A new hematological marker for intrauterine sepsis associated with preterm labor and premature rupture of membranes
Aim. To determine the role of mean platelet volume (MPV) in a complete blood count (CBC) in pregnant women with premature rupture of membranes for predicting intrauterine neonatal sepsis. Materials and methods. A retrospective study was conducted. It included the analysis of 84 birth histories of patients with a single pregnancy, who were admitted to Perinatal Center of Rostov region in the period from 2017 to 2018 due to premature rupture of the fetal membranes at 22.0–28.0 weeks of gestation. In all cases, preterm births occurred before 31.0 weeks of gestation. All patients included in the study were treated and diagnosed according to the Protocol for preterm birth management. Additionally, MPV in a complete blood count in pregnant women was assessed. The course of the neonatal period of life in newborns was analyzed as well. Other platelet-related indicators were not covered in this article. This issue is subject to consideration in further articles. At present, the analysis of changes in other characteristics of platelets in this group of pregnant women and the analysis of neonatal outcomes are being carried out. Results. Comparison of median values of MPV showed statistically significant differences between pregnant women in group I (Me=9.5; Q1=8.3, Q3=10.1) and in group II (Me=6.3; Q1=5.4, Q3= 7.8) (p<0.001). Statistically, the MPV value in a complete blood count before delivery was significantly higher in women, whose babies were born with sepsis compared to the group of women, whose premature babies were born without neonatal sepsis. Conclusion. High MPV in a complete blood count in pregnant women with premature rupture of membranes before delivery is a marker for the sepsis diagnosis in premature babies.Kuznetsova N.B., Bushtyreva I.O., Chernavsky V.V., Dmitrieva M.P., Baranov A.P.
Keywords
Premature rupture of membranes (PROM) is a spontaneous preterm prelabor rupture of membranes before 37 weeks of pregnancy [1–3]. The latency period lasts from disruption of fetal membrane integrity to the onset of labor. Its duration is inversely proportional to the gestation period [4]. The prevalence of PROM ranges from 2 to 15% of all pregnancies [5–8] and is the major cause of preterm labor [9]. PROM followed by preterm labor is the leading cause of morbidity and mortality in the neonatal period [10].
Compared to other causes of preterm birth, PROM is associated with a high risk of neonatal morbidity and mortality only in cases of intrauterine infection, which results in high rates of intrauterine fetal death, early neonatal infection and necrotizing enterocolitis [11].
Annually, there are 4 million newborn deaths worldwide, and about a third of them are due to infections. Sepsis and bacterial meningitis continue to be one of the major causes of neonatal mortality, especially among very low birth weight infants born to mothers, whose pregnancies were complicated with PROM. Sepsis is recognized as one of the most severe pathologies in newborns.
Early diagnosis and treatment of a newborn with suspected sepsis is essential to prevent severe and life-threatening complications. Compared to clear and valuable therapeutic regimens, it is difficult to predict the diagnosis of neonatal sepsis. As a rule, for this purpose complete blood count, procalcitonin test, detection of C-reactive protein, bacteriological examination and verification of the focus of infection are carried out.
Mean platelet volume (MPV) is a measurement of the average size of platelets in a blood sample calculated by automated hematology analyzers using electrical impedance or optical fluorescent method. An increase in platelet volume and size reflects the presence of a thrombotic and inflammatory environment. Based on this, MPV is supposed to be a possible marker of platelet activation and function. Some researchers show that MPV reflects inflammation, disease activity and the effectiveness of anti-inflammatory treatment of certain chronic inflammatory diseases. Although the main pathogenetic mechanisms of change in platelet volume are not yet fully understood, a complex interaction of platelets with pathogens and endothelial cells can result in sepsis and severe pathophysiological cascade characterized by a significant decrease in the number of platelets and their dysfunction [12, 13].
Currently, due consideration is not given to MPV in perinatal clinical practice in establishing diagnoses, developing tactics, and predicting the maternal and fetal condition.
A rather wide range of normal MPV values make it impossible to take this parameter into account in clinical decision making [14].
Aim of study: to determine the role of MPV in a complete blood count in pregnant women with PROM for predicting neonatal sepsis.
Materials and methods
A retrospective study was conducted. It included the analysis of 84 birth histories of patients with a single pregnancy, who were admitted to the Rostov-on-Don State Perinatal Center in the period from 2017 to 2018 due to premature rupture of the fetal membranes at 22–28 weeks of gestation. In all cases, preterm birth occurred before 31 weeks of gestation. The criteria for diagnosis of PROM were: amniotic fluid leak viewed in sterile mirrors, positive results of nitrazine test, assessment of amniotic fluid index using ultrasound. The cases with uncertain diagnosis and multiple pregnancies were not included in the analysis. All patients included in the study underwent a scope of therapeutic and diagnostic procedures stipulated by the Ministry of Health of the RF in the Order No. 572n of November 1, 2011 for "Approval of the Procedure to provide medical care in Obstetrics and Gynecology (except for the use of assisted reproductive technologies) and the Letter No. №15-4\10\2-9480 of December 17, 2013 “Preterm labor”. Additionally, MPV in a complete blood count (CBC) in pregnant women was calculated using automated hematology analyzer MindrayBC-5300.
The following clinical data were analyzed: the gestational age at the time of delivery, the time of anhydrous period, the amniotic fluid index before delivery, the signs of chorioamnionitis, the type of delivery. Laboratory testing data included the maternal leukocyte count, maternal serum MPV level, maternal C-reactive protein level and the data on antibiotic therapy before delivery. Other platelet-related indicators were not covered in this article. This issue is subject to consideration in further articles. At present, the analysis of changes in other characteristics of platelets in this group of pregnant women and the analysis of neonatal outcomes are being carried out.
After birth, the following data were assessed: the newborn’s sex and body weight; the status of the newborn's overall condition according to the Apgar scale at 1, 5 and 10 minutes after birth, Downes score, the length of stay in Emergency Intensive Care Unit (EICU), the presence of intrauterine infection.
Statistical analysis
Parametric and nonparametric tests were used to analyze the obtained data. In particular, Mann-Whitney U-test for comparison of samples that did not follow a normal distribution (Me (Q1; Q3)) and Student's t-test for comparison of samples that followed normal distribution (M ± m) were used, and statistical method of correlation analysis was used for calculation of the correlation coefficient, the values and the coefficient of determination.
The obtained data were processed and the values were calculated using software Statistica 12.
Results
Characteristics of sampling: the study included 84 patients who had delivery at 22–31 weeks of pregnancy, were diagnosed with PROM and their 84 newborns. The women were divided into two groups. Group I (n=39) included the patients, whose babies were born with intrauterine sepsis. Group II (n=45) included the patients, whose babies were born without intrauterine sepsis. The diagnosis of neonatal intrauterine sepsis was based on an increase in C-reactive protein above 5 mg/L, procalcitonin > 2 ng/ml, leukocytosis above 15x109 ml, neutrophil index > 0.2 and an identified focus of infection.
The gestational age at the time of delivery in group I was 27.6±0.28 weeks, in group II – 28,8±0,24 weeks; the time of anhydrous period in group I was 102.4 ±21.9 hours, in group II – 106.7±15.5 hours.
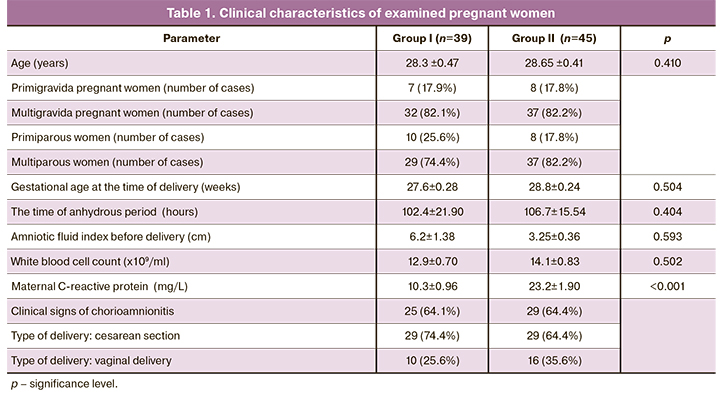
The age, parity, characteristics of the clinical status of pregnant women before delivery, laboratory data, presence/absence of antibiotic therapy are presented in Table 1.
Gestational age at the time of delivery, the time of anhydrous period and the clinical signs of chorioamnionitis (maternal fever ˃ 38°C), fetal tachycardia > 160 bpm, maternal tachycardia > 100 bpm), leukocytosis > 18x109 ml and neutrophil shift in white blood cell count were comparable in patients in groups I and II (Table 1).
26 patients had vaginal delivery and 58 patients had cesarean delivery. The indications for cesarean delivery in both groups were: breech presentation of the fetus – in 6 (10.3%) cases, 1 or more uterine scars – in 12 (20.7%), oligohydramnios – in 19 (32.8%), combined indications – in 21 (36.2%).
The types of delivery in patients in both groups were comparable. Cesarean delivery prevailed among the women in both groups (Table 1).
Leukocyte responses did not differ in groups I and II, but there were statistically significant differences in C-reactive protein levels (10.3±0.96 in group I and 23.2±1.90 in group II) (Table 1).
The following parameters were assessed in newborns: the sex and body weight; Apgar score at 1, 5 and 10 minutes after birth, and Downes score (Table 2).
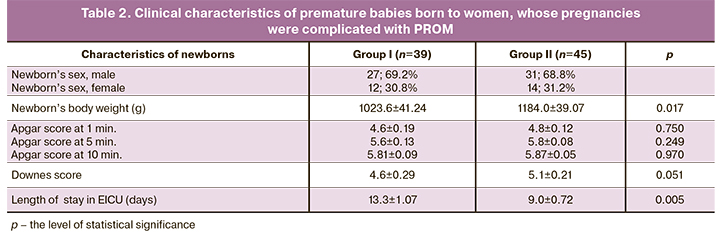
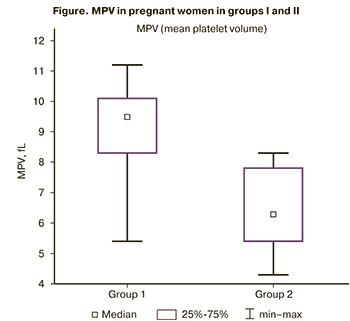 The demographic indicators and the status of newborns at birth, which were assessed using Apgar and Downes scores did not differ in both groups (Table 2). However, the length of postpartum hospital stay was longer in group I (women with intrauterine sepsis) than in group II (women without intrauterine sepsis). The average length of newborns’ stay in EICU born to women in group I was 13.3±1.07 days and in group 2 – 9.0±0.72 days. MPV level in a complete blood count in pregnant women before delivery was assessed using automated hematology analyzer MindrayBC-5300 (Figure).
The demographic indicators and the status of newborns at birth, which were assessed using Apgar and Downes scores did not differ in both groups (Table 2). However, the length of postpartum hospital stay was longer in group I (women with intrauterine sepsis) than in group II (women without intrauterine sepsis). The average length of newborns’ stay in EICU born to women in group I was 13.3±1.07 days and in group 2 – 9.0±0.72 days. MPV level in a complete blood count in pregnant women before delivery was assessed using automated hematology analyzer MindrayBC-5300 (Figure).
Mann–Whitney U-test was used for comparison of MPV that did not follow a normal distribution.
Comparison of median values of MPV using Mann–Whitney U-test showed statistically significant differences between pregnant women in group I (Me=9.5; Q1=8.3, Q3=10.1) and in group II (Me=6.3; Q1=5.4, Q3= 7.8) (p<0.001). In women, whose babies were born with intrauterine sepsis, MPV in complete blood count was significantly higher than in the group of women, whose babies were born without intrauterine sepsis.
Discussion
Sepsis is becoming a serious problem in neonatal intensive care units due to the development of antibiotic-resistant strains of microorganisms. Another problem is early diagnosis of neonatal sepsis. It is difficult to suspect this diagnosis in newborns; and it is even more difficult to diagnose this complication in premature babies. The difficulties arise due to the nonspecific clinical picture and the lack of reliable diagnostic tests leading to loss of time and delayed treatment.
Although a large amount of research has focused on the relationship between neonatal sepsis and thrombocytopenia [15], there are very few studies devoted to the relationship between septic complications and the properties of platelets. Guida J.D. et al. (2003) showed that, the values of MPV are high due to increased platelet production and/or increased platelet destruction in sepsis [16]. Abdelfadil A.M., Abdel Naem E.A. (2018) found that serum MPV level in newborns with clinical neonatal sepsis (11.7±1.3) and culture-confirmed neonatal sepsis (12.2±2.5), was high compared to the control group (healthy neonates) (8.5±2.5) [17]. Another study revealed statistically high values of MPV (10.2 μl) compared to neonates without neonatal sepsis [12].
It should be noted, that there are only few scientific papers about the role of platelets in the blood of newborns and their morphological characteristics, but the articles on the properties of platelets (in particular MPV) in the blood of pregnant women with PROM were not found in the published scientific literature.
Our study showed that pregnant women with PROM, who delivered premature babies with intrauterine sepsis, had high MPV level before delivery. MPV value in complete blood count was significantly higher (Me=9.5; Q1=8.3, Q3=10.1), than in the group of pregnant women, whose babies were born without intrauterine sepsis (6.3; Q1=5.4, Q3=7.8). It can be assumed that increased MPV in the mother's blood before delivery reflects inflammatory status in the mother, that cannot be considered separately from the uteroplacental complex and the fetus, and possibly indirectly indicates the inflammatory status in the fetus. The pathogenesis of increased MPV in women with PROM is not fully clear. However, the reason for an increase in MPV in case of sepsis is the destruction of platelets during their interaction with microorganisms.
Thus, the obtained data – increased MPV in pregnant women with PROM, who gave birth to newborns with intrauterine sepsis allow to consider it to be a predictor of birth of a premature baby with intrauterine sepsis.
Premature birth and prematurity are one of the leading causes of neonatal morbidity and mortality. This is especially important in relation to pregnancies with PROM because, in addition to prematurity, the factors of intrauterine infection are present in newborns.
Conclusion
Pregnant women with PROM at 22.0–28.0 weeks of gestation should be classified as a high risk group for developing intrauterine sepsis. A marker for the development of intrauterine sepsis is an increased MPV in the complete blood count before the onset of labor.
Additional markers (simple ones and not requiring additional costs) that allow assessment of safe prolongation of pregnancy (or the need for urgent delivery) can be of great importance for practical healthcare.
References
- Sim W.H., Araujo Júnior E., Da Silva Costa F., Sheehan P.M. Maternal and neonatal outcomes following expectant management of preterm prelabour rupture of membranes before viability. J. Perinat. Med. 2017; 45(1): 29-44. https://dx.doi.org/10.1515/jpm-2016-0183.
- Musilova I., Kacerovsky M., Stepan M., Bestvina T., Pliskova L., Zednikova B. et al. Maternal serum C-reactive protein concentration and intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017; 12(8): e0182731. https://dx.doi.org/10.1371/journal.pone.0182731.
- Bond D.M., Middleton P., Levett K.M., van der Ham D.P., Crowther C.A., Buchanan S.L. et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome. Cochrane Database Syst. Rev. 2017; 3: CD004735. https://dx.doi.org/10.1002/14651858.CD004735.pub4.
- Practice Bulletin No. 160: Premature rupture of membranes. Obstet. Gynecol. 2016; 127(1): e39-51. https://dx.doi.org/10.1097/AOG.0000000000001266.
- Rajan R., Menon V. Preterm premature rupture of membranes: correlates and pregnancy outcome in a tertiary care setting. Int. J. Res. Med. Sci. 2016; 4(8): 3310-6. https://dx.doi.org/10.18203/2320-6012.ijrms20162285.
- Ashwood E.R., Grenache D.G. Pregnancy and its disorders. In: Tietz textbook of clinical chemistry and molecular diagnostics. 6th ed. Philadelphia: Saunders; 2017: 2004-19.
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins–Obstetrics. Practice Bulletin No. 172: Premature rupture of membranes. Obstet. Gynecol. 2016; 128(4): e165-77. https://dx.doi.org/10.1097/AOG.0000000000001712.
- Chandra I., Sun L. Third trimester preterm and term premature rupture of membranes: Is there any difference in maternal characteristics and pregnancy outcomes? J. Chin. Med. Assoc. 2017; 80(10): 657-61. https://dx.doi.org/10.1016/j.jcma.2016.12.006.
- Kacerovsky M., Musilova I., Andrys C., Hornychova H., Pliskova L., Kostal M. et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am. J. Obstet. Gynecol. 2014; 210(4): 325. e1-325. e10. https://dx.doi.org/10.1016/j.ajog.2013.10.882.
- Wang K.C., Lee W.L., Wang P.H. Early and late preterm premature rupture of membranes. J. Chin. Med. Assoc. 2017; 80(10): 613-4. https://dx.doi.org/10.1016/j.jcma.2017.03.006.
- Schmitz T., Sentilhes L., Lorthe E., Gallot D., Madar H., Doret-Dion M. et al. Preterm premature rupture of the membranes: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 236: 1-6. https://dx.doi.org/10.1016/j.ejogrb.2019.02.021.
- Shalaby M.M., Sobeih A.A., Abdulghany W.E., Behiry E.G., Ismail Y.M., Abd-El-Aziz M.A. Mean platelet volume and serum uric acid in neonatal sepsis: A case-control study. Ann. Med. Surg. (Lond). 2017; 20: 97-102. https://dx.doi.org/10.1016/j.amsu.2017.06.015.
- Kim C.H., Kim S.J., Lee M.J., Kwon Y.E., Kim Y.L., Park K.S. et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One. 2015; 10(3): e0119437. https://dx.doi.org/10.1371/journal.pone.0119437.
- Noris P., Melazzini F., Balduini C.L. New roles for mean platelet volume measurement in the clinical practice? Platelets. 2016; 27(7): 607-12. https://dx.doi.org/10.1080/09537104.2016.1224828.
- Roberts I., Murray N.A. Neonatal thrombocytopenia: causes and management. Arch. Dis. Child. Fetal Neonatal Ed. 2003; 88(5): F359-64. https://dx.doi.org/10.1136/fn.88.5.f359.
- Guida J.D., Kunig A.M., Leef K.H., McKenzie S.E., Paul D.A. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003; 111(6, Pt. 1): 1411-5. https://dx.doi.org/10.1542/peds.111.6.1411.
- Abdelfadil A.M., AbdelNaem E.A. New insight for early diagnosis of neonatal sepsis. J. Paedatr. Neonatal Dis. 2018; 3(1): 104-6. Available at: http://www.annexpublishers.com/articles/JPND/3104-New-Insight-for-Early-Diagnosis-of-Neonatal-Sepsis.pdf
Received 07.07.2020
Accepted 08.02.2021
About the Authors
Natalia B. Kuznetsova, Dr. Med. Sci., Professor at the Simulation Training Center, RostSMU, Ministry of Health of Russia; Chief Physician of the Clinic of Professor Bushtyreva. Tel.: +7(928)770-97-62. E-mail: lauranb@inbox.ru. 344022, Russia, Rostov-on-Don, Nahichevansky str., 29; 344011, Russia, Rostov-on-Don, Sobornyi str., 58/7.Irina O. Bushtyreva, Dr. Med. Sci., Professor, Director of Clinic of Professor Bushtyreva. Tel.: +7(863)288-00-00. E-mail: kio4@mail.ru.
344010, Russia, Rostov-on-Don, Sobornyi str., 58/7.
Viktor V. Chernavsky, Cand. Med. Sci., Associate Professor of the Department of Obstetrics and Gynecology No. 2, RostSMU, Ministry of Health of Russia;
obstetrician-gynecologist, Perinatal Center of Rostov region. Tel.: +7(903)438-10-65. E-mail: chrnv@mail.ru. 344022, Russia, Rostov-on-Don, Nahichevansky str., 29;
344068, Russia, Rostov-on-Don, Bodraya str., 90.
Maria P. Dmitrieva, Junior researcher, RostSMU, Ministry of Health of Russia; obstetrician-gynecologist, Perinatal Center of Rostov region. Tel.: +7(951)830-65-06.
E-mail: dr.dmitrieva@inbox.ru. 344022, Russia, Rostov-on-Don, Nahichevansky str., 29; 344068, Russia, Rostov-on-Don, Bodraya str., 90.
Anton P. Baranov, post-graduate student, Simulation Training Center, RostSMU, Ministry of Health of Russia; obstetrician-gynecologist, Perinatal Center of Rostov region. 344022, Russia, Rostov-on-Don, Nahichevansky str., 29; 344068, Russia, Rostov-on-Don, Bodraya str., 90.
For citation: Kuznetsova N.B., Bushtyreva I.O., Chernavsky V.V., Dmitrieva M.P., Baranov A.P. A new hematological marker for intrauterine sepsis associated with preterm labor and premature rupture of membranes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 4: 84-89 (in Russian)
https://dx.doi.org/10.18565/aig.2021.4.84-89



