A generalized action plan for obstetric hospitals and outpatient clinics during the suspected or confirmed COVID-19 pandemic
This summary paper presents a generalized action plan for hospitals and outpatient clinics during the COVID-19 coronavirus pandemic. Now the pandemic is the most relevant global challenge. Taking into account that the medical world does not have much clinical experience with COVID-19 and other coronaviruses, such as SARS-CoV and MERS-CoV, thoroughness and caution in assessing and treating pregnant women is an extremely urgent topic. The authors of this article summarized the world experience in managing patients with coronavirus disease and brought it into a unique algorithm that was considered most rational.Bettocchi S., Veretzky A., Ivanov D.O., Condo W., Krylov K.Yu., Lisi F., Petraglia F., Reznik V.A., Rukhlyada N.N., Saini S., Sanabria D.
Keywords
General principles
Unlike influenza and other respiratory diseases, the evidence for COVID-19 viral infection is based on a limited number of confirmed cases. Pregnant women do not appear to be at increased risk for the severe course of the disease. However, given the lack of data and clinical experience of dealing with this infection and other coronaviruses, such as SARS-CoV and MERS-CoV, accuracy and precision in the assessment and treatment of pregnant women is relevant.
Obstetric tactics are determined by several main aspects: the severity of the patient’s condition, the condition of the fetus, and gestational age. Pregnancy can be terminated after treatment of the infectious process in case of moderate or severe course of the disease up to 12 weeks gestation due to the high risk of perinatal complications associated with both exposure to viral infection and the embryotoxic effect of drugs. If the patient refuses to terminate the pregnancy, a chorionic villus or placenta villus biopsy is required up to 12–14 weeks or amniocentesis from 16 weeks of gestation to detect fetal chromosomal abnormalities [1].
Termination of pregnancy and delivery at the peak of the disease is associated with an increase in maternal mortality and a large number of complications: worsening of the underlying disease and the complications caused by it, the development and progression of respiratory failure, obstetric bleeding, intrapartum fetal death, postpartum purulent-septic complications [2]. However, if hypoxia cannot be eliminated using mechanical ventilation or in case of progressive respiratory failure, alveolar pulmonary edema, or refractory septic shock, it is essential to perform emergency abdominal delivery (cesarean section) according to the condition of the mother and fetus taking all necessary preventive measures for coagulopathic and hypotonic obstetric bleeding [3].
In pregnancy up to 20 weeks, emergency cesarean section may not be performed, since the pregnant uterus does not affect cardiac output during this period. At 20–23 weeks’ gestation emergency cesarean section is performed to save the life of the mother, but not the fetus, and in the period of more than 24 weeks’ gestation it is done to save the life of the mother and fetus.
In the case of the spontaneous onset of labor during the disease (pneumonia), delivery should be performed via the birth canal under close supervision of the condition of the mother and fetus.
The preferred method of analgesia is regional analgesia in the absence of contraindications. Antiviral, antibacterial, detoxification therapy, respiratory support are carried out according to indications.
In the second stage of labor, it is necessary to limit woman’s bearing down efforts to prevent the development of respiratory and cardiovascular failure. Vacuum extractor or obstetric forceps should be applied to accelerate the labor when necessary.
Cesarean section is performed when there are absolute obstetric indications and as an attempt to save the life of the fetus in case of inevitability of the mother’s lethal outcome [4].
Cesarean section in severe course of the disease should be provided with regional analgesia together with the respiratory support in the absence of signs of severe multiple organ failure (up to 2 points on the SOFA scale) or total intravenous anesthesia with mechanical ventilation in case of severe multiple organ failure [5].
Regardless of gestational age, all patients are administered the prevention of bleeding.
In all cases, the decision on time and mode of delivery is made individually.
Clinical criteria for discharge from hospital of pregnant women and women who have recently had a baby are:
- normal body temperature for 3 days;
- lack of symptoms of respiratory tract impairment;
- normal laboratory findings;
- the absence of obstetric complications (pregnancy, the postpartum period).
The patient is discharged from hospital after a double negative result of a laboratory test for SARS-CoV-2 RNA with PCR method within an interval of at least one day.
The prognosis for the mother and fetus depends on the gestational trimester when the disease occurred, the presence of premorbid background factors (smoking, obesity, underlying diseases of the respiratory system and ENT organs, diabetes mellitus, HIV infection), the severity of the infection process, the presence of complications and time of administering antiviral therapy [6].
Guidelines for obstetric management of patients with confirmed or suspected COVID-19
The medical staff appointed by the hospital administration should check patients and visitors in the admission department in accordance with the latest recommendations. They should identify patients with any of the following symptoms: cough, hyperthermia (body temperature over 38.0 C), shortness of breath. It is necessary to pay attention to the following facts from epidemiological anamnesis:
- visits to other countries or cities over the last 14 days;
- contact with patients with confirmed COVID-19 over the last 14 days;
When confirming one of the above signs, the medical professionals should immediately sanitize their hands, wear gloves and a protective mask and inform an epidemiology specialist [7].
Patients with positive COVID-19 screening and obstetric complaints should be admitted to maternity hospitals equipped with personal protective equipment (PPE). Obstetric complaints include uterine painful contractions with frequency of more than six per hour, uterine bleeding, rupture of membranes, decrease or termination of fetal movement (at the gestational age of ≥ 22 weeks) [8].
Pregnant patients at any gestational age without obstetric complaints and flu-like symptoms, respiratory symptoms should be referred to emergency hospitals. They should be consulted by an obstetrician-gynecologist additionally [9].
Examination of a pregnant patient should be carried out by the attending physician in the presence of the head of the department.
Ultrasonic sensors must be treated with an antiseptic solution in accordance with the manufacturer’s instructions, both before and after the examination of the patient.
Precautions for patients with a positive COVID-19 screening result and obstetric complaints
Preparing a separate room for examination of the patient:
- removal unnecessary equipment, furniture, trolleys, patrimonial balls, mirrors, etc.;
- removal of standard trash bags;
- providing the available amount of PPE;
- the location of all relevant PPE outside the premises in special for this purpose areas;
- providing PPE, including a mask, surgical cap, glasses, sterile gloves, disposable sterile underwear;
- at the entrance to the room where the patient is located, medical workers must put on all PPE in advance;
- when leaving the room where the patient with positive COVID-19 is located, the healthcare provider must remove the cap, protective gloves and sterile underwear; the mask must be disposed of and hands must be sterilized;
- keeping a journal with data on the medical personnel in contact with the patient;
- limit the number of medical workers in contact with the patient;
- furnishing the room with HEPA air filter (High Efficiency Particle Absorption).
Diagnostics
- Make differential diagnosis of acute respiratory diseases.
- Conduct PCR study for COVID-19, urinalysis.
- Do not send test samples through a pneumatic tube system.
- Perform chest radiography of patients with fever, complaints of shortness of breath, cough, as well as in the presence of these symptoms and a positive PCR test for COVID-19.
- Examine the patient for sepsis.
Childbirth [1]
Natural childbirth via the birth canal should take place in a prepared room with negative pressure (a method of isolating a room in which ventilation is performed in such a way that air can enter an isolated room, but not leave it, because the air from the outside will come from areas with a higher pressure to an area with a lower pressure and thereby prevent contaminated air to neighboring rooms) [10].
Patients who are to be performed cesarean section require additional preparation:
- Wear a disposable sterile medical cap in addition to the mask;
- Additional disinfection of the operating room with special solutions;
- ECG - monitoring;
- Consent for surgical intervention (cesarean section), anesthesia, blood transfusion;
- Epidural anesthesia is recommended to minimize aerosol procedures, intubation, etc.
- Limit the number of health workers present;
- Continuous monitoring of the fetus;
- Continuous pulse oximetry;
- Avoid oxygen supply through the nasal cannulas, without recirculating for intrauterine resuscitation;
- General cleaning of the room after transferring the patient to another department.
Anesthesia
COVID-19 diagnosis is not considered a contraindication for performing epidural anesthesia. Taking into account the long-term presence of the anesthetist at a close distance during the procedures, all anesthesiologic manipulations must be performed by the anesthetist who observes the basic precautions and wears the appropriate PPE [11].
Recommendations for performing epidural anesthesia:
Epidural anesthesia should be performed in an operating room where cesarean section is planned.
It is necessary to have two pairs of gloves when performing any anesthetic procedures;
The patient should wear a mask.
Recommendations for performing general anesthesia:
All PPE must be available;
A plastic curtain is required to reduce aerosolization on the top of the patient’s upper body and head;
Pre-oxygenation should occur with a HEPA filter from the side of the patient.
Extubation is equally dangerous or even more dangerous than intubation in terms of virus spread, therefore, the medical personnel located next to the patients during extubation should be minimized and all the above precautions should be observed;
NSAIDs can be safely used in patients without symptoms;
Antiemetics should be used to prevent vomiting in patients who underwent cesarean section. However, because of the potential risk in conditions of COVID infection, the use of dexamethasone for the prevention of postoperative vomiting in patients with suspected or confirmed COVID-19 should be avoided [12];
Putting on / taking off PPE takes time, therefore, emergency situations should be avoided; it is necessary to foresee the needs for PPE and maintain communication among all medical staff. PPE is essential for all medical personnel involved in cesarean surgery [13].
Pregnant patients with severe COVID-19
The intensive care unit which admits a patient with severe COVID-19 at the gestational age of ≥ 22 weeks should be completely prepared.
Before entering the room, personnel are required to disinfect their hands with antiseptic solutions;
The medical staff must take on PPE before entering the intensive care unit;
A respirator is required if the personnel are near the patient for a long time;
All medical surgical instruments necessary for surgical treatment including suture materials must be located outside the room;
If it is necessary to perform cesarean section, the patient should be transferred to a gurney, extra blankets and pillows should be removed.
Intubation should be performed according to the same recommendations as for performing anesthesia;
A newborn should be transferred to a room with negative pressure under intensive care [14].
Placenta
After birth the placenta of a patient with confirmed or suspected COVID-19 should be directed for studying possible pathology.
In addition to the usual referral, the diagnosis of COVID-19 must be clearly written on the container.
The placenta of a suspected or confirmed patient with COVID-19 should not be given out for burial, conservation, or other purposes [15].
Infant
The risks and benefits of temporary separation of the mother and infant should be discussed by healthcare providers with all patients with confirmed and suspected COVID-19 [1].
If a mother is confirmed with COVID-19:
- It is recommended to isolate the patient and the child from each other to prevent the spread of active infection to the newborn.
- If the patient refuses to be isolated from the infant, it is necessary to organize a consultation and ethics committee.
The infant should be placed in a ward with negative air pressure in the intensive care unit.
The infant should be tested for COVID-19.
If the test for COVID-19 is positive and the infant needs respiratory support, the doctors of the intensive care unit should coordinate their actions with the doctors of the children’s hospital for the most adequate care and possible transfer.
If the test for COVID-19 is negative, then the infant must be discharged from hospital within the standard period of time to the father, if he has a negative test for COVID-19.
Persons accompanying the mother of the infant, including her husband, should not be admitted to the intensive care unit to the newborn.
Mothers can be given video access to the infant.
The infant can be fed with expressed breast milk.
If a mother with confirmed or suspected COVID-19 does not have any symptoms of the disease:
Rooming-in with the newborn can be considered if requested.
It is possible to use technical means of control, such as a curtain or a barrier, to separate the infant from the mother.
The infant should be at a distance of more than 1.5 meters.
The mother should practice hand hygiene, wear gloves and a mask before touching the infant or breastfeeding.
The mask should remain in place while in contact with the infant.
If possible, breast milk should be given to the infant through a healthy family member or health care provider, as described below [16].
Breastfeeding
- There is currently no evidence that COVID-19 is transmitted through breast milk. It is safe and appropriate for the mother to give breast milk to the infant. However, coronaviruses can be transmitted from an infected mother to an infant during feeding. It is recommended that a healthy family member or healthcare provider should give the infant expressed breast milk of the mother with confirmed COVID-19. If the mother refuses, she should put on a mask and wash her hands 20 seconds before breastfeeding [17].
Expression of breast milk
- The patient will be provided with a sterilized individual breast pump.
- The nurse will instruct the patient about hand hygiene before touching the pump parts and before each pumping session.
- A separate sterilizer should be available for patients with COVID-19 to sterilize breast pump parts.
- The breast pump must be washed after every use.
- The breast pump parts must be sterilized after every use [18].
Room Cleaning
It is necessary to clean any ward where a patient with suspected COVID-19 was.
After installing HEPA filter, the room must remain empty for one hour for complete air circulation.
- All patients, staff, and visitors will be screened for COVID-19.
- A patient with confirmed or suspected COVID19 may be accompanied by a person (husband, relative), provided that they have no symptoms of the disease. In this case, the patient’s accompanying person may be nearby at the admission department, but should not be admitted to the intensive care unit.
- If an accompanying person leaves the hospital / maternity ward, he will not be allowed to return to the hospital / maternity ward.
- Patients with COVID-19 symptoms should not have visitors.
Etiotropic treatment of COVID-19 in pregnant women, women in labor, and women who have recently had babies [1]
The etiotropic treatment of women with COVID-19 during pregnancy and lactation is not currently developed. Recombinant interferon beta-1b is contraindicated during pregnancy. However, antiviral drugs as an etiotropic therapy can be prescribed, taking into account their effectiveness against the new coronavirus. In other cases, their safety during pregnancy and during breastfeeding should be taken into account. Prescribing the combination of lopinavir and ritonavir is possible if the potential benefit to the mother outweighs the potential risk to the fetus: 400 mg of lopinavir + 100 mg of ritonavir are prescribed every 12 hours for 14 days orally. If the preparation cannot be taken orally, these drugs (400 mg of lopinavir + 100 mg of ritonavir) are administered through a nasogastric tube in the form of a suspension (5 ml) every 12 hours for 14 days. Treatment should be started as early as possible, which to a greater extent ensures recovery. Antiviral drugs for pregnant women with a severe or progressive course of the disease must be prescribed at a later stage of the disease. When prescribing antiviral drugs to lactating women, the decision about breastfeeding depends on the severity of the symptoms.
Pathogenetic treatment of pregnant women, women in labor and women who have recently had babies [1]
The first-line antipyretic drug is paracetamol, which is prescribed at doses of 500-1000 mg up to 4 times per day (not more than 4 g per day).
Symptomatic treatment of pregnant women, women in labor and women who have recently had babies [1]
During pregnancy (II and III trimesters), in the postpartum and postabortion period, it is possible to use mucolytic agents (Ambroxol at a dose of 2–3 ml with isotonic solution in a ratio of 1:1 2–3 times a day) and bronchodilators (ipratropium bromide + phenoterol at a dose of 20 drops in 2–4 ml of isotonic solution twice a day). During pregnancy (I, II and III trimesters), in the postpartum and postabortion period, salbutamol can also be used as a bronchodilator (2.5–5 mg in 5 ml of isotonic solution twice a day). A necessary component of complex therapy is adequate respiratory support. Oxygen saturation should be determined in all pregnant women with clinical manifestations of acute respiratory illness and/or pneumonia [19]. Patient with coronavirus infection is transferred to the intensive care unit if she has rapidly progressing acute respiratory failure (respiratory rate > 25 per minute, SpO2 <92%, as well as multiple organ failure (2 or more points according to the SOFA scale).
Antibiotic therapy in pregnant women, women in labor and women who have recently had babies [1]
In complicated forms of infection, antibiotic therapy should be prescribed during the first 2–3 hours after hospitalization. Patients with a severe course of the disease are given antibacterial drugs intravenously.
In secondary viral and bacterial pneumonia (the most likely causative agents are Streptococcus pneumoniae, Staphylococcus aureus and Haemophilus influenza), it is preferable to use the following antibiotic therapy regimens:
- third-generation cephalosporin ± macrolide;
- protected aminopenicillin ± macrolide;
In tertiary bacterial pneumonia (the most likely pathogens are methicillin-resistant strains of Staphylococcus aureus, Haemophilus influenza), the following drugs are prescribed (in various combinations):
- fourth-generation cephalosporin ± macrolide;
- carbapenems;
- vancomycin;
- linezolid.
Antibacterial drugs contraindicated during pregnancy include tetracyclines, fluoroquinolones, sulfonamides [20, 21].
This algorithm (Fig.) is designed to help practitioners quickly evaluate and treat pregnant women with effect and / or symptoms of COVID-19. If the flu viruses still circulate in the woman’s body, the flu can cause the symptoms of respiratory disorders and doctors are advised to use the treatment and prevention algorithm designed for the flu.
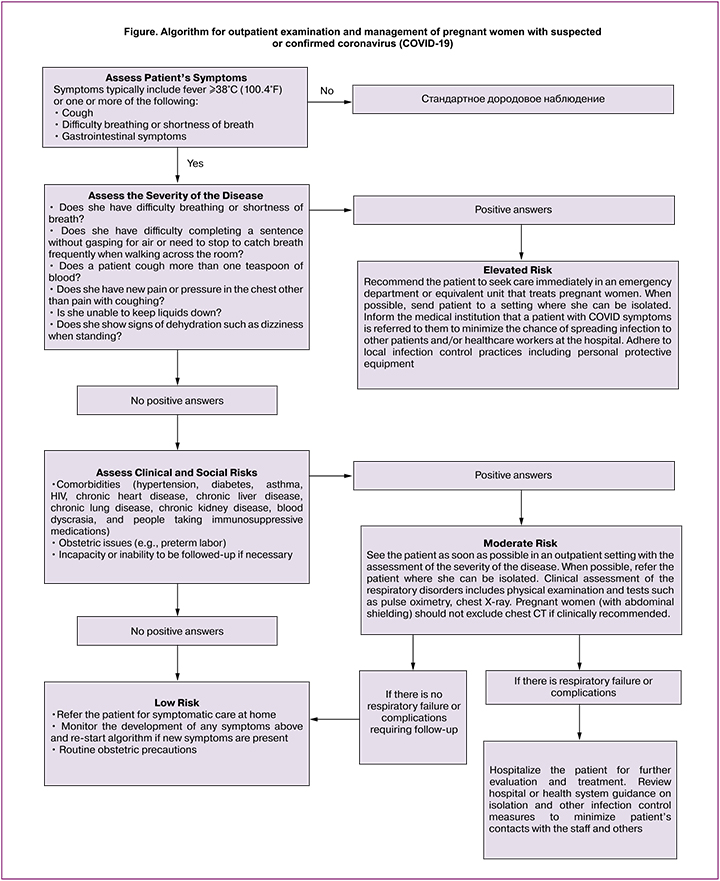
Conclusion
Nowadays, the problem of coronavirus infection is the main issue and the topic of discussion for almost all world powers. It is worth saying that to some extent the healthcare system was not ready for such an increase in the number of patients and the rapid progression of the disease. High incidence of the disease among medical personnel of hospitals and other medical institutions is of crucial importance. This is primarily due to the fact that world health service has not had the opportunity to accumulate clinical and practical experience dealing with the coronavirus infections SARS-CoV and MERSCoV. The world experience in managing patients in obstetric hospitals under the conditions of a coronavirus infection pandemic has been summarized in this article, and a recommendation algorithm has been developed for managing patients with this pathology. The authors hope that the recommendations described in this article will help the heads of medical organizations and medical practitioners in this important period for the whole world.
References
- Временные методические рекомендации «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19)» Версия 5. Утв. Министерством здравоохранения РФ 08.04.2020. [The temporary guidelines «Prevention, diagnosis and treatment of a novel coronavirus infection (COVID-19)» Version 5. Approved by the Ministry of Health of the Russian Federation on April 8, 2020.(in Rissian)].
- Alserehi H., Wali G., Alshukairi A., Alraddadi B. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect. Dis. 2016; 16: 105. https://dx.doi.org/10.1186/s12879-016-1437-y.
- Behzadi M.A., Leyva-Grado V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus infections. Front. Microbiol. 2019; 10: 1327. https://dx.doi.org/10.3389/fmicb.2019.01327.
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020; 395(10223): 507-13. https://dx.doi.org/10.1016/S0140-6736(20)30211-7.
- Chong Y.P., Song J.Y., Seo Y.B., Choi J.P., Shin H.S.; Rapid Response Team. Antiviral treatment guidelines for Middle East respiratory syndrome. Infect. Chemother. 2015; 47(3): 212-22. https://dx.doi.org/10.3947/ic.2015.47.3.212.
- World Health Organization. Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-CoV) infection is suspected: interim guidance. WHO/MERS/Clinical/15.1 Revision 1. WHO; 2019.
- Commonwealth of Australia. Department of Health. Novel coronavirus (2019-nCoV). Available at: https://www.health.gov.au/health-topics/novel-coronavirus-2019-ncov
- FDA. Novel coronavirus (2019-nCoV). Available at: https://www.fda.gov/emergency-preparedness-andresponse/mcm-issues/novel-coronavirus-2019-ncov
- Federal Ministry of Health. Current information on the coronavirus. Available at: https://www.bundesgesundheitsministerium.de/en/en/press/2020/coronavirus.html
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020; 2020; 14(1): 72-3. https://dx.doi.org/10.5582/bst.2020.01047.
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. et al. Cinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223): 497-506. https://dx.doi.org/10.1016/S0140-6736(20)30183-5.
- Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020; 92(4): 433-40. https://dx.doi.org/10.1002/jmv.25682.
- Jeong S.Y., Sung S.I., Sung J.H., Ahn S.Y., Kang E.S., Chang Y.S. et al. MERS-CoV infection in a pregnant woman in Korea. J. Korean Med. Sci. 2017; 32(10): 1717-20. https://dx.doi.org/10.3346/jkms.2017.32.10.1717.
- Lei J., Li J., Li X., Qi X. CT Imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020; 295(1): 18. https://dx.doi.org/10.1148/radiol.2020200236. Available at: https://pubs.rsna.org/doi/10.1148/radiol.2020200236
- Chang L., Yan Y., Wang L. Coronavirus disease 2019: Coronaviruses and blood safety. Transfus. Med. Rev. 2020; Feb 21. pii: S0887-7963(20)30014-6. https://dx.doi.org/10.1016/j.tmrv.2020.02.003.
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020; 382(13): 1199-207. https://dx.doi.org/10.1056/NEJMoa2001316.
- Li X., Zai J., Wang X., Li Y. Potential of large ‘first generation’ human-to-human transmission of 2019-nCoV. J. Med. Virol. 2020; 92(4): 448-54. https://dx.doi.org/10.1002/jmv.25693.
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends. 2020; 14(1): 69-71. https://dx.doi.org/10.5582/bst.2020.01020.
- National Health Commission of the People’s Republic of China. Available at: http://en.nhc.gov.cn
- Wu P., Hao X., Lau E.H.Y., Wong J.Y., Leung K.S.M., Wu J.T. et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020; 25(3). https://dx.doi.org/10.2807/1560-7917.ES.2020.25.3.2000044.
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China. J. Med. Virol. 2020; 92(5): 479-90. https://dx.doi.org/10.1002/jmv.25707.
Received 24.04.2020
Accepted 14.05.2020
About the Authors
Stefano Bettocchi, full professor, II Unit of obstetrics and gynecology at the Policlinico of Bary, University ‘Aldo Moro’, Bary, Italy.Attila Vereczkey, professor, Founder and Medical director of the Human Reproduction Institute – Versys Clinics. 1138, Budapest, Hungary. Tel.: +36203881036.
E-mail: Attila.vereczkey@versysclinics.com.
Dmitriy O. Ivanov, Doctor of Medical Sciences, professor, Chief Neonatologist of the Ministry of Health of Russia, Rector of Saint-Petersburg State Pediatric Medical University, Ministry of Health of Russia.
2 Litovskaya str., St. Petersburg, 194100, Russian Federation.
William Kondo, professor, Vita Batel hospital. 80420-60, R. Alferes Angelo Sampaio, 1986 Batel, Curitiba – PR, Brazil.
Kirill Yu.Krylov, PhD, candidate of medical sciences, researcher, obstetrician-gynecologist, Saint Petersburg I.I. Dzhanelidze Research Institute of Emergency Medicine.
Tel.: + 7(911)168-70-73. E-mail: drkrylov@mail.ru.
3 Budapeshtskaya str., St. Petersburg, 192242, Russian Federation.
Franco Lisi, professor, director of IVF Unit Altamedica. 00198, Viale Liegi 45, Roma, Italy. E-mail: Franco.lisi01@gmail.com.
Felice Petraglia, professor, president of society of endometriosis and uterine disorders, Obstetrics and gynecology Department Exp. Clinical Biomed. Sci. University of Florence. 50134, Firenze, Italy. E-mail: petraglia@unifi.it.
Vitaly A. Reznik, assistant professor, candidate of medical sciences, Head physician of Saint-Petersburg State Pediatric Medical University, Ministry of Health of Russia.
2 Litovskaya str., St. Petersburg, 194100, Russian Federation.
Nikolai N., Rukhliada, professor, Chief Researcher of the Department of Gynecology, I.I. Dzhanelidze Saint Petersburg Research Institute of Emergence Care; Head of the Department of Obstetrics and Gynecology with a Course of pediatric gynecology, Chief specialist, Saint Petersburg State Pediatric Medical University,
Ministry of Health of Russia. Tel.: + 7(911)913-20-20. E-mail: nickolasr@mail.ru.
3 Budapeshtskaya str., St. Petersburg, 192242, Russian Federation.
Sushila Saini, Professor of the Jaipur doorbeen hospital, 8, devi nagar, new sanganer road, opposite metro pillar 79. Jaipur, India.
Daniel Sanabria S., MD, gynecologist, Obstetrics and Human reproduction department Santa Fe de Bogota Foundation University of the Andes, Bogota, Columbia.
For reference: Bettocchi S., Veretzky A., Ivanov D.O., Condo W.,
Krylov K.Yu., Lisi F., Petraglia F., Reznik V.A., Rukhlyada N.N., Saini S.,
Sanabria D. A generalized action plan for obstetric hospitals and outpatient clinics during the suspected or confirmed COVID-19 pandemic.
Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2020; 5: 34-41 (In Russian).
https://dx.doi.org/10.18565/aig.2020.5.34-41



