Проблема бесплодия во всем мире является одной из самых актуальных. Согласно статистическим данным Всемирной организации здравоохранения (ВОЗ), около 48 млн пар и 186 млн людей страдают бесплодием [1], в России каждая шестая пара сталкивается с проблемами планирования семьи [2]. Овариальная стимуляция (ОС) является неотъемлемой частью экстракорпорального оплодотворения (ЭКО), позволяющей получить достаточное количество ооцитов, которые затем оплодотворяются in vitro методами ЭКО/ИКСИ. К настоящему времени нет международного или общенационального консенсуса по проведению программ ОС, в результате чего подбор оптимальной дозы фолликулостимулирующего гормона (ФСГ) для получения приемлемого количества ооцитов остается крайне сложным и субъективным, нередко решение о подборе необходимой дозы препарата принимается исходя из личного клинического опыта врача. Особенность ОС заключается в назначении гормональных препаратов, чаще всего фармацевтических аналогов человеческого ФСГ (чФСГ), при этом ответ яичников зависит от многих факторов: овариального резерва пациентки, массы тела, наличия различных сопутствующих заболеваний и т.д. При бедном ответе яичников значительно снижаются шансы получить бластоцисты хорошего качества, которые впоследствии могут привести к беременности и рождению здорового ребенка; при чрезмерном ответе яичников на стимуляцию может развиться крайне опасная для здоровья женщины ситуация – синдром гиперстимуляции яичников (СГЯ).
Фоллитропин дельта (Рековелль, Ferring Pharmaceuticals) представляет собой новый препарат рекомбинантного чФСГ (р-чФСГ), экспрессируемый с использованием клеточной линии человека (PER.C6), для которого разработан алгоритм индивидуального расчета дозировки с учетом концентрации антимюллерова гормона (АМГ) в сыворотке крови пациентки и ее массы тела. Использование алгоритма индивидуального дозирования позволяет уменьшить риск развития СГЯ без снижения эффективности стимуляции яичников.
В данной работе представлены обобщающие результаты клинических исследований ESTHER-1, ESTHER-2, MARCS, STORK и GRAPE, в ходе которых оценивались эффективность применения и профиль безопасности фоллитропина дельта при стимуляции яичников в программах преодоления бесплодия методами вспомогательных репродуктивных технологий (ВРТ) в различных странах.
Доказательное исследование применения рчФСГ для контролируемой стимуляции яичников в Европе и мире (ESTHER-1)
Особенности фармакокинетического/фармакодинамического профиля и отсутствие релевантного эталонного соединения для оценки биоактивности фоллитропина дельта с помощью теста Стилмана–Поли (Sleelman–Pohley) предопределили расчет дозировки по массе фармацевтической субстанции. Алгоритм дозирования фоллитропина дельта подразумевает расчет индивидуальной дозировки в зависимости от массы тела и концентрации АМГ в сыворотке крови пациентки, которая ежедневно вводится подкожно на протяжении всей стимуляции суперовуляции яичников. Эффективность индивидуального расчета дозировки фоллитропина дельта по сравнению со стратегией стандартного дозирования фоллитропина альфа оценивалась в первом проспективном исследовании, получившем название ESTHER-1 (от англ. Evidence-based Stimulation Trial with Human Recombinant Follicle-Stimulating Hormone in Europe and Rest of the World-1) [3].
Исследование ESTHER-1 проводилось в 37 исследовательских центрах, расположенных в 11 странах по всему миру: Бельгии, Бразилии, Канаде, Чехии, Дании, Франции, Италии, Польши, Российской Федерации, Испании и Великобритании. Участниками исследования стали 1326 женщин в возрасте 18–40 лет с индексом массы тела от 17,5 до 32,0 кг/м2 и регулярными менструальными циклами продолжительностью 24–35 дней, которые впервые обратились за помощью с применением методов ЭКО/ИКСИ, при этом причиной бесплодия был один из следующих диагнозов: бесплодие неясного генеза, трубный фактор бесплодия, эндометриоз I–II степени и мужское бесплодие. Дополнительными главными критериями включения в исследование были наличие обоих яичников и концентрация эндогенного ФСГ в сыворотке крови в ранней фолликулярной фазе не более 15 МЕ/л. Пациентки с эндометриозом III–IV степени, привычным невынашиванием беременности в анамнезе и использованием гормональных препаратов (за исключением гормонов щитовидной железы) во время последнего менструального цикла перед началом стимуляции не включались в состав участников исследования [3].
Женщины распределялись случайным образом в соотношении 1:1 в анализируемую и контрольную группы с учетом возраста: 35, 35–37 и 38–40 лет. Таким образом, в группу приема фоллитропина дельта и группу приема фоллитропина альфа были включены 665 и 661 пациентка соответственно. Сформированные группы пациенток были сопоставимы по возрасту, массе тела, индексу массы тела (ИМТ), количеству антральных фолликулов (КАФ) и концентрации АМГ (табл. 1).

В группе приема фоллитропина дельта препарат вводился подкожно в дозировке, индивидуально рассчитанной на основании сывороточной концентрации АМГ и массы тела пациентки в течение всего цикла стимуляции. При концентрации АМГ менее 15 пмоль/л ежедневная доза фоллитропина дельта была максимально допустимой и составляла 12 мкг; при концентрации АМГ в сыворотке крови выше 15 пмоль/л дозировка препарата подбиралась из расчета 0,10–0,19 мкг/кг/сут, но не более максимально допустимой 12 мкг. В группе приема фоллитропина альфа препарат вводился подкожно в стандартной дозировке (150 МЕ/сут) в течение первых 5 дней, с 6-го дня стимуляции дозировка могла быть скорректирована в зависимости от ответа яичников на стимуляцию, с максимальной допустимой суточной дозой фоллитропина альфа 450 МЕ/сут. Во всех группах исследования стимуляция яичников препаратами р-чФСГ начиналась на 2–3-й день менструального цикла, а препарат антагониста гонадотропин-рилизинг-гормона (ГнРГ) (Цетротид) назначался с 6-го дня ОС в дозировке 0,25 мг подкожно ежедневно. Триггер назначался при наличии трех и более фолликулов с диаметром от 17 мм. Женщинам с количеством фолликулов менее 25 с диаметром от 12 мм в качестве триггера вводили рекомбинантный хорионический гонадотропин человека (ХГЧ) (хориогонадотропин альфа, Овитрель, EMD Serono) в дозировке 250 мкг, подкожно. Женщинам с 25–35 фолликулами, диаметр которых оценивался более 12 мм, вводили препарат агониста ГнРГ (трипторелина ацетат, Ferring Pharmaceuticals) в дозировке 0,2 мг. Цикл исключали из исследования при ожидаемом количестве фолликулов более 35 (с диаметром от 12 мм) и плохом ответе яичников на стимуляцию, когда были опасения не получить три или более фолликулов с диаметром ≥7 мм к 20-му дню стимуляции. Пункцию фолликулов проводили через 36±2 ч после введения триггера, оплодотворение ооцитов проводили методами ЭКО или ИКСИ с использованием спермы партнера или донора. При введении в качестве триггера агониста ГнРГ все полученные эмбрионы криоконсервировали.
Для женщин, получивших ХГЧ, одна бластоциста была перенесена на 5-й день для всех женщин в возрасте ≤37 лет, а для женщин в возрасте ≥38 лет при наличии бластоцисты качества 3BB и выше. В остальных случаях было перенесено 2 бластоцисты. Оставшиеся бластоцисты криоконсервировали.
В качестве первичных конечных точек были выбраны частота продолжающейся беременности на 10–11-й неделе после переноса и частота имплантации эмбриона, определяемая как количество внутриутробных жизнеспособных плодов через 10–11 недель после переноса. Анализ полученных данных продемонстрировал сопоставимую эффективность стимуляции препаратами фоллитропина дельта и фоллитропина альфа в отношении первичных конечных точек (табл. 2).
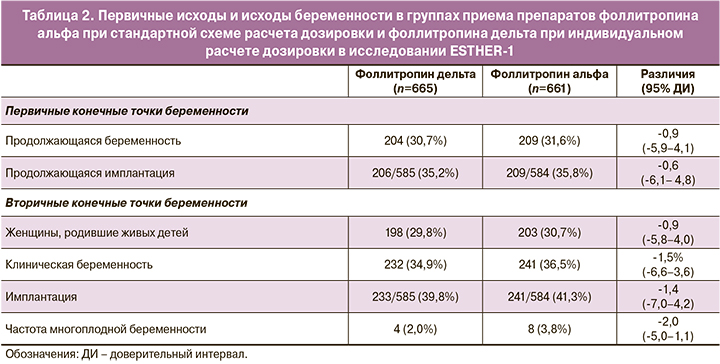
Вторичными конечными точками стали исход беременности, частота живорождений (определяется как рождение по меньшей мере одного живого ребенка), целевой ответ яичников (8–14 ооцитов), диапазон ответа яичников ниже/выше целевого (<4, ≥15 или ≥20 ооцитов), эмбриологические показатели, безопасность и нежелательные явления, доля женщин с развитием раннего и позднего СГЯ (включая СГЯ средней/тяжелой степени, классифицированные с использованием системы Голана) и/или профилактическими вмешательствами в отношении раннего СГЯ (т. е. отмена цикла из-за избыточного ответа яичников, замена триггера на агонист ГнРГ или применение агониста дофамина у женщин с ≥20 фолликулами диаметром ≥12 мм).
Достижение целевого ответа яичников с получением 8–14 ооцитов во время пункции позволяет получить достаточное количество ооцитов для развития оптимального числа бластоцист и в то же время снизить риск чрезмерного ответа яичников и избежать СГЯ. Как показано в таблице 3, целевой ответ яичников чаще достигался в группе с индивидуальным расчетом дозировки фоллитропина дельта по сравнению со стандартным дозированием фоллитропина альфа (43,3 и 38,4% при р=0,019 соответственно). Стоит отметить, что целевой ответ с получением в среднем 8 ооцитов также был достигнут в группе пациенток с низким АМГ <15 пмоль/л.
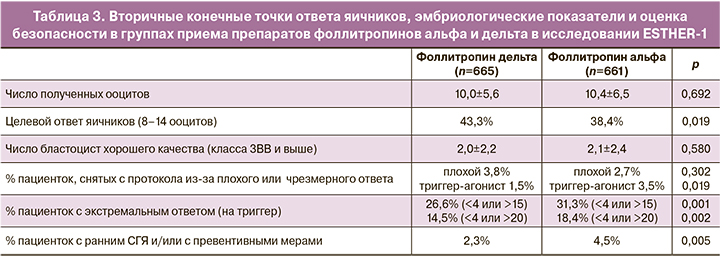
Согласно представленным данным, в группе приема фоллитропина дельта во время пункции реже получали менее 4 ооцитов (18 и 12% случаев в группах приема фоллитропина альфа и дельта соответственно), а также значительно различалось количество пункций с большим количеством полученных ооцитов. Так, в 35 и 28% случаев в группах приема фоллитропина альфа и фоллитропина дельта соответственно во время пункции получали более 15 ооцитов, из которых в 16 и 10% случаев количество клеток достигало более 25 ооцитов. Анализ зависимости числа получаемых ооцитов и концентрации АМГ в сыворотке крови пациенток показал, что применение индивидуального протокола подбора дозировки фоллитропина дельта позволяет избежать резкого увеличения количества ооцитов при увеличении уровня АМГ, что снижает риски развития СГЯ, при этом снижения числа бластоцист хорошего качества не происходит (рис. 1).
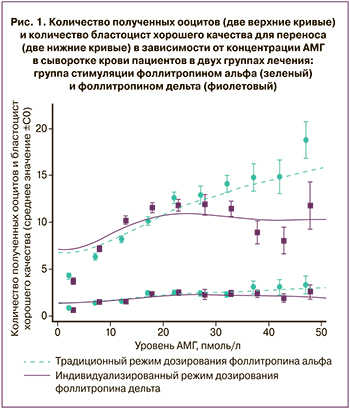
Таким образом, в ходе исследования ESTHER-1 доказаны эффективность индивидуального расчета дозировки фоллитропина дельта по сравнению с обычным режимом дозирования фоллитропина альфа, а также сопоставимость исследуемых препаратов в отношении первичных конечных точек с достижением более целенаправленного ответа яичников с меньшими рисками развития СГЯ без значительного снижения количества бластоцист хорошего качества.
Доказательное исследование применения рчФСГ для контролируемой стимуляции яичников в Европе и мире (ESTHER-2)
Изучение профиля безопасности фоллитропина дельта было продолжено в рамках исследования, получившего название ESTHER-2 (от англ. Evidence-based Stimulation Trial with Human Recombinant Follicle-Stimulating Hormone in Europe and Rest of the World-2).
Участниками исследования стали пациентки, которые принимали участие в испытании эффективности препарата в рамках исследования ESTHER-1, но не достигли продолжающейся беременности. Для оценки иммунного ответа, а также эффективности фоллитропина дельта при повторных циклах стимуляции с точки зрения ответа яичников, частоты наступления беременности и живорождений пациенткам назначался второй курс приема фоллитропина дельта, а затем и третий, если в предыдущую стимуляцию не наступала беременность (рис. 2). Таким образом, исследование ESTHER-2 проводилось в 32 исследовательских центрах, расположенных в 10 странах: Бельгии, Бразилии, Канаде, Чехии, Дании, Италии, Польше, Российской Федерации, Испании и Великобритании [4].
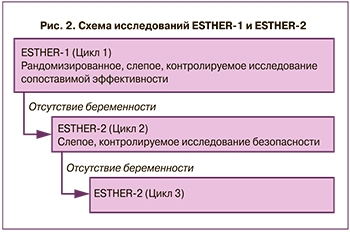
Женщины распределялись случайным образом в соотношении 1:1 в анализируемую и контрольную группы с учетом возраста. Пациенты с тяжелым СГЯ в предыдущем цикле или с любым клинически значимым изменением любого из критериев отбора в рамках ESTHER-1 или клинически значимыми изменениями в истории болезни с момента предыдущего цикла исключались из исследования. Таким образом, в группу приема фоллитропина дельта и группу приема фоллитропина альфа были включены 252 и 261 пациенток соответственно при второй стимуляции; 95 и 93 пациентки – при третьей. В отличие от исследования ESTHER-1, в исследовании ESTHER-2 фоллитропин альфа назначался с учетом овариального ответа в предыдущем цикле (табл. 4).

Доля пациенток, которые сохранили ту же дозировку/начальную дозировку во 2-м цикле стимуляции, составила 40,9% против 33,3% при приеме фоллитропина дельта и фоллитропина альфа соответственно; в третьем цикле стимуляции – 43,2% против 41,9% соответственно.
В качестве первичной конечной точки была определена доля женщин с индуцированными лечением анти-ФСГ антителами после двух повторных циклов стимуляции яичников, вторичная конечная точка при исследовании иммуногенности фоллитропина дельта оценивала долю женщин с нейтрализующими антителами и антителами, индуцированными лечением, по циклам (общими и нейтрализующими). Антитела к ФСГ измеряли до и после введения препарата пациенткам, которым проводилось до трех повторных циклов лечения. Показано, что частота появления антител к ФСГ после стимуляции фоллитропином дельта составила 1,05% – в 1-м, 0,79% – во 2-м и 1,05% – в 3-м цикле. У всех пациентов с антителами к ФСГ титры были неопределяемыми или очень низкими и не имели нейтрализующей способности. Повторное лечение препаратом фоллитропина дельта пациентов с ранее существовавшими или вызванными лечением антителами к ФСГ не увеличивало титр антител, не было связано со снижением ответа яичников и не вызывало побочных эффектов, связанных с иммунитетом (табл. 5) [5].
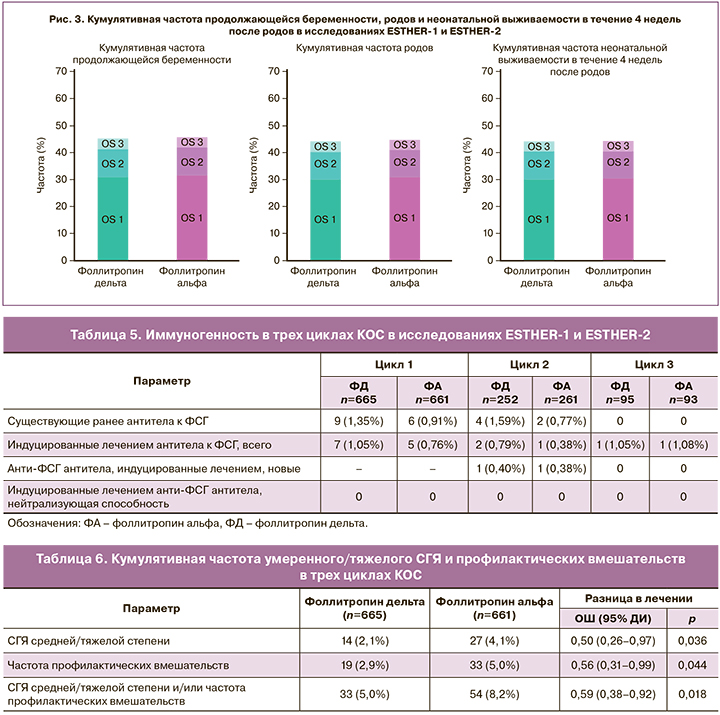
Другими вторичными конечными точками исследования стали наступление беременности, частота живорождений, ответ яичников на стимуляцию, эмбриологические показатели, побочные явления, частота применения превентивных мер для предотвращения развития СГЯ и частота возникновения СГЯ.
Сравнение кумулятивных данных по частоте достижения продолжающейся беременности, родов и неонатальной выживаемости в течение 4 недель после родов в результате третьей стимуляции препаратами фоллитропина альфа и дельта показало сопоставимую эффективность (рис. 3) [4].
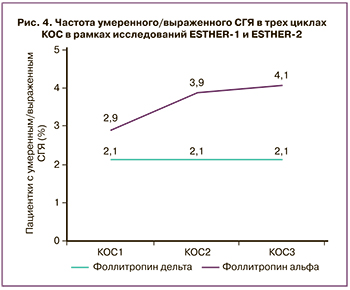
Индивидуальный подбор дозы фоллитропина дельта позволяет значительно снизить частоту развития СГЯ, а также минимизировать проведение профилактических вмешательств для его предотвращения на протяжении всех трех циклов контролируемой овариальной стимуляции (КОС). В частности, частота умеренного/выраженного СГЯ была стабильной во всех трех циклах КОС при использовании фоллитропина дельта (примерно 2,1%), тогда как в группе фоллитропина альфа имелась тенденция к повышению частоты развития СГЯ (рис. 4).
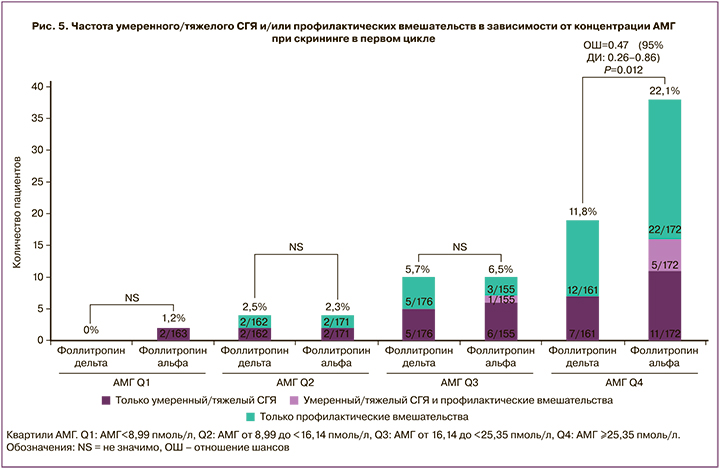
Кумулятивная частота умеренного/тяжелого СГЯ и профилактических вмешательств в трех циклах КОС представлена в таблице 6. Расчет индивидуальной дозы фоллитропина дельта по сравнению со стандартным протоколом фоллитропина альфа показал статистически значимые преимущества: развитие СГЯ средней/тяжелой степени составило 0,50 (95% ДИ 0,26–0,97 при р=0,036), необходимость профилактических вмешательств – 0,56 (95% ДИ 0,31–0,99 при р=0,044), СГЯ средней/тяжелой степени и/или профилактические вмешательства – 0,59 (95% ДИ 0,38–0,92 при р=0,018). Наибольшая статистическая значимость наблюдается в группе пациенток с АМГ более 25,35 пмоль/л (рис. 5).
Таким образом, результаты исследования ESTHER-2 показали низкую иммуногенность, а также подтвердили эффективность и безопасность индивидуального расчета дозировки фоллитропина дельта в повторяющихся циклах стимуляции яичников.
Комбинированное исследование препаратов Менопур и Рековелль (MARCS)
Результаты исследования ESTHER-1 показали сопоставимую эффективность индивидуально подобранной дозировки фоллитропина дельта по сравнению с традиционным режимом дозирования фоллитропина альфа в отношении частоты развивающейся беременности и живорождения, в то время как частота развития СГЯ была значимо ниже в группе индивидуального дозирования с помощью фоллитропина дельта. Однако есть данные, что у пациенток со сниженным овариальным резервом и неадекватным ответом в предыдущих циклах исходы контролируемой стимуляции яичников значительно улучшаются при добавлении лютеинизирующего гормона (ЛГ) во время стимуляции.
В комбинированном исследовании препаратов Менопур и Рековелль (MARCS, от англ. Menopur and Rekovelle Combination Study) авторы изучали новую схему, которая учитывала массу тела и сывороточную концентрацию АМГ каждой пациентки для определения подходящих доз гонадотропинов при стимуляции овуляции. Так называемые смешанные протоколы стимуляции яичников, в которых также дополнительно используют препараты человеческого мочевого высокоочищенного менопаузального гонадотропина (ЧМГ-ВО), как правило, применяются при попытке получить более качественные ооциты и эмбрионы и, следовательно, увеличить частоту наступления беременности по сравнению с использованием только рекомбинантного ФСГ у пациентов со сниженным овариальным резервом или у пациентов с неадекватным ответом яичников в предыдущих циклах стимуляции.
MARCS – многоцентровое открытое исследование, в которое были включены 110 пациенток в возрасте 18–40 лет с подтвержденным диагнозом «бесплодие» (включая эндометриоз I–II степени, трубный и мужской факторы бесплодия), регулярным менструальным циклом продолжительностью 24–35 дней, наличием обоих яичников и концентрацией ФСГ в ранней фолликулярной фазе менее 10 МЕ/л. Пациентки с эндометриозом III–IV степени, привычным невынашиванием беременности (более трех последовательных выкидышей) в анамнезе, высоким риском развития СГЯ (при концентрации АМГ≥35 пмоль/л) и использованием гормональных препаратов (за исключением гормонов щитовидной железы) во время последнего менструального цикла перед началом стимуляции не включались в состав участников исследования.
Все участницы исследования ежедневно получали индивидуальную дозировку фоллитропина дельта подкожно, рассчитанную по алгоритму в зависимости от массы тела и сывороточной концентрации АМГ, а также ЧМГ-ВО (Менопур): 75 МЕ/сут при дозе фоллитропина дельта ниже 12 мкг, 150 МЕ/сут при дозе фоллитропина дельта 12 мкг и массе тела менее 100 кг и 225 МЕ/сут ЧМГ-ВО вводили пациенткам с массой тела более 100 кг (табл. 7). Стимуляцию начинали со 2-го дня цикла и на 6-й день оценивали ее эффективность по количеству растущих фолликулов и сывороточной концентрации эстрадиола. В зависимости от уровня эстрадиола могла быть скорректирована дозировка ЧМГ-ВО с 6-го дня стимуляции при максимально допустимой дозировке 225 МЕ/сут. Триггер овуляции назначался при наличии трех и более фолликулов с диаметром от 17 мм. В качестве триггера вводился препарат ХГЧ в дозировке 5000–10 000 МЕ при концентрации эстрадиола менее 10 000 пмоль/л или агонист ГнРГ в дозировке 0,2 мг при больших значениях сывороточного эстрадиола. В качестве контрольной группы использовались данные из исследования ESTHER-1.
В качестве первичной конечной точки определяли количество бластоцист качества выше 3ВВ, полученных на 5-е и 6-е сутки культивирования эмбрионов. Вторичными конечными точками стали овариальный ответ, эмбриологические показатели и безопасность данного подхода стимуляции овуляции, включая оценку длительности стимуляции, суммарную дозу фоллитропина дельта, процент пациентов с вынужденной корректировкой дозировки, частоту использования препаратов агонистов ГнРГ в качестве триггера овуляции, количества полученных зрелых ооцитов при пункции, среднее количество эмбрионов на 3-и сутки культивирования, из которых впоследствии сформировались бластоцисты. Профиль безопасности стимуляции оценивался по частоте и серьезности развития раннего и позднего СГЯ (включая СГЯ средней/тяжелой степени, классифицированные с использованием системы Голана).
Сравнение основных показателей исследований ESTHER и MARCS показало, что в исследовании ESTHER пациентки были более молодыми и с более высоким уровнем АМГ, что, вероятно, стало причиной более высоких дозировок (общей и стартовой) фоллитропина дельта у пациенток в исследовании MARCS. В частности, максимально допустимая доза фоллитропина дельта (12 мкг/сут) в исследовании MARCS была назначена в 71% случаев, а также отмечалась более длительная продолжительность стимуляции и более высокая частота замены триггера. В то же время в исследовании MARCS было большее число полученных ооцитов и бластоцист. Интересно, что в отличие от исследования ESTHER-1 случаев СГЯ средней или тяжелой степени тяжести при применении комбинированного протокола Рековелль и Менопур отмечено не было (табл. 8).
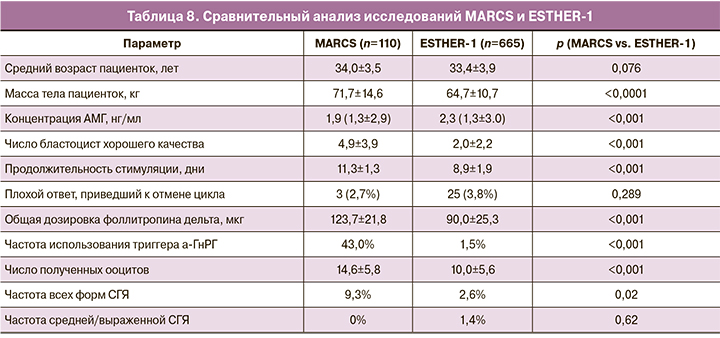
Стоит отметить, что в исследовании MARCS во всех возрастных группах в среднем получали 4 бластоцисты хорошего качества, при этом средний возраст и вес были выше, а концентрация АМГ ниже, чем у пациентов в исследовании ESTHER-1. В среднем количество бластоцист хорошего качества в исследовании MARCS составило 4,9±3,9, что практически в 2 раза больше среднего числа полученных бластоцист по всем возрастным группам в исследовании ESTHER-1 (2,0±2,2), при этом частота отмены цикла из-за бедного ответа яичников на стимуляцию была ниже [6].
Таким образом, в исследовании MARCS показано статистически значимое увеличение количества бластоцист хорошего качества на 5-й или 6-й день стимуляции с чуть повышенным риском развития СГЯ, не требующего медицинского вмешательства или госпитализации, при использовании смешанного протокола стимуляции яичников препаратами фоллитропина дельта и ЧМГ-ВО. Данная схема стимуляции особенно актуальна для пациентов старшей возрастной группы в возрасте старше 35 лет, однако для подтверждения эффективности выбора дозировки ЧМГ-ВО для пациенток моложе 35 лет необходимы дополнительные исследования в соответствующей возрастной группе.
Исследование по установлению зависимости ответа яичников от дозы фоллитропина дельта в циклах контролируемой овариальной стимуляции у японских женщин (STORK)
Эффективность индивидуального расчета дозировки в основном доказана на данных испытаний, проведенных в странах Европы, Северной и Южной Америки. Исследования ESTHER и MARCS не проводились в азиатских странах, где характеристики пациентов и/или диагностические маркеры овариального резерва могут значительно отличаться. В частности, азиатские женщины обычно характеризуются более низкими значениями ИМТ и разным составом тела при одинаковом ИМТ по сравнению с европейскими женщинами. Кроме того, сообщалось о значительно более низких концентрациях АМГ у китайских женщин по сравнению с женщинами европеоидной расы [7] и европейскими женщинами того же возраста [8]. Кроме того, исследования, проведенные в США и Великобритании, выявили этнические различия в результатах лечения, что подтверждает необходимость дополнительных исследований в популяциях из разных географических регионов. Так, в исследовании STORK сравнивалась реакция яичников, связанная с индивидуальной дозировкой фоллитропина дельта, по сравнению с обычным дозированием фоллитропина бета у японских женщин в циклах КОС.
Исследование по установлению зависимости ответа яичников от дозы фоллитропина дельта при проведении стимуляции яичников у японских женщин проводилось в 2014–2015 гг. в 10 исследовательских центрах в Японии с участием 158 женщин в возрасте 20–39 лет, с индексом массы тела от 17,5 до 32,0 кг/м2, регулярным менструальным циклом продолжительностью 24–35 дней, которые обратились за помощью с применением методов оплодотворения ЭКО/ИКСИ, при этом причиной бесплодия был один из следующих диагнозов: бесплодие неясного генеза, трубный фактор бесплодия, эндометриоз I/II степени и мужское бесплодие. Дополнительными главными критериями включения в исследование были наличие обоих яичников и концентрация эндогенного ФСГ в сыворотке крови в ранней фолликулярной фазе 1–12 МЕ/л и концентрация АМГ в диапазоне 5,0–44,9 пмоль/л. Пациентки с эндометриозом III–IV степени, привычным невынашиванием беременности, тремя и более попытками с применением методов оплодотворения ЭКО/ИКСИ в анамнезе и использованием гормональных препаратов (за исключением гормонов щитовидной железы) во время последнего менструального цикла перед началом стимуляции не включались в состав участников исследования [9].
Женщины распределялись случайным образом в соотношении 1:1:1:1, в зависимости от сывороточной концентрации АМГ: группа с низким значением АМГ (5,0–14,9 пмоль/л) и группа с высоким значением АМГ (15,0–44,9 пмоль/л), в которых ежедневно подкожно вводили по 6, 9 или 12 мкг/сут фоллитропина дельта (Рековелль) или 150 МЕ/сут фоллитропина бета на протяжении всего периода стимуляции. Во всех группах исследования стимуляция яичников препаратами р-чФСГ начиналась на 2–3-й день менструального цикла, а препарат антагониста ГнРГ (торговое наименование Ganirest, MSD K.K) назначался с 6-го дня ОС в дозировке 0,25 мг подкожно ежедневно. В качестве триггера препарат мочевого ХГЧ в дозировке 5000 МЕ подкожно назначался при наличии трех и более фолликулов с диаметром от 17 мм. Женщинам с количеством фолликулов более 25 и их диаметром от 12 мм назначался препарат агониста ГнРГ (Suprecur, Sanofi/Mochida Pharmaceuticals Co.) в дозировке 300 мг. Цикл исключали из исследования при ожидаемом количестве фолликулов более 35 (с диаметром от 12 мм) и при плохом ответе яичников на стимуляцию, когда были опасения не получить три или более фолликула с диаметром ≥10 мм к 10-му дню стимуляции.
Первичной конечной точкой данного исследования было число полученных ооцитов; вторичными конечными точками стали число зигот, особенность роста фолликулов, изменение сывороточной концентрации некоторых гормонов яичников, длительность стимуляции, частота наступления беременности и рождения. Профиль безопасности стимуляции оценивался по частоте и серьезности развития раннего и позднего СГЯ, наличию побочных реакций, в том числе в местах введения препаратов, а также появлению ассоциированным с лечением анти-ФСГ-антител [9].
При оценке параметров ответа яичников (число полученных ооцитов, число оплодотворенных ооцитов, концентрация эстрадиола, ингибина А и прогестерона) отмечена положительная зависимость от дозировки фоллитропина дельта (табл. 9). В то же время у пациентов контрольной группы (фоллитропин бета 150 МЕ/сут) в среднем было получено 11 ооцитов, что сопоставимо с результатами в группах приема фоллитропина дельта в дозировке 9–12 мкг/сут. Разделение пациенток по группам в зависимости от сывороточной концентрации АМГ позволило зафиксировать получение от 3 до 6 дополнительных ооцитов у пациенток с высоким уровнем АМГ при сравнении с пациентками с более низкими концентрациями гормона на фоне приема одинаковых доз фоллитропина дельта.
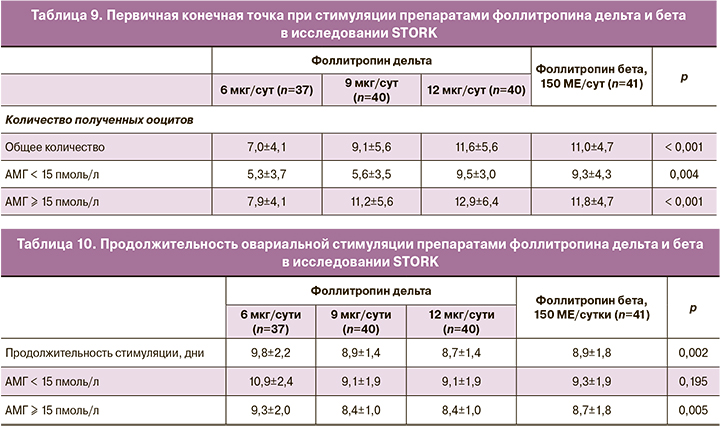
При оценке продолжительности стимуляции также очевидна прямая зависимость от дозировки препарата фоллитропина дельта. В частности, средняя продолжительность стимуляции значительно уменьшалась в группах приема 9–12 мкг/сут фоллитропина дельта. Сывороточная концентрация АМГ также имела прямое влияние на длительность стимуляции (табл. 10) [9].
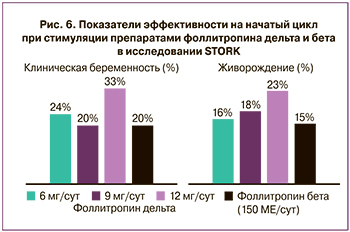
Частота наступления клинической беременности в ответ на начатый цикл в группе фоллитропина дельта варьировалась в группах приема, но была не ниже контрольной группы. Так, в группах приема 6, 9 и 12 мкг/сут частота наступления клинической беременности составила 24, 20 и 33% соответственно, в то время как в группе приема фоллитропина бета – 20%. Процент живорождения в группе фоллитропина дельта также незначительно превышал показатели в группе фоллитропина бета. Таким образом, максимально исследованная дозировка фоллитропина дельта 12 мкг/сут позволила достигнуть максимального ответа яичников, что было показано по частоте наступления беременности и живорождения (рис. 6).
Все случаи СГЯ регистрировались как нежелательные явления и классифицировались по степени тяжести. Частота СГЯ возрастала с увеличением дозировки фоллитропина дельта, причем в большинстве случаев (82%) развитие СГЯ наблюдалось у пациенток в группах с высокой концентрацией АМГ. Режим индивидуального расчета дозировки фоллитропина дельта привел к улучшенному профилю безопасности при сравнении с единой дозировкой в группе приема фоллитропина бета. В частности, в контрольной группе фоллитропина бета частота раннего СГЯ составляла 22%, при этом частота развития СГЯ в группах приема фоллитропина дельта составляла ниже 20% при любой дозировке, что ниже среднепопуляционной. Стоит отметить, что ни один из ранних случаев не был классифицирован как тяжелый (табл. 11).

Следующая фаза исследования STORK проходила в 2017–2019 гг. и проводилась в 17 исследовательских центрах Японии, ее участниками стали 347 женщин в возрасте 20–40 лет с аналогичными критериями включения и исключения [10].
Женщины распределялись случайным образом в соотношении 1:1 в зависимости от сывороточной концентрации АМГ: группа с низким значением АМГ (<15 пмоль/л) и группа с высоким значением АМГ (>15 пмоль/л). Ежедневная дозировка фоллитропина дельта составляла 12 мкг/сут в группе с низким значением АМГ и 0,10–0,19 мкг/кг/сут в зависимости от массы тела и концентрации АМГ в группе пациенток с высоким уровнем гормона. Стимуляция продолжалось от 6 до 12 дней, в контрольной группе пациенткам подкожно ежедневно вводили фоллитропин бета в дозировке 150 МЕ/сут в течение первых 5 дней, после чего дозировку могли корректировать с шагом 75 МЕ и максимально допустимой дозой 375 МЕ/сут.
Первичной конечной точкой данного исследования было число полученных ооцитов, вторичными конечными точками стали длительность стимуляции, суммарная доза гонадотропинов, распределение по группам числа полученных ооцитов, неадекватный ответ яичников (менее 4 ооцитов в группе с низкими значениями АМГ и более 15–20 ооцитов в группе с высокими значениями концентрации АМГ), частота наступления беременности и рождения. Профиль безопасности стимуляции оценивался по частоте и серьезности развития раннего и позднего СГЯ, превентивным мерам при риске развития СГЯ, отменам цикла или переноса эмбриона в свежем цикле, местной переносимости.
Одной из целей исследования было показать сопоставимую эффективность фоллитропина дельта и фоллитропина бета в отношении числа полученных ооцитов (при этом нижняя граница разницы между группами для демонстрации преимущества должна быть не менее 3 ооцитов), что и было продемонстрировано, как будет показано ниже. Общее количество ооцитов, полученных в группе фоллитропина дельта, составляло 9,3, тогда как в группе фоллитропина бета данный показатель составил 10,5. Что касается целевого ответа яичников, при котором получают 8–14 ооцитов, то в группе фоллитропина дельта его достигли в 40,8% случаев, тогда как в группе фоллитропина бета этот показатель составил 42,8% (табл. 12).
Среднее число ооцитов, полученных у пациенток с нормальным и высоким овариальным резервом, было в среднем на 2 меньше в группе фоллитропина дельта, чем в группе фоллитропина бета: 10,8±5,9 и 12,9±6,4 соответственно. Поскольку при высокой концентрации АМГ (>15 пмоль/л) повышается риск чрезмерного ответа яичников и СГЯ, пациенток, у которых получили больше 15 и больше 20 ооцитов, рассматривали по отдельности. Так, в группе приема фоллитропина дельта процент пациенток, у которых получили более 20 ооцитов, был значительно меньше. В группах приема препаратов пациентками с низким овариальным резервом в числе полученных ооцитов статистически значимых различий не зафиксировано. Данные по частоте наступления беременности и живорождения в группах приема фоллитропина дельта и бета были сопоставимыми и коррелировали с ранее полученными данными в предыдущем исследовании STORK: 23,5 и 18,6% в группах приема фоллитропина дельта и бета соответственно.
Несмотря на сравнительно одинаковую продолжительность стимуляции суперовуляции яичников и корректировки дозировки только в группе приема фоллитропина бета, ежедневная дозировка и общая суммарная дозировка в группе приема фоллитропина дельта были значительно ниже (табл. 13). Стоит отметить, что в группе приема фоллитропина бета стартовую дозу корректировали в 46,3% случаев.
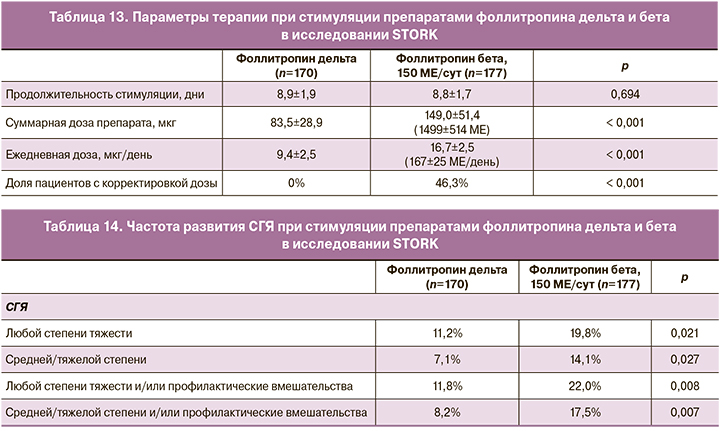
Частота СГЯ по всем показателям (частота развития СГЯ, степени тяжести, с применением профилактических мероприятий и т.д.) была значительно ниже в группе приема фоллитропина дельта (табл. 14) [10].
Таким образом, данное рандомизированное контролируемое исследование доказало эффективность фоллитропина дельта в отношении количества полученных ооцитов при сравнении с фоллитропином бета. Индивидуальный подбор дозы позволяет получать сопоставимое количество ооцитов, при этом значительно (в некоторых группах до 50%) снизить риск развития СГЯ, а также использовать меньшие количества гонадотропинов, что делает лечение с применением методов ЭКО/ИКСИ экономически более дешевым, а значит, более доступным [7].
Исследование по изучению фоллитропина дельта с его новым индивидуальным режимом дозирования в популяции азиатских пациентов (GRAPE)
Помимо разнообразия популяционных характеристик, клинические практики, такие как день переноса, количество перенесенных эмбрионов и другие важные аспекты, потенциально влияющие на показатели успеха, различаются в зависимости от географических регионов, что еще больше подчеркивает необходимость индивидуальных региональных клинических испытаний. Целью другого крупного рандомизированного контролируемого исследования, получившего название GRAPE, стало изучение фоллитропина дельта с его новым индивидуальным режимом дозирования в популяции азиатских пациентов, а также сравнение его эффективности и безопасности с фоллитропином альфа при использовании стандартного подхода дозирования среди пациенток нескольких азиатских стран: Китая, Южной Кореи, Тайваня и Вьетнама [11].
В исследовании GRAPE приняли участие 1009 пациенток в возрасте 20–40 лет с подтвержденным диагнозом «бесплодие» (включая эндометриоз I–II степени, трубный и мужской факторы бесплодия, бесплодие неясного генеза), регулярным менструальным циклом продолжительностью 24–35 дней, наличием обоих яичников и концентрацией ФСГ в ранней фолликулярной фазе от 1 до 15 МЕ/л, которые раньше не обращались за лечением с использованием методов ВРТ. Пациентки с эндометриозом III–IV степени, привычным невынашиванием беременности в анамнезе и наличием одного или более фолликулов диаметром более 10 мм на момент рандомизации не включались в исследование.
Пациентки были разделены поровну на три возрастные группы: менее 35 лет, 35–37 лет и 38–40 лет: 499 пациенток в группе приема фоллитропина дельта и 510 пациенток в контрольной группе приема фоллитропина альфа. Расчет дозировки фоллитропина дельта проводился на основании сывороточной концентрации АМГ и массы тела на момент рандомизации: при концентрации АМГ менее 15 пмоль/л дозировка фоллитропина дельта составляла 12 мкг независимо от массы тела; при концентрации АМГ в сыворотке крови более 15 пмоль/л дозировка составляла 0,10–0,19 мкг/кг/сут при минимально допустимой суточной дозировке 6 мкг и максимальной суточной дозировке 12 мкг. Стартовая дозировка в группе фоллитропина альфа составила 150 МЕ/сут, по истечении 5 дней стимуляции дозировка могла корректироваться в зависимости от индивидуального ответа яичников до максимально возможной суточной дозировки 450 МЕ/день. Все пациентки получали стандартный протокол с назначением антагонистов с 6-го дня стимуляции в дозировке 0,25 мг/сут до окончания стимуляции. Триггер назначали при наличии более трех фолликулов с диаметром от 17 мм. В качестве триггера выступали препараты ХГЧ при количестве фолликулов до 25 или агонисты рецептора ГнРГ при большем количестве. В случае бедного ответа яичников с визуализацией 1–2 фолликулов с диаметром от 17 мм при ультразвуковом исследовании или наличии более 35 фолликулов с диаметром более 12 мм пациента исключали из исследования.
Первичной конечной точкой данного исследования была частота продолжающейся беременности на сроке 10–11 недель после переноса эмбриона в свежем цикле, вторичными конечными точками стали частота наступления беременности, частота живорождения и неонатальная выживаемость в течение 4 недель, ответ яичников на стимуляцию (число ооцитов и зигот, достижение целевого ответа с получением 8–14 ооцитов, чрезмерный ответ яичников на стимуляцию с получением более 15 и 20 ооцитов у пациенток с концентрацией более 15 пмоль/л), изменения эндокринного профиля (эстрадиол, ингибины А и В, прогестерон), ежедневная дозировка и суммарная доза гонадотропинов. Профиль безопасности стимуляции оценивался по наличию побочных реакций, частоте и серьезности развития раннего и позднего СГЯ, наличию местных реакций в местах введения препаратов.
Средняя продолжительность стимуляции в группах приема фоллитропинов альфа и дельта составила 8,7±5,5 и 7,0±4,8 дня соответственно, с общей дозой препаратов 109,9±2,9 мкг (фоллитропин альфа) и 77,5±24,4 мкг (фоллитропин дельта) (табл. 15). Желаемый эффект по количеству полученных ооцитов (от 8 до 14 ооцитов) достигнут в 42,6 и 46,7% случаев при стимуляции фоллитропинами альфа и дельта соответственно. У женщин с риском развития СГЯ (с концентрациями АМГ >5 пмоль/л) после стимуляции фоллитропином альфа на пункции получали более 15 и 20 фолликулов в 39,1 и 18,5% соответственно; при стимуляции фоллитропином дельта – в 20,2 и 6,7% соответственно. Полученные данные коррелируют с результатами предыдущих исследований ESTHER-1 и STORK, которые показывают эффективность индивидуального расчета дозировки фоллитропина у пациентов с риском СГЯ. В ходе исследований ранняя степень СГЯ без обращения за медицинской помощью выявлена у 4,0% в группе приема фоллитропина дельта и 6,5% – в группе приема фоллитропина альфа; с превентивными мерами – 5,0 и 9,6% соответственно.
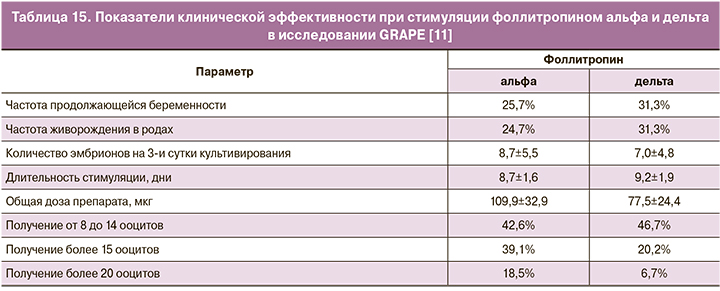
Таким образом, исследование GRAPE дополнительно доказывает, что, помимо снижения риска развития СГЯ на ранних стадиях, прием фоллитропина дельта в индивидуализированной схеме приема фиксированных доз позволяет повысить вероятность успеха в циклах ЭКО/ИКСИ для возрастных групп с более низким потреблением гонадотропина по сравнению с обычным дозированием фоллитропина альфа.
Фоллитропин дельта – рекомбинантный препарат чФСГ, продуцируемый с использованием линии клеток человека, показывает высокую эффективность и безопасность как в клинических исследованиях, так и в реальной клинической практике. Благодаря своим фармакокинетическим свойствам, а также четкому индивидуализированному алгоритму дозирования (основанному на уровне сывороточного АМГ и массе тела), использование фоллитропина дельта для ОС значительно снижает вероятность недостаточного или чрезмерного ответа яичников. Этот фактор имеет особенно важное значение при лечении таких пациенток, как женщины с низким овариальным резервом, с эндометриозом – группа риска по недостаточному ответу яичников, а также с высоким овариальным резервом и с синдромом поликистозных яичников (СПКЯ) – группа риска по чрезмерному ответу.
Фоллитропин дельта показал свою эффективность при применении у различных групп пациенток: с низким, нормальным и высоким овариальным резервом, в моно- и в микст-протоколах.
Клинический опыт применения фоллитропина дельта
Клинический опыт применения фоллитропина дельта у пациенток при низком овариальном резерве в смешанных протоколах
По данным исследований, ЛГ-активность в протоколах ОС необходима для того, чтобы обеспечить лучшее качество ооцитов и эмбрионов, а также более высокий уровень живорождения, особенно у пациенток старшего репродуктивного возраста или с низким показателем АМГ. Ниже представлен опыт применения фоллитропина дельта в смешанных протоколах у пациенток, установленным первичным и вторичным бесплодием с уровнем АМГ 0,67 нг/мл и ниже, с регулярным менструальным циклом и индексом массы тела в диапазоне 19,82–26,0. Возраст пациенток составил 42–43 года, у двух женщин в анамнезе отмечены проводимые ранее циклы ЭКО, в дополнение у двух пациенток были проведены хирургические вмешательства гинекологического профиля: правосторонняя аднексэктомия у одной пациентки и лапарогистероскопия, иссечение эндометриоидных узлов, удаление левой овариальной кисты – у другой (табл. 16). Назначенная терапия включала применение ЧМГ-ВО (Менопур) и фоллитропина дельта в комбинации с 1-го дня стимуляции. Стартовая доза ЧМГ-ВО составляла 150 МЕ и корректировалась при необходимости на 5–6-й день стимуляции по итогам ультразвукового исследования; доза фоллитропина дельта подбиралась в соответствии с алгоритмом и оставалась постоянной на протяжении всего протокола.
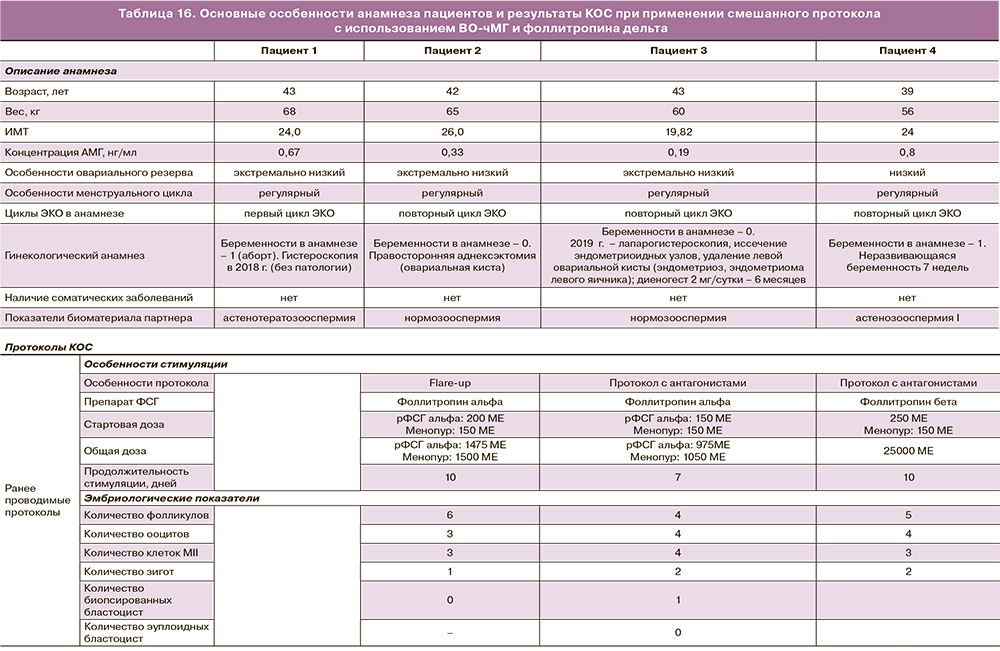
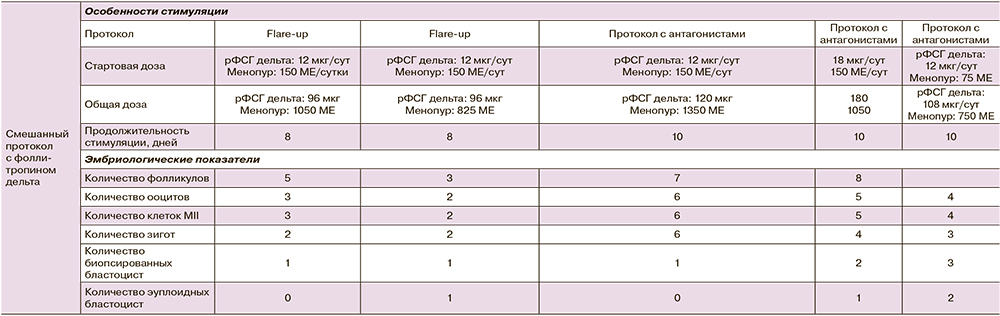
Полученные результаты дополнительно подтверждают эффективность применения фоллитропина дельта в комбинации с препаратами ЧМГ-ВО для индивидуализации ОС в рамках смешанного протокола, что позволяет получить эмбрионы хорошего качества, в том числе у пациенток с экстремально низким овариальным резервом и отягощенным анамнезом, в частности, с эндометриозом.
Клинический опыт применения фоллитропина дельта у пациенток с нормальным овариальным резервом в моно- и в смешанных протоколах
Ниже представлен опыт применения фоллитропина дельта в смешанных и моно- протоколах у пациенток с нормальным уровнем АМГ. Назначенная терапия включала применение ЧМГ-ВО (Менопур) и фоллитропина дельта в комбинации с 1-го дня стимуляции либо только фоллитропина дельта в дозировке, подобранной в соответствии с алгоритмом (табл. 17).
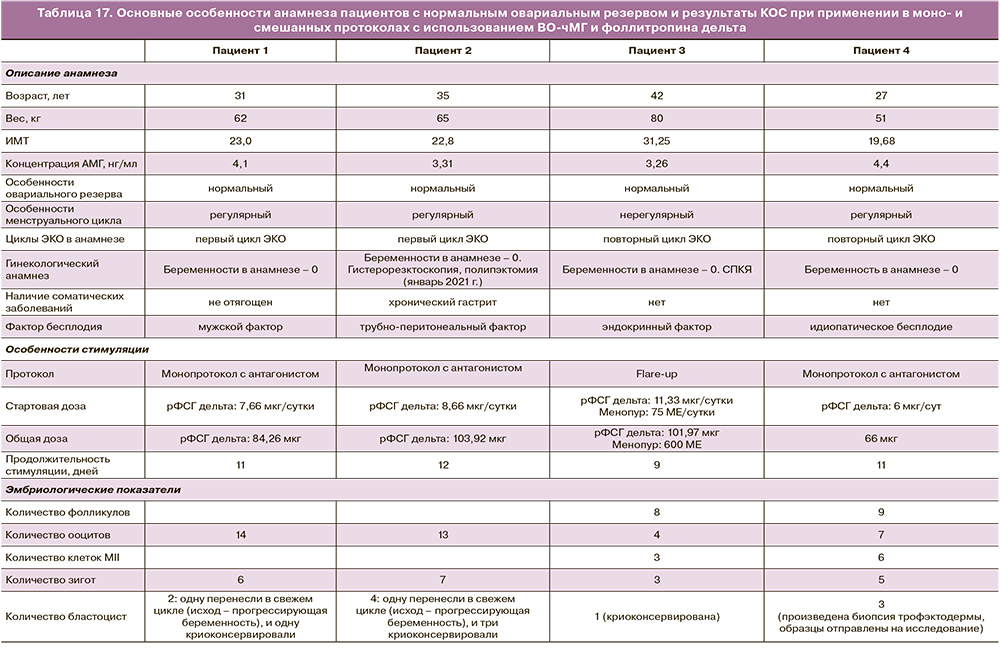
Клинический опыт применения фоллитропина дельта у пациенток с высоким овариальным резервом в монопротоколах
Ниже представлен опыт применения фоллитропина дельта в монопротоколах у пациенток с высоким уровнем АМГ (6,2 нг/мл и выше). Назначенная терапия включала применение фоллитропина дельта с первого дня стимуляции, в дозировке, подобранной в соответствии с алгоритмом (табл. 18).

Заключение
Полученный опыт применения фоллитропина дельта подтверждает эффективность фоллитропина дельта как в моно-, так и в смешанных протоколах у пациенток с низким, нормальным и высоким овариальным резервом. Фоллитропин дельта помогает достигнуть предсказуемого овариального ответа с минимизированием риска развития СГЯ, включая пациенток с эндокринным фактором бесплодия (в том числе с СПКЯ).



