The impact of COVID-19 on the outcomes of assisted reproductive technology programs
Objective: To investigate the outcomes of assisted reproductive technology (ART) programs in patients with a history of COVID-19 of various severity.Dolgushina N.V., Ermakova D.M., Lomova N.A., Menzhinskaya I.V., Vtorushina V.V.
Materials and methods: This prospective study enrolled 240 infertile patients. They were divided into group 1 comprising patients without a history of COVID-19 (n=105) and group 2 (n=135) including patients who less than 12 months before the ART cycle had mild (subgroup 2a, n=85) or moderate (subgroup 2b, n=50) COVID-19. The level of specific antibodies to SARS-CoV-2, parameters of oogenesis, early embryogenesis, and clinical outcomes of HRT were evaluated.
Results: The parameters of oogenesis and embryogenesis, pregnancy and delivery rates did not differ between groups 1 and 2. A weak negative correlation was detected between the level of IgG-antibodies to SARS-CoV-2 and the number of obtained oocytes and embryos. Patients with an interval between COVID-19 and ART cycle ≤6 months had a significantly higher relative number of poor-quality blastocysts than women with >6 months interval. Patients who experienced moderate COVID-19 had a high early miscarriage rate of (12%).
Conclusion: COVID-19 can adversely affect reproductive outcomes, lead to a decrease in the number of oocytes and embryos obtained in ART cycles and their quality, and increase the risk of early miscarriage. More research is needed to investigate the mechanisms underlying the adverse effects of COVID-19 and the post- COVID syndrome.
Keywords
COVID-19 pandemic has stimulated research on the impact of maternal exposure to SARS-CoV-2 infection during pregnancy on female reproductive health. Of particular interest are studies investigating the impact of the SARS-CoV-2 virus on human oogenesis and embryogenesis. The high expression of protein receptors for SARS-CoV-2 (ACE2, TMPRSS2, CD147) in ovarian granulosa and epithelial cells, theca cells and luteal cells has been established [1]. SARS-CoV-2 infection is supposed to have an adverse effect by penetrating into ovarian tissues and oocytes through these receptors [2]. In animal models, ACE2 expression and activity have been shown to increase in the uterus and placenta during pregnancy [3]. In light of this, reproductive organs are considered potential targets of SARS-CoV-2.
Isolated studies have investigated the effects of COVID-19 on the female reproductive system, but their results were contradictory and inconsistent [4–7]. There is also insufficient evidence on the potential of SARS-CoV-2 infection of human embryos through oocytes and vertical transmission of infectious agents from an infected mother to her fetus during pregnancy [8–10]. COVID-19 can increase the risk of pregnancy complications such as spontaneous miscarriage and preterm birth. The incidence of preterm birth in pregnant women with COVID-19 was higher than in women without COVID-19 and ranged from 11.5% [11] to 17% [12]. The rate of early spontaneous miscarriage in pregnant women infected with SARS-CoV-2 in the first trimester was as high as 14% and higher than in uninfected women (8%) [13]. The incidence of complications (miscarriages, preterm births) has been shown to be influenced by the age of women and the severity of COVID-19 [14].
Studies investigating the outcomes of ART programs in patients with a history of COVID-19 are also scarce. In a meta-analysis of 2022, no effect of coronavirus infection was observed on the outcomes of assisted reproductive technology (ART) [15]. However, another study showed a tendency for an adverse effect of COVID-19 on oocyte yield in ART programs [16]. It has been shown that post-COVID syndrome is associated with cardiovascular complications, chronic fatigue, and neurological disorders [17]. One of the manifestations of this syndrome may be reproductive dysfunction, leading to decreased fertility and recurrent pregnancy loss [18]. Thus, the study of reproductive function in women who were exposed to SARS-CoV-2 infection is an important and urgent task.
In view of the above, the present study aimed to investigate the outcomes of ART programs in patients with a history of COVID-19 of various severity.
Materials and methods
The study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology. A total of 240 infertile patients were enrolled in the prospective observational study. The patients were stratified into 2 groups categorized by their history of COVID-19. Group 1 comprised patients without a history of COVID-19 (n=105) and group 2 (n=135) included patients with a history of COVID-19, which occurred less than 12 months before the ART cycle. Group 2 was further stratified into subgroups with mild (subgroup 2a, n=85) or moderate (subgroup 2b, n=50) COVID-19.
The inclusion criteria were age 18–40 years, normal ovarian reserve [anti-Mullerian hormone (AMH)≥1.2 ng/ml, follicle-stimulating hormone (FSH)] <12 mmU/ml, antral follicle count (AFC)≥5 in both ovaries), a history of COVID-19 at 12 months or less before entering the ART for Group 2 patients. Exclusion criteria were a history of COVID-19 vaccination, contraindication to ART, morbid obesity (body mass index ≥40.0 kg/m2), donor program, surrogacy program, HIV infection.
Data on COVID-19 were obtained from patients' medical records, confirmed by information entered into the USHIS, and by determination of serum IgG antibodies against SARS-CoV-2 above the positivity index (PI). The criterion for mild COVID-19 was a subfebrile temperature (<38°C) in the absence of clinical manifestations of a moderate course of infection. The criteria for moderate COVID-19 included a temperature above 38°C, exertion dyspnea, signs of pneumonia with minimal or moderate lung involvement (CT 1–2) and the absence of clinical manifestations of a severe SARS-CoV-2 infection [19].
All patients were examined according to the Women's Infertility Clinical Guidelines (2021) [20].
Antibodies to SARS-CoV-2 in serum samples were determined using the "Reagent kit for detection of class G antibodies to spike protein SARS-CoV-2 by enzyme immunoassay" ("DS-IFA-ANTI-SARS-CoV-2-G(S)", produced by RPC Diagnostic Systems (Russia) for qualitative detection of antibodies in human blood serum (plasma) by enzyme immunoassay (EIA). The result of the analysis was evaluated by the PI value calculated by the formula: PI=OD of the sample/cutoff, where OD of sample is the optical density of the sample. The result was considered positive with PI>1.2, negative with PI <0.8, and equivocal with PI from 0.8 to 1.2.
Ovarian stimulation was performed according to the protocol using a gonadotropin-releasing hormone antagonist (ant-GnRH), recombinant FSH (rFSH), and/or preparations containing luteinizing hormone (LH): combined rFSH/rLH or human menopausal gonadotropin. Ovarian stimulation was performed 6 (2–9) months after COVID-19. The dose of gonadotropins was selected individually, taking into account age, medical history, and parameters of ovarian reserve. Gonadotropins were administered from days 2–3 of the menstrual cycle, anti-GnRH was administered as soon as the lead follicle reached 14 mm in diameter daily until the day of the ovulation trigger (inclusive) as soon as the lead follicle reached 19 mm in diameter. Chorionic gonadotropin (CG) (8000–10000 IU) once-only or a combination of CG and gonadotropin-releasing hormone agonist (a-GnRH) was used as an ovulation trigger. Transvaginal puncture (TVP) of follicles was performed 36 h after ovulation trigger injection under ultrasound guidance.
Aspirated follicular fluid was evaluated by an embryologist using a stereomicroscope. The analysis included an assessment of the number of oocyte-cumulus complexes (OCCs) and oocyte maturity after denudation. Parallel centrifugation, flotation, and processing of the partner sperm were performed. Fertilization of all mature oocytes was conducted using the in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Normal fertilization was recorded by the presence of two equally sized pronuclei in the cytoplasm 16–18 h after fertilization. After fertilization, the zygotes were transferred to culture medium (COOK, Australia) for further culture. After 120–122 h (on day 5), the embryos were morphologically evaluated taking into account the morphological characteristics of the embryos according to Gardner blastocyst classification system: blastocyst maturity, intracellular mass quality, and trophectoderm quality [21].
On the fifth day of culture, one or two embryos were transferred to the uterine cavity using a soft catheter in a stimulated cycle. Vaginal micronized progesterone (600 mg per day) or oral dydrogesterone (30 mg per day) was administered to support the period after embryo transfer.
Pregnancy was confirmed by a positive β-hCG test ((serum hCG level ≥20 IU/L) on day14 after embryo transfer. At 21 days after embryo transfer, clinical pregnancy was recorded by fetal ultrasound in the uterine cavity.
Statistical analysis
Statistical analysis was performed using Statistica 10 (USA) software. Categorical variables were presented as counts and percentages. Categorical variables were compared by the χ2 test. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test and graphical analysis of the data. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD), otherwise the median (Me) with interquartile range (Q1; Q3) were reported. The Mann–Whitney and Kruskal–Wallis tests were used to compare unpaired samples. The Spearman correlation coefficient was calculated to assess the correlation between the variables. The odds ratio (OR) with 95% confidence interval (95% CI) was determined to compare binary data. The logistic regression method was used to calculate the OR for confounder control. Differences between groups were considered statistically significant at p<0.05.
Results
The mean age of the patients in the two groups was 34 years and ¼ of the patients were of late reproductive age (37 years or older) (Table 1). Analysis of clinical and anamnestic data showed that patients with a history of COVID-19 had a higher body mass index (BMI), a higher proportion of women who consumed alcohol, and a higher incidence of ENT and allergic diseases than patients without a history of COVID-19. Endocrine diseases were diagnosed equally frequently in both groups (>20%).
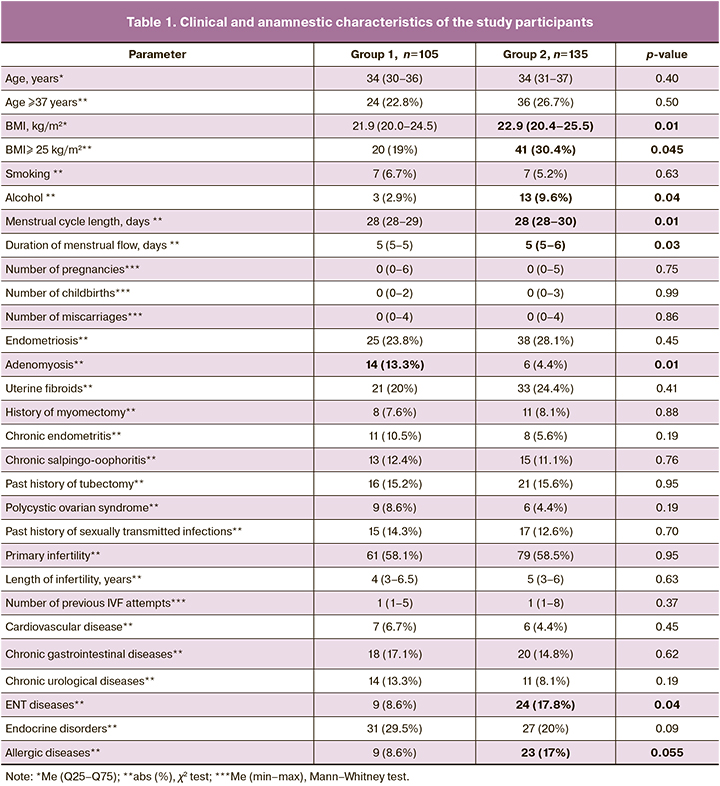
Women in groups 1 and 2 did not differ in the mean number of pregnancies, births, and miscarriages and in the rates of miscarriages [3/105(2.9%) and 9/135(6.7%); p=0.18]. Women in group 2 had significantly longer menstrual cycle length and duration of menstrual bleeding. Endometriosis and uterine fibroids were the most frequently diagnosed gynecological diseases in both groups (≥20%). Patients in group 2 were significantly less likely to have chronic endometritis, salpingo-oophoritis, and polycystic ovary syndrome (p<0.01). Analysis of gynecological morbidity showed that groups 1 and 2 differed significantly only in the frequency of adenomyosis, which was diagnosed in group 1 more often than in group 2.
The scheme and duration of ovarian stimulation administration did not differ between the study groups. Data on spermatogenesis, oogenesis, and early embryogenesis are presented in Table 2. There was no difference in the proportion of patients with any form of pathospermia. Absolute and relativeparameters of oogenesis and embryogenesis were comparable in patients of two groups and subgroups of group 2, although group 1 had slightly higher values of mature oocytes and mature oocyte-to-total oocyte ratio. The proportion of ICSIs was high in groups 1 and 2, which was due to the high number of couples with pathospermia in the partner, 72/105 (68.6%) and 86/135 (63.7%), respectively (p=0.43).
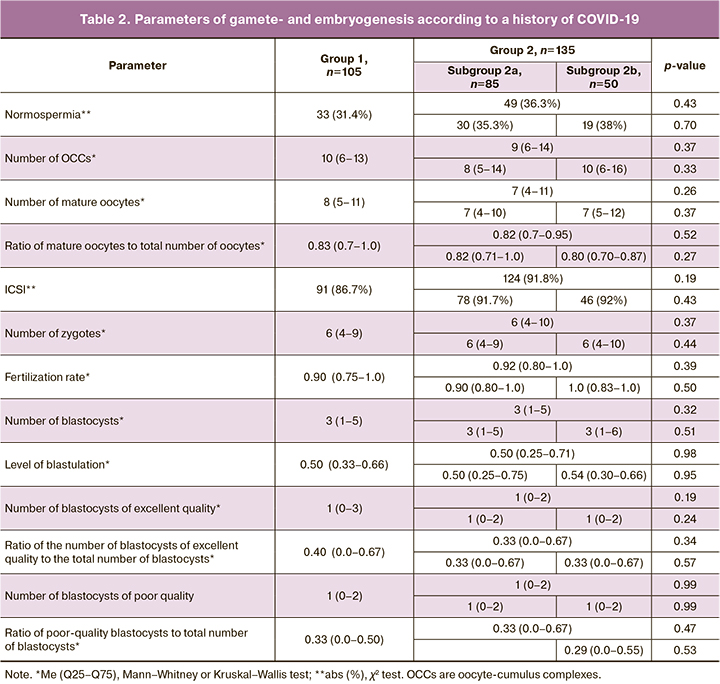
The mean PI values for specific antiviral antibodies were 0.16 (0.13) and 6.8 (4.1) in groups 1 and 2, respectively. The PI of antibodies in group 2 was significantly higher than in group 1 (p<0.001). The correlation between the level of specific antiviral antibodies and the parameters of oogenesis and embryogenesis in the patients was analyzed (Table 3). A weak significant negative correlation was detected between the level of specific antiviral antibodies and the number of obtained oocytes, mature oocytes, zygotes, and blastocysts, i.e., the patients with a higher level of antibodies had a smaller number of obtained oocytes and embryos.
We also analyzed the correlation between the parameters of oogenesis and embryogenesis in COVID-19 patients and the time interval from the disease to TVP. Patients were divided into 2 subgroups classified by the time interval from COVID-19 to TVP, subgroup 1, with an interval of more than 180 days (n=50) and subgroup 2, with an interval of 180 days or less (n=85).
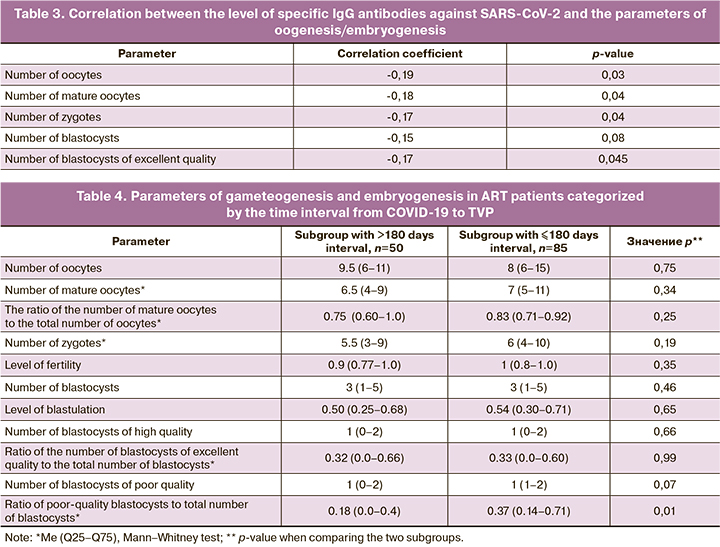
Patients with COVID-19 less than 6 months before TVP were shown to have a significantly higher ratio of poor-quality blastocysts (category C) to total blastocysts than patients with a longer time interval before TVP. There was an inverse correlation between this index and the time factor (the interval from COVID-19 disease to TVP) with r=-0.22 (p=0.01).
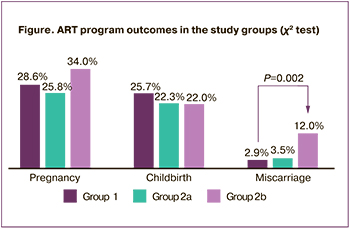
Analysis of the outcomes of the ART program revealed that pregnancy and delivery rates were not significantly different in group 1 and subgroups 2a and 2b (Figure). It is important to note that in subgroup 2b, patients with a history of moderate COVID-19 had the highest rate of spontaneous miscarriages before 12 weeks. All six miscarriages in subgroup 2b occurred in singleton pregnancies; in subgroup 2a, one miscarriage occurred in twin pregnancies and two in singleton pregnancies. The miscarriage rate in subgroup 2b compared to group 1 was 2.1 (95% CI 1.05–4.4). When adenomyosis was included as a confounder, the miscarriage rate in subgroup 2b compared to group 1 was 2.2 (95% CI 1.07–4.7).
Discussion
The accumulated research evidence mostly suggests that there is no adverse effect of COVID-19 on the outcomes of the ART program [5, 15, 22]. This is also evidenced by studies that compared the results of ART programs before and during the COVID-19 pandemic [23, 24]. However, there are also publications that discuss the possible adverse impact of a previous infection on the reproductive function of women [18]. One of the mechanisms of this effect may be the psychological stress experienced by women during the pandemic. According to a population study, during the pandemic, 46% of the women surveyed experienced menstrual irregularities and 53% of those had an increase in premenstrual symptoms [25]. Youngster M. et al. (2022) reported that oocyte yield in ART programs may be time-dependent [16]. Although coronavirus infection caused by SARS-CoV-2 did not affect treatment outcomes, including oocyte yield, fertilization, maturation rate, and clinical pregnancy rate in ART cycles, the possibility of a delayed adverse effect of infection on oocyte yield was observed.
Long COVID-19 syndrome is associated with many complications, including various cardiovascular abnormalities, chronic fatigue, and neurological disorders [17]. One of the manifestations of this syndrome can be reproductive disorders, manifested by decreased fertility and recurrent miscarriage [18]. Individual cases of reduced oocyte quality and ovarian function after COVID-19 infection, as well as cases of infertility or premature ovarian failure in fertile young patients after COVID-19 infection have been described (26, 27).
Exposure to SARS-CoV-2 infection during pregnancy has been shown to increase the risk of spontaneous miscarriage. The rate of early spontaneous miscarriage in pregnant women with COVID-19 is 1.7-fold higher compared to uninfected women [13]. The incidence of complications (miscarriages, preterm births) can be influenced by the age of the women and the severity of COVID-19 [14]. Women with severe symptoms of the disease, especially those over 35 years of age, tend to have a higher incidence of pregnancy complications.
Our study findings are consistent with the above-mentioned data. It was found that the parameters of oogenesis, embryogenesis, pregnancy rate, and delivery rate did not differ between patients with and without a history of COVID-19. However, there was a statistically significant weak negative correlation between the level of specific antiviral antibodies and the number of oocytes, zygotes, and blastocysts, which suggests an inverse relationship between the strength of the specific antiviral immune response, which is largely determined by the severity of the disease, and the yield of oocytes and embryos in ART.
Our results are also consistent with the recently published data of Herrero Y. et al. (2022), according to which a lower number of obtained and mature oocytes was observed in the group of women with high levels of specific antiviral antibodies, compared to the group of women with low or no levels of these antibodies [28]. This study shows the presence of antibodies to SARS-CoV-2 in the follicular fluid of COVID-19-treated women, changes in its composition, in particular a decrease in interleukin (IL)-1β and vascular endothelial growth factor (VEGF), and a possible effect of follicular fluid on protein expression in granulosa cells. SARS-CoV-2 infection is assumed to adversely affects the follicular microenvironment and therefore alter the regulation of ovarian function.
Importantly, the significant inverse correlation found in the present study between the ratio of the number of poor-quality blastocysts to the total number of blastocysts and the time interval from COVID-19 to TVP also indicates an adverse effect of the SARS-CoV-2 virus on oocytes, embryos and their quality and is important to decide the time of entry in ART in women with a history of severe COVID-19.
In addition, the results of this study indicate that when managing pregnancies in patients who have had moderate COVID-19 12 months or less before pregnancy, it is important to consider the higher risk of early spontaneous miscarriage compared with women who have not had COVID-19, which may be related in part to autoimmune activation in the post COVID-19 recovery.
Conclusion
A history of COVID-19 of less than 12 months before pregnancy, especially in its moderate form, can have an adverse impact on reproductive outcomes, increase the risk of early spontaneous miscarriage, result in fewer oocytes, fewer embryos in ART programs, and reduced oocyte quality during the early post-COVID-19 recovery time. A history of COVID-19 of less than 6 months is associated with an increased proportion of poor-quality blastocysts. More studies are needed to investigate the mechanisms of this adverse effect of the novel coronavirus infection and the post-COVID-19 syndrome.
References
- https://www.genecards.org/
- Долгушин Г.О., Романов А.Ю. Влияние SARS-COV-2 на репродукцию человека. Акушерство и гинекология. 2020; 11: 6-12. https://dx.doi.org/10.18565/aig.2020.11.6-12. [Dolgushin G.O., Romanov A.Yu. The influence of SARS-COV-2 on human reproduction. Obstetrics and gynecology. 2020; 11: 6-12 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.6-12
- Levy A., Yagil Y., Bursztyn M., Barkalifa R., Scharf S., Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008; 295(6): 1953-61. https://dx.doi.org/10.1152/ajpregu.90592.2008.
- Bentov Y., Beharier O., Moav-Zafrir A., Kabessa M., Godin M., Greenfield C.S. et al. Ovarian follicular function is not altered by SARS–CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum. Reprod. 2021; 36(9):2506-13. https://dx.doi.org/10.1093/humrep/deab182.
- Wang M., Yang Q., Ren X., Hu J., Li Z., Long R. et al. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: A retrospective cohort study. EClinicalMedicine. 2021; 38: 101013. https://dx.doi.org/10.1016/j.eclinm.2021.101013.
- Li K., Chen G., Hou H., Liao Q., Chen J., Bai H. et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod. Biomed. Online. 2021; 42(1): 260-7. https://dx.doi.org/10.1016/j.rbmo.2020.09.020.
- Ding T., Wang T., Zhang J., Cui P., Chen Z., Zhou S. et al. Analysis of ovarian njury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: An observational study. Front. Med. (Lausanne). 2021; 8: 635255. https://dx.doi.org/10.3389/fmed.2021.635255.
- Demirel C., Tulek F., Celik H.G., Donmez E., Tuysuz G., Gökcan B. Failure to detect viral RNA in follicular fluid aspirates from a SARS-CoV-2-positive oman. Reprod. Sci. 2021; 28(8): 2144-6. https://dx.doi.org/10.1007/s43032-021-00502-9.
- Barragan M., Guillén J.J., Martin-Palomino N., Rodriguez A., Vassena R. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum. Reprod. 2021; 36(2): 390-4. https://dx.doi.org/10.1093/humrep/deaa284.
- Boudry L., Essahib W., Mateizel I., Van de Velde H., De Geyter D., Piérard D. et al. Undetectable viral RNA in follicular fluid, cumulus cells, and endometrial tissue samples in SARS-CoV-2–positive women. Fertil. Steril. 2022; 117(4): 771-80. https://dx.doi.org/10.1016/j.fertnstert.2021.12.032.
- Mullins E., Perry A., Banerjee J., Townson J., Grozeva D., Milton R. et al. Pregnancy and neonatal outcomes of COVID-19: The PAN-COVID study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022; 276: 161-7. https://dx.doi.org/10.1016/j.ejogrb.2022.07.010.
- Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020; 370: m3320. https://dx.doi.org/10.1136/bmj.m3320.
- Balachandren N., Davies M.C., Hall J.A., Stephenson J.M., David A.L., Barrett G. et al. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: a UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum. Reprod. 2022; 37(6): 1126-33. https://dx.doi.org/10.1093/humrep/deac062.
- Shams T., Alhashemi H., Madkhali A., Noorelahi A., Allarakia S., Faden Y. et al. Comparing pregnancy outcomes between symptomatic and asymptomatic COVID-19 positive unvaccinated women: Multicenter study in Saudi Arabia. J. Infect. Public Health. 2022; 15(8): 845-52. https://dx.doi.org/10.1016/j.jiph.2022.06.002.
- Hu W., Zhu Y., Wu Y., Wang F., Qu F. Impact of COVID-19 pandemic on the pregnancy outcomes of women undergoing assisted reproductive techniques (ARTs): a systematic review and meta-analysis. J. Zhejiang Univ. Sci. B. 2022; 23(8): 655-65. https://dx.doi.org/10.1631/jzus.B2200154.
- Youngster M., Avraham S., Yaakov O., Landau Rabbi M., Gat I., Yerushalmi G. et al. IVF under COVID-19: treatment outcomes of fresh ART cycles. Hum. Reprod. 2022; 37(5): 947-53. https://dx.doi.org/10.1093/humrep/deac043.
- Khetpal V., Berkowitz J., Vijayakumar S., Choudhary G., Mukand J.A., Rudolph J.L. et al. Long-term cardiovascular manifestations and complications of COVID-19: spectrum and approach to diagnosis and management. R. I. Med. J. 2022; 105(7): 16-22.
- Bechmann N., Maccio U., Kotb R., Dweik R. Al, Cherfane M., Moch H. et al. COVID-19 infections in gonads: consequences on fertility? Horm. Metab. Res. 2022; 54(8): 549-55. https://dx.doi.org/10.1055/a-1891-6621.
- Временные методические рекомендации «Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19)» Версия 7 (утв. Министерством здравоохранения РФ 3 июня 2020 г.). Доступно по:https://base.garant.ru/74212510/ [Temporary guidelines "Prevention, diagnosis and treatment of new coronavirus infection (COVID-19)" Version 7 (approved by the Ministry of Health of the Russian Federation on June 3, 2020)(in Russian)]. Available at: https://base.garant.ru/74212510/
- https://cr.minzdrav.gov.ru/recomend/641_1
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Kolanska K., Hours A., Jonquière L., Mathieu d’Argent E., Dabi Y., Dupont C. et al. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod. Biomed. Online. 2021; 43(6): 1117-21. https://dx.doi.org/10.1016/j.rbmo.2021.09.001.
- Aharon D., Gounko D., Lee J.A., Copperman A.B., Flisser E. The impact of the coronavirus disease 19 pandemic on early pregnancy outcomes among patients undergoing in vitro fertilization treatment. Womens Health Rep.(New Rochelle). 2021; 2(1): 473-8. https://dx.doi.org/10.1089/whr.2021.0054.
- Setti P.E.L., Cirillo F., Immediata V., Morenghi E., Canevisio V., Ronchetti C. et al. First trimester pregnancy outcomes in a large IVF center from the Lombardy County (Italy) during the peak COVID-19 pandemic. Sci. Rep. 2021; 11(1): 16529. https://dx.doi.org/10.1038/s41598-021-96134-9.
- Phelan N., Behan L.A., Owens L. The impact of the COVID-19 pandemic on women’s reproductive health. Front. Endocrinol. (Lausanne). 2021; 12: 642755. https://dx.doi.org/10.3389/fendo.2021.642755.
- Madaan S., Jaiswal A., Kumar S., Talwar D., Halani D. Premature ovarian failure-A long COVID sequelae. Med. Sci. 2021; 25(112): 1286-90.
- Wilkins J., Al-Inizi S. Premature ovarian insufficiency secondary to COVID‐19 infection: An original case report. Int. J. Gynecol. Obstet. 2021; 154(1): 179-80. https://dx.doi.org/10.1002/ijgo.13719.
- Herrero Y., Pascuali N., Velázquez C., Oubiña G., Hauk V., de Zúñiga I. et al. SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim. Biophys. Acta Mol. Basis Dis. 2022; 1868(1): 166295.https://dx.doi.org/10.1016/j.bbadis.2021.166295.
Received 29.08.2022
Accepted 16.09.2022
About the Authors
Nataliya V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director – Head of the Department for Scientific Projects Administration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, n_dolgushina@oparina4.ru,https://orcid.org/0000-0003-1116-138X, 117997, Russia, Moscow, Academician Oparin str., 4.
Darya M. Ermakova, Postgraduate Student, Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, daria.ermakova.97@bk.ru, https://orcid.org/0000-0002-8558-4687,
117997, Russia, Moscow, Academician Oparin str., 4.
Natalia A. Lomova, PhD, Senior Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health
of the Russian Federation, n_lomova@oparina4.ru, https://orcid.org/0000-0002-6090-586X, 117997, Russia, Moscow, Academician Oparin str., 4.
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of the Russian Federation, i_menzinskaya@oparina4.ru, https://orcid.org/0000-0002-5385-0370, 117997, Russia, Moscow, Academician Oparin str. 4.
Valentina V. Vtorushina, Ph.D., Clinical Pathologist, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry
of Health of the Russian Federation, v_vtorushina@oparina4.ru, https://orcid.org/0000-0002-8406-3206, 117997, Russia, Moscow, Academician Oparin str., 4.
Authors' contributions: Ermakova D.M. – patient recruitment, manuscript drafting; Dolgushina N.V. – planning the manuscript, reviewing and final approval of the article, statistical analysis; Lomova N.A. – patient recruitment, manuscript drafting; Menzhinskaya I.V. – manuscript drafting and editing; Vtorushina V.V. – conducting the laboratory investigations.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: This work was supported by the "Contribution to the Future" charitable foundation as part of the "Stop Coronavirus Together" campaign.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P
Patient consent for publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Dolgushina N.V., Ermakova D.M.,
Lomova N.A., Menzhinskaya I.V., Vtorushina V.V.
The impact of COVID-19 on the outcomes of assisted reproductive technology programs.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 10: 115-122 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.115-122



