 log and above were considered high. The level of significance was set at p<0.05.
Results
Among 819 patients, HPV DNA detection was recorded 1269 times; at the same time, the HPV viral load was determined to be low in 164 cases, moderate in 564, and high in 541. The distribution of the viral loads of different HPV genotypes according to the histologically confirmed diagnosis is presented in Table 1.
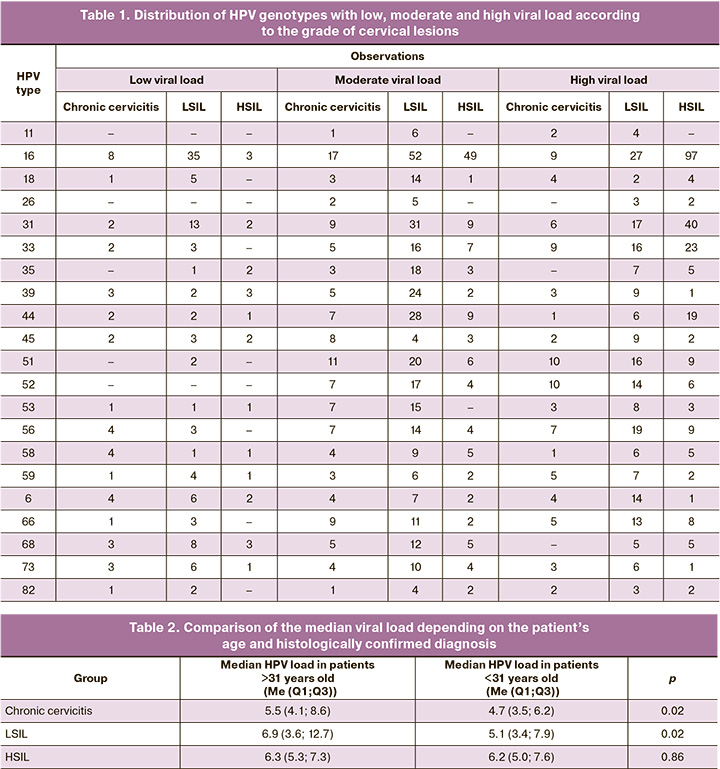
The mean age of the patients included in the study was 31 (range: 26–37) years. At the same time, the viral load among women in Group 1 was significantly lower at the age of over 31 years than in patients under 31 years of age (p=0.02). A similar pattern was observed in group 2 (p=0.02). However, in Group 3, there was no statistically significant difference in the level of viral load according to age (p=0.86) (Table 2).
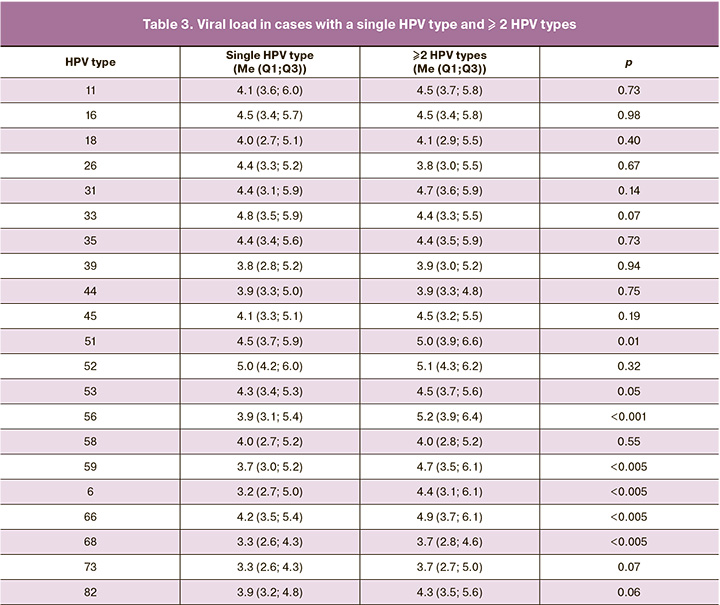
A single HPV genotype was detected in 588/819 (71.8%) cases, of which the viral load was low in 78/588 (13.3%), moderate in 231/588 (39.2%), and high in 279/588 (47.4%) samples.
It should be noted that when comparing viral load between cases with one and more HPV types identified, there were no statistically significant differences for genotypes 16, 31, and 44, which were the most prevalent and were associated with the least favorable prognosis for progression of cervical lesions according to data from our previous study [22]. However, a statistically significant difference in viral loads was found for the 51 HPV genotypes, with the median quantitative DNA presence being 4.5 (3.7; 5.9) for cases with single HPV type identified and 5.0 (3.9; 5.9); 6.6) with two or more identified genotypes (p<0.05). Similar results were obtained for 56 (p<0.001), 59 (p<0.05), 6 (p<0.05), 66 (p<0.05), 68 (p<0.005), 73 (p<0.05), and 82 (p<0.05) HPV genotypes, respectively (Table 3).
Among the patients in Group 1, 141/186 (75.8%) had only one HPV genotype, 45/186 (24.2%) had two or more HPV genotypes. In cases where HPV genotype 1 was detected, the viral load was low in 22/141 (15.6%) patients, moderate in 68/141 (48.2%) patients, and high in 51/141 (36.2%) patients. HPV detection occurred 109 times in samples in which two or more HPV genotypes were simultaneously present. Among the women with multiple HPV genotypes, the viral load was low in 20/109 (18.3%), moderate in 54/109 (49.5%), and high in 35/109 (32.1%)).
Among women in Group 2, only one type of HPV was detected in 213/341 (62.5%) patients, and in 128/341 (37.5%), two or more HPV genotypes were detected. In cases where HPV genotype 1 was detected, the viral load was low in 46/213 (21.6%) patients, moderate in 100/213 (46.9%) patients, and high in 67/213 (31.5%) patients. HPV was detected 421 times in LSIL samples with multiple HPV genotypes. Among the women with multiple HPV genotypes, the viral load was low in 54/421 (12.8%) cases, moderate in 223/421 (52.9%), and high in 144/421 (34.2%).
Among the 292 women in Group 3, only one type of HPV was detected in 234/292 (80.1%) cases and two or more types of HPV in 58/292 (19.9%) samples. Among patients in whom one HPV genotype was detected, the viral load was low in 10/234 (4.3%), moderate in 63/234 (26.9%) and high in 161/234 (68.8%) patients (Fig. 1). HPV was detected 151 times in HSIL samples in which multiple HPV genotypes were detected. In patients with several identified HPV genotypes, the viral load was low in 12/151 (7.9%), moderate in 56/151 (37.1%), and high in 83/151 (54.9%) patients (Fig. 2).
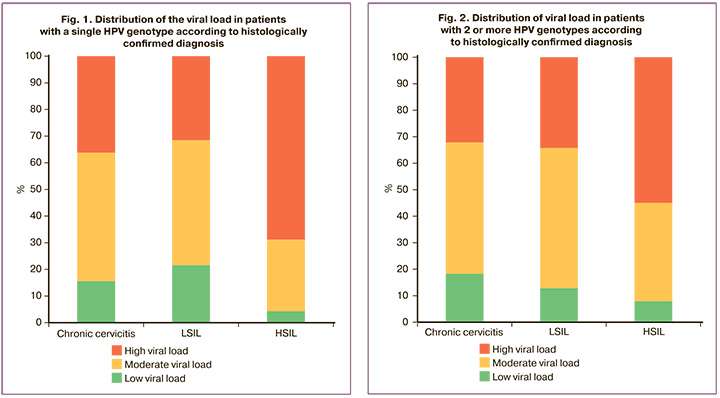
When comparing the incidence of low, moderate, and high viral loads in the structure of the studied cervical pathology between cases with detection of one or more HPV types, no statistically significant difference was found (p>0.05).
It was found that detection of a high viral load was associated with a statistically significantly higher risk of HSIL than LSIL, regardless of HPV genotype (OR=5.26; 95% CI 3.19–8.64). A similar trend was observed with moderate viral loads (OR=2.32; 95% CI 1.78–3.03). In addition, patients with low viral loads had a statistically significant higher incidence of LSILs than HSILs (OR=3.09; 95% CI 1.91–4.99).
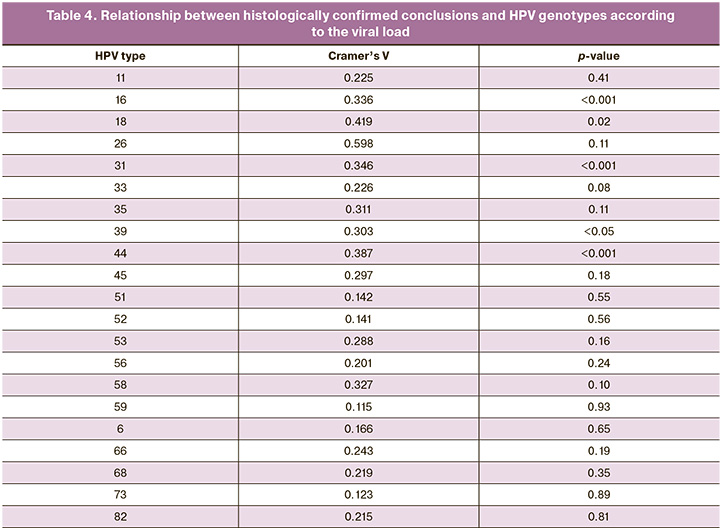
For each HPV genotype, the relationship between the histologically confirmed findings and HPV viral load was calculated (Table 4). The data obtained indicated the presence of a positive relationship of average strength between the viral load of HPV genotype 16 and the grade of cervical lesions (p<0.0001). Thus, with a high viral load of type 16 HPV, the development of HSIL was observed with the highest frequency, whereas at low and moderate loads, LSIL and chronic cervicitis were more common. It should be noted that HSIL was extremely rare at low viral loads. A positive, relatively strong relationship was found between the viral load of HPV genotype 18 and the conclusion of the histological examination protocol (p<0.05). Thus, LSIL was more often detected at low and moderate loads; however, there were no cases of HSIL with a low viral load of HPV genotype 18. A positive association of average strength was also obtained for the 31, 39, and 44 HPV genotypes between the level of viral load and the degree of cervical lesions (p<0.001, p<0.05, and p<0.001, respectively). LSIL was more often observed at low and moderate viral loads of HPV type 31, but at high viral loads, HSIL was the most frequently detected. A characteristic feature of HPV genotype 39 was a higher frequency of HSIL and chronic cervicitis with a low viral load, and a dominant position of LSIL with a moderate one. For HPV genotype 44, a low viral load was more often associated with chronic cervicitis, a moderate viral load with LSIL, and a high viral load with HSIL. For the other HPV genotypes, this relationship was not statistically significant.
Discussion
Our findings indicate an association between HPV load and severity of cervical lesions. Although we did not observe a statistically significant difference in the frequency of various levels of viral load based on the detection of one or multiple HPV genotypes, our study revealed important results suggesting an increased risk of detecting more severe cervical lesions with a rising HPV viral load (OR=2.32 for moderate and OR=5.26 for high viral load). These findings align with those of numerous similar studies [19–21]. For instance, Liu et al. (2021) reported that the HPV viral load independently contributes to the risk of developing CIN III and cervical cancer. Notably, the likelihood of detecting CIN III significantly varied based on the classification of moderate or high viral load (OR=2.85 and OR=7.05, respectively) [23].
It is worth noting that for HPV types 16, 31, and 44, we found no statistically significant difference in the mean viral load between cases with one or multiple identified HPV genotypes. Conversely, for types 51, 56, 59, 66, 6, 68, 73, and 82, the trend indicated an increase in the average viral load with a higher number of identified HPV genotypes. These discrepancies in the comparative analysis results may stem from both insufficient sample size and unexplored factors, such as the potential synergistic or antagonistic relationships between HPV genotypes from different phylogenetic groups, which were not considered in our study.
However, we were able to identify a statistically significant pattern in which higher levels of HPV viral load were associated with more severe cervical lesions for HPV genotypes 16, 18, 31, 39, and 44.
Furthermore, our study revealed that, with age, the HPV viral load becomes significantly lower in patients with chronic cervicitis and LSIL (p=0.02 for both groups). However, these findings contradict the recent literature, such as the study by Zhou et al. (2023), which reported a 5.5-fold increase in the likelihood of detecting CIN III in patients with a high viral load of HPV genotype 16 compared to those with a low quantitative presence of HPV type 16 DNA [21]. Importantly, a high viral load of HPV genotype 16 was associated with a significantly less favorable prognosis for CIN III development in patients over 30 years of age (OR 30.7 vs. 86.7, p<0.00001). The authors also concluded that HPV genotypes 33, 31, 18, 35, and 58 showed a statistically significant trend toward an increased likelihood of higher-grade cervical lesions with an escalating viral load. However, such trends were not observed for other HPV types, likely because of the limited sample size in our study.
In our investigation, no statistically significant results were found for HPV types 6, 11, 26, 33, 35, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82 concerning the relationship between cervical lesion severity and viral load levels, presumably because of the insufficient number of identified cases of these HPV types.
Conclusion
HPV viral load has emerged as a crucial diagnostic marker, which is currently underappreciated due to challenges in interpreting quantitative HPV DNA results and the complexities of research in this domain. Nevertheless, the undeniable link between HPV load and cervical lesion severity emphasizes the need for further in-depth exploration of this issue.
References
- Shen T.-T., Long C.-Y., Wu M.-P. Favorable cervical cancer mortality-to-incidence ratios of countries with good human development index rankings and high health expenditures. BMC Women’s Health. 2023;23(1):284. https://dx.doi.org/10.1186/s12905-023-02423-y.
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72(1):7-33. https://dx.doi.org/10.3322/caac.21708.
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209-49. https://dx.doi.org/10.3322/caac.21660.
- WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition. World Health Organization; 2021.
- Portnoy A., Pedersen K., Trogstad L., Hansen B.T., Feiring B., Laake I. et al. Impact and cost-effectiveness of strategies to accelerate cervical cancer elimination: a model-based analysis. Prev. Med. 2021;144:106276.https://dx.doi.org/10.1016/j.ypmed.2020.106276.
- Hall M.T., Simms K.T., Lew J.B., Smith M.A., Brotherton J., Saville M. et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:19-27. https://dx.doi.org/10.1016/S2468-2667(18)30183-X.
- Burger E.A., Smith M.A., Killen J., Sy S., Simms K.T., Canfell K., Kim J.J. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):e213-e222.https://dx.doi.org/10.1016/S2468-2667(20)30006-2.
- Castanon A., Rebolj M., Sasieni P. Is a delay in the introduction of human papillomavirus-based cervical screening affordable? J. Med. Screen. 2019;26(1):44-9. https://dx.doi.org/10.1177/0969141318800355.
- Kojalo U., Tisler A., Parna K., Kivite-Urtane A., Zodzika J., Stankunas M. et al. An overview of cervical cancer epidemiology and prevention in the Baltic States. BMC Public Health. 2023;23(1):660. https://dx.doi.org/10.1186/s12889-023-15524-y.
- Bosch F.X., Muñoz N., de Sanjosé S., Izarzugaza I., Gili M., Viladiu P. et al. Risk factors for cervical cancer in Colombia and Spain. Int. J. Cancer. 1992;52(5):750-8. https://dx.doi.org/10.1002/ijc.2910520514.
- Cuzick J., Terry G., Ho L., Hollingworth T., Anderson M. Type-specific human papillomavirus DNA in abnormal scrapes as a predictor of high-grade cervical intraepithelial neoplasia. Br. J. Cancer 1994;69(1):167-71.https://dx.doi.org/10.1038/bjc.1994.28.
- Амирханян А.С., Прилепская В.Н., Байрамова Г.Р., Бурменская О.В., Костава М.Н., Асатурова А.В. Хронический цервицит: современные возможности диагностики и лечения. Акушерство и гинекология. 2018;4:22-7. [Amirkhanyan A.S., Prilepskaya V.N., Bairamova G.R., Burmenskaya O.V., Kostava M.N., Asaturova A.V. Chronic cervicitis: current opportunities for diagnosis and treatment. Obstetrics and Gynecology. 2018;(4):22-7.(in Russian)]. https://dx.doi.org/10.18565/aig.2018.4.22-27.
- Андреев А.О., Байрамова Г.Р., Зарецкий А.Р., Ребриков Д.В. Современные представления о ВПЧ как о мультифакторном предикторе развития плоскоклеточных интраэпителиальных поражений шейки матки. Акушерство и гинекология. 2022;11:60-6. [Andreev A.O., Bairamova G.R., Zaretsky A.R., Rebrikov D.V. Current concepts of HPV as a multifactorial predictor for squamous intraepithelial lesions of the cervix. Obstetrics and Gynecology. 2022;(11):60-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.60-66.
- Lorincz A.T., Castle P.E., Sherman M.E., Scott D.R., Glass A.G., Wacholder S. et al. Viral load of human papillomavirus and risk of CIN3 or cervical cancer. Lancet. 2002;360(9328):228-9. https://dx.doi.org/10.1016/S0140-6736(02)09463-1
- Castle P.E., Schiffman M., Wheeler C.M. Hybrid capture 2 viral load and the 2-year cumulative risk of cervical intraepithelial neoplasia grade 3 or cancer. Am. J. Obstet. Gynecol. 2004;191(5):1590-7. https://dx.doi.org/10.1016/j.ajog.2004.05.018.
- Castle P.E., Schiffman M., Scott D.R., Sherman M.E., Glass A.G., Rush B.B. et al. Semiquantitative human papillomavirus type 16 viral load and the prospective risk of cervical precancer and cancer. Cancer Epidemiol. Biomarkers Prev. 2005;14(5):1311-4. https://dx.doi.org/10.1158/1055-9965.EPI-04-0799
- Sherman M.E., Wang S.S., Wheeler C.M., Rich L., Gravitt P.E, Tarone R., Schiffman M. Determinants of human papillomavirus load among women with histological cervical intraepithelial neoplasia 3: dominant impact of surrounding low-grade lesions. Cancer Epidemiol. Biomarkers Prev. 2003;12(10):1038-44.
- Коган Е.А., Файзуллина Н.М., Ли Ц., Демура Т.А., Жарков Н.В., Козаченко А.В., Чернова В.Ф., Байрамова Г.Р., Прилепская В.Н. Ранняя диагностика ВПЧ-ассоциированной патологии шейки матки у женщин до 30 лет и старше. Акушерство и гинекология. 2015;9:62-7. [Kogan E.A., Faizullina N.M., Li Ts., Demura T.A., Zharkov N.V., Kozachenko A.V., Chernova V.F., Bairamova G.R., Prilepskaya V.N. Early diagnosis of HPV-associated disease of the cervix uteri in women aged less than 30 years or older. Obstetrics and Gynecology. 2015;(9):62-7. (in Russian)].
- Adcock R., Cuzick J., Hunt W.C., McDonald R.M., Wheeler C.M.; New Mexico HPV Pap Registry Steering Committee. Role of HPV genotype, multiple infections, and viral load on the risk of high-grade cervical neoplasia. Cancer Epidemiol. Biomarkers Prev. 2019;28(11):1816-24.https://dx.doi.org/10.1158/1055-9965.EPI-19-0239.
- Oyervides-Muñoz M.A., Pérez-Maya A.A., Sánchez-Domínguez C.N., Berlanga-Garza A., Antonio-Macedo M., Valdéz-Chapa L.D. et al. Multiple HPV infections and viral load association in persistent cervical lesions in Mexican women. Viruses. 2020;12(4):380. https://dx.doi.org/10.3390/v12040380.
- Zhou Y., Shi X., Liu J., Zhang L. Correlation between human papillomavirus viral load and cervical lesions classification: A review of current research. Front. Med. (Lausanne). 2023;10:1111269. https://dx.doi.org/10.3389/fmed.2023.1111269
- Андреев А.О., Байрамова Г.Р., Ильясова Н.А., Асатурова А.В., Трофимов Д.Ю. Ретроспективный анализ распространенности вируса папилломы человека у женщин с патологией шейки матки. Акушерство и гинекология. 2023;11:140-9. [Andreev A.O., Bayramova G.R., Ilyasova N.A., Asaturova A.V., Trofimov D.Yu. Retrospective analysis of human papillomavirus prevalence in women with cervical pathology. Obstetrics and Gynecology. 2023;(11):140-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.225.
- Liu Y., Xu C., Pan J., Sun C., Zhou H., Meng Y. Significance of the viral load of high-risk HPV in the diagnosis and prediction of cervical lesions: a retrospective study. BMC Women’s Health. 2021;21(1):353. https://dx.doi.org/10.1186/s12905-021-01493-0.
Received 10.11.2023
Accepted 27.12.2023
About the Authors
Guldana R. Bayramova, Dr. Med. Sci., Merited Doctor of the Russian Federation, Professor at the Department of Obstetrics and Gynecology of the Department of Professional Education, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Clinical Care Supervisor at the Research and Outpatient Department, V.I. KulakovNMRC for OG&P, Ministry of Health of Russia, +7(926)660-48-77, bayramova@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0003-4826-661X
Alexander O. Andreev, Ph.D. Student, specialty “obstetrics and gynecology”, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(903)613-00-26,
sasha.grash2010@yandex.ru, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0002-9835-440X
Natalya A. Ilyasova, Researcher at the Department of International Cooperation, Obstetrician-Gynecologist at the Research and Outpatient Department, V.I. Kulakov
NMRC for OG&P, Ministry of Health of Russia, natalia_ilyasova@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0003-0665-3515
Anna V. Tregubova, Junior Researcher at the 1st Pathological Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(495)438-23-11,
a_tregubova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0003-4601-1330
Alina S. Badlaeva, Junior Researcher at the 1st Pathological Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, https://orcid.org/0000-0001-5223-9767
Dmitry Yu. Trofimov, Corresponding Member of the RAS, Professor, Dr. Bio. Sci., Director of the Institute of Reproductive Genetics, V.I. Kulakov NMRC for OG&P,
Ministry of Health of Russia, +7(495)438-49-51, d_trofimov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.



